INTRODUCTION
Angiostrongylus cantonensis is a parasitic nematode of the pulmonary arteries in rodents and the leading aetiological agent of eosinophilic meningitis in humans. Humans become infected by ingestion of the L3 present in vegetables, water or through ingestion of raw or undercooked infected molluscs as intermediate host or other animals that may serve as paratenic hosts (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008). To date more than 2000 cases of the disease have been reported, mostly in Southeastern Asia (Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008). Cerebral angiostrongyliasis is an emerging global public health problem since the disease has been increasingly observed around the world, including countries where the disease had not been recognized previously, such as in Brazil, the Caribbean, Ecuador and South Africa (Caldeira et al. Reference Caldeira, Mendonça, Goveia, Lenzi, Graeff-Teixeira, Lima, Mota, Pecora, Medeiros and Carvalho2007; Diaz, Reference Diaz2008; Wang et al. Reference Wang, Lai, Zhu, Chen and Lun2008; Pincay et al. Reference Pincay, García, Narváez, Decker, Martini and Moreira2009; Maldonado et al. Reference Maldonado, Simões, Oliveira, Motta, Fernandez, Pereira, Monteiro, Torres and Thiengo2010; Archer et al. Reference Archer, Appleton, Mukaratirwa and Hope2011).
In humans the parasite is unable to complete its life cycle and eventually dies in the meninges. As a consequence the L1 are not found in feces preventing a simple parasitological diagnosis through identification of the parasite in stool samples. Fourth-stage larvae are rarely seen in the cerebrospinal fluid (CSF) and yet this is considered the gold standard for diagnosis (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009). In general A. cantonensis eosinophilic encephalitis can be distinguished from encephalitis caused by viruses, bacteria and protozoan parasites. Nevertheless differential aetiological diagnosis is required since there are other tissue-dwelling parasites that may cause eosinophilic meningitis, such as Gnathostoma and Baylisascaris (Graeff-Teixeira et al. Reference Graeff-Teixeira, da Silva and Yoshimura2009).
Several studies have identified specific and sensitive targets for immunodiagnosis of angiostrongyliasis. Most of the tests described in the literature use crude extract preparations obtained from the nematode. Recombinant protein antigens are needed, but their identification is hindered by the lack of molecular information on Angiostrongylus spp. Less than 800 nucleotide sequences, 2631 ESTs, 699 protein sequences and only 43 genes of Angiostrongylus were deposited at GenBank as of March 2013, including the mitochondrial genome sequences of A. costaricensis and A. cantonensis (Lv et al. 2012). To date, most Angiostrongylus proteomics studies have been performed using tandem mass spectrometry using orthologous sequences of related organisms for peptide mass comparison (Rebello et al. Reference Rebello, Barros, Mota, Carvalho, Perales, Lenzi and Neves-Ferreira2011; Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a). Unfortunately, the value of results from such analyses is limited because the amount of Angiostrongylus nucleotide or amino acid data in searchable databases is so small.
Next generation sequencing approaches have allowed rapid sequencing and annotation of genomes from a myriad of organisms. Data collected from such platforms allow researchers to close significant gaps towards the identification of previously unidentified open reading frames that translate into potential diagnostic targets. In this study we combined random high-throughput sequencing of the A. cantonensis genome together with proteomics analyses and cDNA walking methodology for novel immunoreactive protein discovery; this strategy is sometimes known as the dirty genome sequencing approach (Greub et al. Reference Greub, Kebbi-Beghdadi, Bertelli, Collyn, Riederer, Yersin, Croxatto and Raoult2009).
MATERIALS AND METHODS
Biological materials
Adult A. cantonensis worms were recovered from experimentally infected Rattus norvegicus. These worms were originally obtained from the Department of Parasitology, Akita Medical School, Japan and have been maintained in our laboratory since 1997. Wistar rats and Biomphalaria glabrata were used as definitive and intermediate hosts, respectively. Rats were infected with 104 larvae by gavage inoculation. Animals were euthanized 42 days after infection and female worms were isolated from their lungs and stored at −80 °C in RNAlater (Qiagen, Inc., Valencia, CA) to preserve the mRNA, according to manufacturer's instructions until use.
DNA sequencing
Total genomic DNA of A. cantonensis was extracted from female worms using the Gentra Puregene Tissue Kit (Qiagen, Valencia, CA, USA), according to the supplementary protocol for purification of archive-quality DNA from nematodes (www.qiagen.com/literature/render.aspx?id=103616). From genomic DNA, random shotgun libraries were produced using standard protocols for nebulization, AMPure XP magnetic bead cleanup (Beckman Coulter, Inc., Beverly, MA, USA), end-polishing, adaptor ligation and size selection. Roche-454 bead libraries were amplified using emulsion PCR followed by bead isolation and sequencing using long-read GS-FLX Titanium chemistry. Illumina libraries were flowcell amplified using a cBot single read cluster generation kit. These were then sequenced using v4 cycle sequencing chemistry on a Genome Analyzer IIe sequencer. Roche-454 data was cumulatively assembled using the Roche Newbler de novo assembler. Data assemblies which combine Roche-454 reads and the shorter Illumina reads were performed using CLC Genomics Workbench v 4.9 (Cambridge, MA, USA).
Sequence analysis
Contigs were examined by the open access software AUGUSTUS version 2.4 (Stanke et al. Reference Stanke, Diekhans, Baertsch and Haussler2008) (http://bioinf.uni-greifswald.de/augustus) to predict ORFs using NCBI Caenorhabditis elegans sequences as a model. For protein annotation the NCBI database was accessed to identify homology to other organisms associated with the protein sequence generated here using the PyCogent toolkit (Knight et al. Reference Knight, Maxwell, Birmingham, Carnes, Caporaso, Easton, Eaton, Hamady, Lindsay and Liu2007). The absolute and relative frequencies were calculated in the R project statistical software (Winham and Motsinger-Reif, Reference Winham and Motsinger-Reif2011). Immunogenic protein data were obtained in other related projects (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012b) that used two-dimensional electrophoresis and tandem mass spectrometry (MS/MS) experiments for antigenic protein identification. From these experiments the generated peptide database was re-searched against the AUGUSTUS genome data annotation using MyriMatch software (Tabb et al. Reference Tabb, Fernando and Chambers2007). Final annotation of each peptide, score ranking and artifact exclusion were done using the IDPicker software (Ma et al. 2009).
RNA isolation and cDNA synthesis
Approximately 30 mg of A. cantonensis worms were homogenized in 600 μL of Lysis Bufffer RA1 using a T8 homogenizer (IKA WORKS, Inc., Wilmington, NC, USA) and total RNA was isolated using the NucleoSpin RNA II Kit (Machery-Nagel, Inc., Bethlehem, PA, USA), according to the manufacturer's protocol. Concentration of isolated RNA was measured on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 5 μg of total RNA were converted into single-stranded cDNA using the SuperScript III First-Strand Synthesis SuperMix (Invitrogen, Carlsbad, CA, USA) with an oligo(dT)20 primer according to manufacturer's recommendations. The cDNA obtained was aliquoted and stored at −20 °C.
cDNA walking
In order to achieve the complete sequence of each protein of interest, C. elegans sequences from GenBank were used to search against the A. cantonensis sequences by the tblastn program. Two sets of three gene-specific primers were then designed based on each partial mRNA sequence identified and used to extend the 5′ and 3′ portions, respectively, using the DNA Walking (DW) SpeedUp Kit (Seegene, Rockville, MD, USA). The multiple sequences obtained from both 5′ and 3′ walking experiments for each gene were aligned using the MegAlign computer program package (DNASTAR, Inc., Madison, WI, USA) to obtain a full-length consensus cDNA sequence.
Two full-length sequences were obtained directly by AUGUSTUS software, corresponding to the 14-3-3 phosphoserine-binding protein and a protein containing a nascent polypeptide-associated complex domain (NAC). Primers were designed to clone and confirm cDNA sequences by sequencing. Primers: 14-3-3: Forward: 5´CAC CAT GAC GGA CAA CAG GGG CGA; Reverse: 5´TCA GTT GGC ACC CTC TCC TTG TTC. NAC: Forward: 5´CAC CAT GGT TGC CGC GGT GGA AGT; Reverse: 5´TTA ACA AAT AAC TGA GAA TCAA. Complete sequences of the amplicons were achieved by cycle sequencing using BigDye V3.1 chemistry (Applied Biosystems, Foster City, CA). Sequences were assembled, and then submitted to GenBank.
Data access
This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession ANFR00000000. The version described in this paper is the first version, ANFR01000000.
RESULTS
Genomic sequences
The genomic DNA of A. cantonensis was randomly sequenced by pyrosequencing using 454 (Roche) technology. About 2 million reads were assembled into 141 351 contigs with an average length of 0·8 kb each. From these contigs using AUGUSTUS software, 28 080 putative ORFs were obtained, of which 3370 were annotated according to their homology with other sequences deposited in GenBank as shown in Fig. 1 (details in supplementary material – in Online version only). More than 90% of the annotated sequences matched previously deposited sequences from nematodes other than A. cantonensis. Most of the sequences had homology to Caenorhabditis spp. (73%) sequences, but other nematode sequences were also represented (12%), such as Loa loa, Brugia malayi, Haemonchus contortus, Ancylostoma spp., Ascaris suum, among others (for more detail see supplemental material – in Online version only). Also, homology to other animal sequences, including arthropods, echinoderms, fish, and mammals, and 107 sequences (3·18%) homologous to bacteria organism were observed. The identified genes were separated in categories of their identity to relative homologous organisms (Fig. 1).

Fig. 1. Annotation of Open Reading Frame Sequence Homologies. Graphic shows the percentage of sequences homologous to different organisms.
Immunoreactive protein identification
Previous experiments using a proteomics approach identified 43 spots considered immunodiagnostic targets (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012b). From these spots 34 proteins were identified after interrogating GenBank (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012b). Those peptide sequences were then re-searched using our A. cantonensis genomic sequences. 206 putative ORFs identified by the AUGUSTUS software matched sequences from previous MS/MS data. From these, only 50 ORFs were identified that shared homology with peptides of other organisms using GenBank data; 156 ORFs matched only peptide sequences from the A. cantonensis MS/MS database.
Identification and extension of A. cantonensis antigenic sequences
After sequence assembly we focused the database analysis to search for sequences of diagnostic interest from previous mass spectrometry analyses of antigenic proteins (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeirab): galectins, the 14-3-3-like proteins, and a protein containing NAC domain were chosen for recombinant expression in the future.
Six galectins were identified in our A. cantonensis database. Partial sequences of each individual lec gene lec-1 (n=5), lec-2 (n=6), lec-3 (n=9), lec-4 (n=5), lec-8 (n=7) were aligned using the MegAlign program searched with orthologous C. elegans sequences. In addition, A. cantonensis galectin sequences from the GenBank EST database were used for comparison (Table 1). Based on these alignments, the full-length lec-1, lec-2 and lec-8 gene sequences were predicted. Alignment of the partial lec-8 sequence revealed a 118 bp deletion within GenBank EST DN190616. To verify the actual predicted proteins, three pairs of primers were designed and used to amplify corresponding full-length cDNAs. The partial gene sequences of A. cantonensis LEC-3, LEC-4 and LEC-5 were extended to full-length sequences by cDNA walking. Each was PCR amplified using 5′ and 3′ terminal gene-specific primer pairs. The complete sequences of the amplicons were obtained by individual sequencing and then submitted to GenBank under accession numbers: lec-1(JN133961), lec-2 (JN133962), lec-3 (JN133963), lec-4 (JN133964), lec-5 (JN133965) and lec-8 (JN133966). The other two targets protein 14-3-3 and NAC full-length ORFs were directly obtained by AUGUSTUS prediction. Primers were designed for cDNA amplification of each predicted ORF and amplicons were sequenced for confirmation of the sequence and finally deposited in GenBank under the accession numbers JN133968 and JN133967, respectively.
Table 1. Partial and full-length A. cantonensis galectin sequences
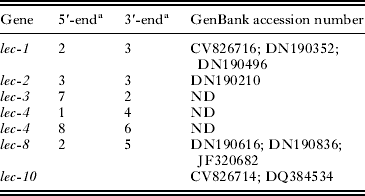
a Numbers of partial galectin sequences detected in our database. ND, not detected.
DISCUSSION
Recently Greub and co-workers applied a combined strategy of genome sequencing and proteomics to discover immunogenic proteins of the emerging pathogen Parachlamydia acanthamoebae and named the approach the ‘dirty genome approach’. This strategy demonstrated that incomplete genome sequence information could be used as a starting point to discover protein targets, especially for diagnostic target discovery (Greub et al. Reference Greub, Kebbi-Beghdadi, Bertelli, Collyn, Riederer, Yersin, Croxatto and Raoult2009).
As our previous mass spectrometry analyses of antigenic proteins have identified targets for angiostrongyliasis diagnosis (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012b), galectins, the 14-3-3 and a protein containing NAC domain were chosen for future recombinant expression because of their promising immunodiagnostic features (Schechtman et al. Reference Schechtman, Winnen, Tarrab-Hazdai, Ram, Shinder, Grevelding, Kunz and Arnon2001; Siles-Lucas et al. Reference Siles-Lucas, Merli and Gottstein2008; Wang et al. Reference Wang, Cheng, Lu and Tang2009). In our previous studies, these proteins were identified based on homology with orthologous sequences even though GeneBank-deposited DNA sequences of Angiostrongylus spp. have been increasing after 2000 EST sequences deposited by Wang in 2011 and the whole mitochondrial genome of both A. cantonensis and costaricensis were published (Lv et al. Reference Lv, Zhang, Zhang, Liu, Liu, Hu, Wei, Steinmann, Graeff-Teixeira, Zhou and Utzinger2012) including 12 protein coding genes, 22 transfer RNAs and 2 ribosomal RNAs. However, none of those Angiostrongylus deposited sequences matched with our search and actual protein sequence was needed to produce recombinant proteins for diagnostics. Also, in our original studies we sequenced some peptides that did not match anything in the databases.
After contig assembly, the peptide sequences obtained from mass spectrometry analysis were compared with our A. cantonensis genomic database. AUGUSTUS software predicted 3370 proteins and 206 of those had homology with the peptide sequences we originally obtained by mass spectrometry (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a, Reference Morassutti, Levert, Perelygin, da Silva, Wilkins and Graeff-Teixeira2012b). This is in stark comparison to the 26 proteins we identified using the existing GenBank entries (for annotation details, please see supplementary material – in Online version only). Thus, the remaining 156 protein sequences that matched the peptides generated by the MS/MS experiments were found only as a result of having the A. cantonensis genomic sequence database. This result indicates these 156 proteins are different from all published sequences in the GenBank and may be either exclusive to Angiostrongylus species or may be the first deposited sequences of a previously unrecognized relative. Although there is no known identity or function for these proteins, our proteomic data supports the AUGUSTUS prediction, especially since some of these were found from the native protein source, further demonstrating the value of this approach.
Galectins are a family of sugar-binding proteins with affinity for N-acetyl lactosamines, an interaction mediated via a conserved carbohydrate-recognition domain (CRD). It has a particular interest in parasite–host relationships because helminth galectins such as those from B. malayi and Onchocerca volvulus have been implicated as potential immune modulators (Klion and Donelson, Reference Klion and Donelson1994; Hewitson et al. Reference Hewitson, Harcus, Curwen, Dowle, Atmadja, Ashton, Wilson and Maizels2008). Also, a C. elegans galectin-8 has been shown to be involved in host defence during bacterial infection (Ideo et al. Reference Ideo, Fukushima, Gengyo-Ando, Mitani, Dejima, Nomura and Yamashita2009). In addition, galectins have also been identified as targets for vaccination as observed for the sheep gastrointestinal nematode Trichostrongylus colubriformis (Kiel et al. Reference Kiel, Josh, Jones, Windon, Hunt and Kongsuwan2007) as well as targets for diagnosis. The LEC-10 protein of A. cantonensis has been shown to be immunoreactive (Hao et al. Reference Hao, Wu, Chen and Wang2007) and we identified a Lec5 as a potential target for immunodiagnosis (Morassutti et al. Reference Morassutti, Levert, Pinto, da Silva, Wilkins and Graeff-Teixeira2012a).
In this paper we identified and extended the coding sequence of at least six galectins from A. cantonensis. Potentially more galectin sequences may be identified in the future taking into account that 11 members of the galectin family were previously described in the C. elegans genome (Nemoto-Sasaki et al. Reference Nemoto-Sasaki, Hayama, Ohya, Arata, Kaneko, Saitou, Hirabayashi and Kasai2008). Interestingly both 5′ and 3′ terminal segments between ESTs DN190616 (previously deposited) and DN190836 are identical, while internal sequences are different. Most likely that these two ESTs originated by alternatively spliced transcripts of the lec-8 gene since EST DN190616 is missing an internal exon as compared with EST DN190836. Alternative splicing in an invertebrate galectin gene had been reported for the cnidarian organism Hydra, which generates two nemato-galectin transcripts and these are mutually exclusively expressed (Hwang et al. Reference Hwang, Takaku, Momose, Adamczyk, Özbek, Ikeo, Khalturin, Hemmrich, Bosch and Holstein2010). In addition, we were able to amplify only a single lec-8 transcript corresponding to EST DN190836, which may suggest it could be expressed in a stage-dependent manner.
The A. cantonensis predicted genes matched a number of bacterial sequences (3.18%), some attaining about 80% similarity to the genus Burkholderia spp. and Neisseria spp. bacteria (see supplementary material – in Online version only). Interestingly, although previous research failed to demonstrate the presence of the endosymbiont organism Wolbachia spp. in A. cantonensis (Foster et al. Reference Foster, Kumar, Ford, Johnston, Ben, Graeff-Teixeira and Taylor2008), another study isolated a gram-positive bacteria from the intestines of A. cantonensis, indicating worm microbiota is reduced in number and diversity (Graeff-Teixeira personal communication). However the identity of this bacillus was not identified. The observation of sequences with homology to bacterial sequences from genomic preparations of the whole worm DNA might be indicative of DNA co-isolation either because it is endosymbiont or contamination from the host. Further studies are in progress to amplify and identify this possible prokaryotic organism.
In this study we generated not only the completed sequences of some possible antigenic protein targets of A. cantonensis, but also greatly increased the amount of genomic sequence data available in A. cantonensis databases. This will enhance future genomic, proteomic, transcriptomic or metabolomic studies for Angiostrongylus and will facilitate the search for diagnostic and possibly therapeutic targets for angiostrongyliasis.
FINANCIAL SUPPORT
Financial support was provided by Brazilian agencies: CNPq (scholarship 201760/2009-6) and US Food Safety Initiative, CDC and APHL- USA. C. Graeff-Teixeira is a recipient of a CNPq PQ 1D fellowship and of grants 300456/2007-7 and 477260/2007-1.