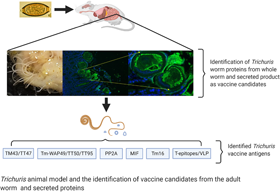
Introduction
Epidemiology
Trichuris trichiura, known as whipworm, is one of the three major soil-transmitted gastrointestinal helminths (STH, T. trichiura, Ascaris lumbricoides and Necator americanus/Ancylostoma duodenale) and causes infection in over 360 million people annually worldwide (IHME, 2019). STHs are highly prevalent neglected tropical diseases (NTDs) remaining one of the most common infectious pathogens in humans globally (Hotez, Reference Hotez2018b; Hotez et al., Reference Hotez, Bethony, Oliveira, Brindley and Loukas2008a, Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008b). Together, the three major STH infections rank high among the NTDs in terms of disability adjusted life years (DALYs). The most recent projections from the Institute of Health Metrics and Evaluation (IHME) estimate that the global burden of trichuriasis in 2019 alone was 236 000 DALYs (IHME, 2019). Trichuris is most commonly encountered in rural subtropical and tropical areas with overlapping extreme poverty in which sanitation facilities are inadequate (Pullan et al., Reference Pullan, Smith, Jasrasaria and Brooker2014; Zawawi and Else, Reference Zawawi and Else2020). Trichuriasis is highly prevalent in Southeast Asian nations of Myanmar, Malaysia, Philippines, Laos, and Vietnam; Nepal and Bangladesh in South Asia; Somalia and Cameroon in Africa; and Venezuela, Ecuador, and Honduras in Latin America (IHME, 2019) (Fig. 1).

Fig. 1. Geographic distribution of trichuriasis, from the GBD 2019; Age-standardized DALY rates (per 100 000) by location, both sexes combined. 2019. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2020. Available from http://ghdx.healthdata.org/gbd-results-tool. http://www.healthdata.org/results/gbd_summaries/2019/trichuriasis-level-4-cause.
Children harbour the largest number of T. trichiura infection (Bundy et al., Reference Bundy, Cooper, Thompson, Anderson and Didier1987). The basis for this age-dependent predilection is not well understood, and different theories have been proposed based on biological, environmental, or socioeconomic factors. Additionally, there is increasing evidence that in utero exposure may be linked to an increased risk of acquisition of worm infections (Weatherhead and Hotez, Reference Weatherhead and Hotez2015). In a St. Lucia village, about 90% of infections were diagnosed in children aged 5–15 years old. Children also harbour the highest worm burden (Fig. 2) (Bundy et al., Reference Bundy, Cooper, Thompson, Anderson and Didier1987; Stephenson et al., Reference Stephenson, Holland and Cooper2000), leading to a significant impact on childhood morbidity. However, more recent analyses suggest that high level of years lost from disability (YLDs) or related morbidity metrics can extend beyond early childhood and into adolescent and young adult age cohorts (IHME, 2019) (Fig. 3). For this illness, DALYs and YLDs are considered equivalent since no deaths are currently described directly.

Fig. 2. Prevalence and intensity of T. trichiura infection by age in St. Lucia. From Stephenson et al. (Reference Stephenson, Holland and Cooper2000), Used with permission.

Fig. 3. Composition of years lost from disability (YLDs) by age group and sex, 2019. From Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2020. Available from http://ghdx.healthdata.org/gbd-results-tool; http://www.healthdata.org/results/gbd_summaries/2019/trichuriasis-level-4-cause.
Parasite−host interaction
T. trichiura is transmitted through a faecal−oral cycle. The life cycle of T. trichiura infection starts with the passage of un-embryonated eggs in the stool. Once in the soil, the eggs embryonate and become infective after 15–30 days. After oral ingestion of the eggs from environmental exposure, via food or hands with contaminated soil, the eggs will hatch in the small intestine. First-stage larvae (L1) are released and penetrate the intestinal epithelial cells where they will create an intracellular niche and mature through the larval (L2, L3 and L4) and adult stages. The adult worms live in the caecum and ascending colon where the females will begin to oviposit 60–70 days after infection (Stephenson et al., Reference Stephenson, Holland and Cooper2000; Else et al., Reference Else, Keiser, Holland, Grencis, Sattelle, Fujiwara, Bueno, Asaolu, Sowemimo and Cooper2020).
The adult whipworm is slender with a tapered whip-like anterior end which embeds into the mucosa of the caecum. The engagement of the anterior whip with the host intestinal mucosa forms a syncytial environment in which whipworms take mucosal cells or blood as nutrition source (Stephenson et al., Reference Stephenson, Holland and Cooper2000; Else et al., Reference Else, Keiser, Holland, Grencis, Sattelle, Fujiwara, Bueno, Asaolu, Sowemimo and Cooper2020). Once inserted into the mucosa, whipworm secrets abundant proteins in the form of excretory−secretory (ES) products. ES products aid in immunomodulation of the host immune system to facilitate their parasitism in the human body (Else et al., Reference Else, Keiser, Holland, Grencis, Sattelle, Fujiwara, Bueno, Asaolu, Sowemimo and Cooper2020). Secretome analysis of T. muris adult worm ES products by mass spectrometry identified 73 unique proteins, with 62 of them sharing homology to other nematode species, revealing high secretome conservation within nematodes (Tritten et al., Reference Tritten, Tam, Vargas, Jardim, Stevenson, Keiser and Geary2017). More than 14 high confidence miRNA were also identified in the T. muris adult ES products that are believed to be involved in host immunomodulation (Tritten et al., Reference Tritten, Tam, Vargas, Jardim, Stevenson, Keiser and Geary2017). Beyond proteins, more than 35 non-protein small polar metabolites were found within the ES products of T. muris adult worms, 17 of them exhibited various pharmacological activities (Tritten et al., Reference Tritten, Tam, Vargas, Jardim, Stevenson, Keiser and Geary2017; Wangchuk et al., Reference Wangchuk, Kouremenos, Eichenberger, Pearson, Susianto, Wishart, McConville and Loukas2019). Another proteomic analysis on T. muris ES products using LC-MS/MS identified 147 proteins of which most were ‘trypsin-like peptidase’, ‘thioredoxin-like’ and ‘tetratricopeptide repeat domains’ proteins, but also hundreds of exosome-like extracellular vesicles (EVs) (Eichenberger et al., Reference Eichenberger, Talukder, Field, Wangchuk, Giacomin, Loukas and Sotillo2018). The molecular components of ES and EV are important mediators in parasite−host communication and aid in immune evasion by parasitic organisms such as Trichuris.
Trchuris infection induced T helper 2 (Th2) or T helper 1 (Th1) immune response in the host determines the resistance or susceptibility to the infection. Th-2 cytokines are associated with resistance to infection and rapid parasite expulsion. Cytokines that have been found to play a major role in Trichuris control include IL-4, IL-5, IL-9 and IL-13. Blocking IL-4 receptor during Trichuris infection polarizes to a Th-1 response and promotes chronic infection. On the contrary, the administration of IL-4 to susceptible mouse strains results in a predominant Th-2 response and clearance of infection (Klementowicz et al., Reference Klementowicz, Travis and Grencis2012; McSorley and Maizels, Reference McSorley and Maizels2012; Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). Additionally, neutralization of IL-9 using IL-9-specific antibody prevents Trichuris worm expulsion from the caecum (Richard et al., Reference Richard, Grencis, Humphreys, Renauld and Van Snick2000). Type 2 cytokines induce gut hypercontractility, increase mucus production and promote epithelial cell turn over which leads to rapid expulsion of parasites (Khan et al., Reference Khan, Richard, Akiho, Blennerhasset, Humphreys, Grencis, Van Snick and Collins2003; Cliffe and Grencis, Reference Cliffe and Grencis2004).
However, during chronic trichuriasis, high concentrations of IFN-γ, IL-12 and IL-18, characteristic of Th1 response, have been identified. IL-18 drives suppression of IL-4 and IL-13 (Th2-related cytokines) reducing rapid parasite expulsion which makes the host more susceptible to persistent infection (Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). But when IFN-γ is depleted there is a reduction in IL-18 which creates a more resistant immunologic profile in the host (Helmby et al., Reference Helmby, Takeda, Akira and Grencis2001). Studies have shown that T. muris infected colonic tissues resembles mouse models of inflammatory bowel disease, with a defective epithelial barrier and a predominant Th1-related cytokines infiltrate. Mouse models of chronic trichuriasis have massive crypt hyperplasia driven by parasite-derived IFN-γ homologue (Grencis and Entwistle, Reference Grencis and Entwistle1997).
Clinical manifestations
Most infections with T. trichiuria are asymptomatic. The clinical symptoms usually develop with moderate to heavy infections. The most common manifestations are asthenia, abdominal pain and diarrhoea (Jourdan et al., Reference Jourdan, Lamberton, Fenwick and Addiss2018). Trichuriasis has been associated with a form of inflammatory bowel disease which is linked to chronic diarrhoea and decreased nutrition intake, resulting in anaemia, physical and cognitive growth restriction in children. Heavy infections with T. trichiura can cause Trichuris dysentery syndrome (TDS) leading to severe malnutrition, bloody diarrhoea, tenesmus and rectal prolapse (Richard et al., Reference Richard, Grencis, Humphreys, Renauld and Van Snick2000; Stephenson et al., Reference Stephenson, Holland and Cooper2000; Khuroo et al., Reference Khuroo, Khuroo and Khuroo2010; Weatherhead and Hotez, Reference Weatherhead and Hotez2015; Zeehaida et al., Reference Zeehaida, Zueter, Zairi and Zunulhisham2015). T. trichiura infection promotes poverty by impacting cognitive and physical growth, reducing educational performance and impairing economic productivity of the society further perpetuating the cycle of poverty. Trichuriasis has emerged as substantial public health problem in areas of poverty globally. Because of the significant individual and societal impact of trichuriasis, it is critical to work towards disease control and eradication.
Current management approach
Benzimidazoles (albendazole and mebendazole) are the most commonly used anthelmintic therapy for the treatment of T. trichiura (Keiser and Utzinger, Reference Keiser and Utzinger2008) and have been recommended by the World Health Organization (WHO) as part of mass drug administration (MDA) and preventive chemotherapy approach to control STH infections (Keiser and Utzinger, Reference Keiser and Utzinger2008). Despite highly commendable efforts led by WHO to make therapeutic drugs available to everyone at risk, in 2019 the WHO estimated that of the 613 million children who required regular deworming, only two-thirds actually received treatment. In addition, there is evidence that pregnant women do not consistently receive anthelminthic treatments even though the WHO recommends deworming after the first trimester of pregnancy in high prevalence areas (Brooker et al., Reference Brooker, Hotez and Bundy2008; Hotez et al., Reference Hotez, Alvarado, Basanez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fevre, Furst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014).
However, preventive chemotherapy and targeted treatment strategies alone are not sufficient to achieve elimination of trichuriasis due to the following reasons:
(i) Single-dose albendazole and mebendazole is highly efficacious against A. lumbricoides (95.7% and 96.2%, respectively), however, both have poor efficacy against T. trichiura (30.7% and 42.1% respectively) (Keiser and Utzinger, Reference Keiser and Utzinger2008; Soukhathammavong et al., Reference Soukhathammavong, Sayasone, Phongluxa, Xayaseng, Utzinger, Vounatsou, Hatz, Akkhavong, Keiser and Odermatt2012; McCarty et al., Reference McCarty, Turkeltaub and Hotez2014; Clarke et al., Reference Clarke, Doi, Wangdi, Chen, Clements and Nery2019).
(ii) High rates of post-treatment re-infection have been observed, especially in areas of intense transmission, requiring a higher frequency of deworming (WHO, 2006; Yap et al., Reference Yap, Du, Wu, Jiang, Chen, Zhou, Hattendorf, Utzinger and Steinmann2013).
(iii) There are concerns about the potential development of Trichuris resistance to benzimidazoles as control programmes continue to be scaled up worldwide. This resistance threat has also been seen in areas of intense transmission with post-treatment infection and higher frequency of deworming (Vercruysse et al., Reference Vercruysse, Albonico, Behnke, Kotze, Prichard, McCarthy, Montresor and Levecke2011; Geary, Reference Geary2012; Clarke et al., Reference Clarke, Doi, Wangdi, Chen, Clements and Nery2019).
(iv) Some studies have shown that drug combination are superior to single-dose albendazole, however, there are multiple operational and financial barriers that would need to be considered for large-scale deworming programme (Clarke et al., Reference Clarke, Doi, Wangdi, Chen, Clements and Nery2019; Keller et al., Reference Keller, Palmeirim, Ame, Ali, Puchkov, Huwyler, Hattendorf and Keiser2020).
On the basis of these limitations, the current approach to deworming using treatment alone will not lead to the elimination of STH infections. Therefore, new technologies are required (Keenan et al., Reference Keenan, Hotez, Amza, Stoller, Gaynor, Porco and Lietman2013). There is increasing concerns about the suitability of preventive chemotherapy programmes for trichuriasis that rely exclusively on monotherapy with benzimidazole anthelminthic drugs (Keller et al., Reference Keller, Palmeirim, Ame, Ali, Puchkov, Huwyler, Hattendorf and Keiser2020; Patel et al., Reference Patel, Coulibaly, Schulz, N'Gbesso, Hattendorf and Keiser2020). In areas of Asia and Africa where ivermectin is being deployed for treatment of lymphatic filariasis or onchocerciasis, the combination of albendazole and ivermectin may offer superior results against trichuriasis, although there is recognition that we will require additional agents for the elimination of trichuriasis and other STH infections (Palmeirim et al., Reference Palmeirim, Hurlimann, Knopp, Speich, Belizario, Joseph, Vaillant, Olliaro and Keiser2018). The development of a preventive vaccine given to children before exposure to the helminths or during programmes linked to deworming (vaccine-linked chemotherapy to prevent helminth reinfection) would lead to acquisition of immunity at an earlier age and reduce community infection, representing a key technology for shaping global trichuriasis control and elimination strategies (Keenan et al., Reference Keenan, Hotez, Amza, Stoller, Gaynor, Porco and Lietman2013).
However, compared to other infectious pathogens, the vaccine development for trichuriasis has made modest progress and no human vaccine candidates have so far entered the critical path towards the clinic. A significant hurdle is the absence of an adequate animal model due to T. trichiura tropism for humans. Another challenge for trichuriasis vaccine development is the lack of effective antigens identified to induce protective immunity against trichuriasis. Lastly, consensus on what would be a suitable product development strategy and the minimal or preferred target product profile for such a vaccine has not been reached.
Animal model
Establishing an appropriate animal model is key for vaccine development against human whipworm. Trichuridae family are host-specific nematodes. Human whipworm T. trichiura predominately infects human, although have been found in the intestine of some non-human primates (NHP) (Ghai et al., Reference Ghai, Simons, Chapman, Omeja, Davies, Ting and Goldberg2014). The T. trichiura collected from infected macaques, baboons and humans are morphologically indistinguishable and some studies have suggested that primate and human T. trichiura share the same evolutionary line. Knowing that NHP can sustain infection provides a potential animal model for human whipworm research, however, genetic analysis of these pathogens suggests the phylogenetic structure of T. trichiura is complicated (Foth et al., Reference Foth, Tsai, Reid, Bancroft, Nichol, Tracey, Holroyd, Cotton, Stanley, Zarowiecki, Liu, Huckvale, Cooper, Grencis and Berriman2014; Ghai et al., Reference Ghai, Simons, Chapman, Omeja, Davies, Ting and Goldberg2014; Xie et al., Reference Xie, Zhao, Hoberg, Li, Zhou, Gu, Lai, Peng and Yang2018). Nevertheless, there is no NHP model established for laboratory infection of T. trichiura up until now. Despite the recent advancements of NHP trichuriasis models, they largely remain impractical due to cost, availability, ethical reasons and large space required for breeding colonies (VandeBerg and Williams-Blangero, Reference VandeBerg and Williams-Blangero1997).
However, human trichuriasis can be modelled using naturally occurring whipworm species in other animals, such as T. suis in pig (Beer, Reference Beer1976) and T. muris in mice (Else et al., Reference Else, Wakelin and Roach1989). Porcine T. suis and human T. trichiura are morphologically and genetically closely related (Beer, Reference Beer1976) potentially leading to cross-infections between humans and pigs (Beer, Reference Beer1976). However, T. suis fecundity in humans remains uncertain as it typically does not develop into mature adult worms in the human host (Beer, Reference Beer1976; Nejsum et al., Reference Nejsum, Betson, Bendall, Thamsborg and Stothard2012). However, it is possible that T. suis may develop to stages capable of secreting ES products that influence the host immune response which could provide insights into potential vaccine targets as well as reveal important pathogen−host interactions at the mucosal barrier (Leroux et al., Reference Leroux, Nasr, Valanparambil, Tam, Rosa, Siciliani, Hill, Zarlenga, Jaramillo, Weinstock, Geary, Stevenson, Urban, Mitreva and Jardim2018). This concept forms the basis of several human clinical trials which used T. suis egg infection to treat inflammatory bowel diseases (IBD), allergic airway disease, allergic rhinitis, asthma, and multiple myeloma due to the known immunomodulatory properties of the ES products (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b; Bager et al., Reference Bager, Arnved, Ronborg, Wohlfahrt, Poulsen, Westergaard, Petersen, Kristensen, Thamsborg, Roepstorff, Kapel and Melbye2010; Bourke et al., Reference Bourke, Mutapi, Nausch, Photiou, Poulsen, Kristensen, Arnved, Ronborg, Roepstorff, Thamsborg, Kapel, Melbye and Bager2012; Jouvin and Kinet, Reference Jouvin and Kinet2012; Bager, Reference Bager2013; Voldsgaard et al., Reference Voldsgaard, Bager, Garde, Akeson, Leffers, Madsen, Kapel, Roepstorff, Thamsborg, Melbye, Siebner, Sondergaard, Sellebjerg and Sorensen2015; Huang et al., Reference Huang, Zeng, Chen, Zhu and Zhu2018). However, despite the immunomodulatory properties of T. suis ES products, the meta-analysis on randomized, double-blind, placebo-controlled trials of T. suis ova therapy (TSO) showed no statistical benefit for IBD patients (Huang et al., Reference Huang, Zeng, Chen, Zhu and Zhu2018). T. muris is the rodent whipworm and shares a similar oral−faecal life cycle as well as extensive homology at genomic and transcriptomic levels as human T. trichiura (Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). As a result of these similarities, the T. muris mouse model has been largely used as a surrogate for immunogenicity and efficacy models for vaccine development against human trichuriasis (Dixon et al., Reference Dixon, Johnston and Else2008; Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). The model was specifically used to better characterize the natural history of infection with and without inhibiting key mediators in protective immunity (Else et al., Reference Else, Wakelin and Roach1989). Genetically altered mice have a range of T. muris susceptibility (Else et al., Reference Else, Wakelin and Roach1989). Genes within the H-2 allele and some non-H-2 genes cause resistance to T. muris infection (Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). Mice with H-2q, H-2b H-2 alleles have been found to expel parasites faster than mice having H-2k and H-2d alleles (Else and Wakelin, Reference Else and Wakelin1988). Furthermore, targeted deletion of genes related to the immunological response significantly affect parasite expulsion kinetics (Else et al., Reference Else, Hultner and Grencis1992). As a result different genetic knockout strains implications on T. muris susceptibility, experimental mouse models of trichuriasis can be classified as high-responder (HR), low-responder (LR), or non-responder (NR) strains to T. muris infection (Else and Wakelin, Reference Else and Wakelin1988). HR mice such as BALB/c mainly elicit a type-2 immune response, associated with elevated IL-4 and IgG1, enhancing early expulsion of worms from the intestines prior to maturation (Patel et al., Reference Patel, Kreider, Urban and Gause2009; Dixon et al., Reference Dixon, Little and Else2010). HR strains are ideal for examining the natural protective immune response to T. muris infection as well as the immunological response to vaccination and post-vaccine challenge models. LR mice (such as C57BL/10 and B10.B) or NR (AKR) induce a type-1 immune response (Else et al., Reference Else, Finkelman, Maliszewski and Grencis1994; Patel et al., Reference Patel, Kreider, Urban and Gause2009) which allows survival of worms in the large intestine and leads to chronic patent infections. Susceptible NR strains, such as AKR, are useful in testing the efficacy of potential vaccines by measuring reduction in worm burden but are less effective in understanding vaccine-induced protective immunity (type-2 immunity) due to the polarized type-1 immune response (Else et al., Reference Else, Finkelman, Maliszewski and Grencis1994; Robinson et al., Reference Robinson, Bellaby and Wakelin1995; Wangchuk et al., Reference Wangchuk, Kouremenos, Eichenberger, Pearson, Susianto, Wishart, McConville and Loukas2019). Therefore, selection of mouse strains for vaccine development studies needs to balance host susceptibility to infection with the development of vaccine-induced protective immunity (Robinson et al., Reference Robinson, Bellaby and Wakelin1995).
Beside the genetic background, the sex of mouse also affects worm expulsion dynamics in the intestines, with female mice being more resistant to infection while males are more susceptible (Robinson et al., Reference Robinson, Bellaby and Wakelin1995; Bancroft et al., Reference Bancroft, Artis, Donaldson, Sypek and Grencis2000; Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). This sex difference susceptibility to T. muris infection is likely related to discrepancies in production of the type-2 cytokine IL-13 (Bancroft et al., Reference Bancroft, McKenzie and Grencis1998, Reference Bancroft, Artis, Donaldson, Sypek and Grencis2000) and type-1 cytokines IFN-γ or TNF-α (Hayes et al., Reference Hayes, Bancroft and Grencis2007; Hepworth and Grencis, Reference Hepworth and Grencis2009). Studies have revealed that male-associated dihydrotestosterone hormones inhibit dendritic cell (DC) activation of T cells and skew T cell differentiation towards Th1 response via IL-18-dependent mechanisms. Female-related hormones increase the generation of Th2 response leading to enhanced Trichuris resistance (Hepworth et al., Reference Hepworth, Hardman and Grencis2010). This sex-specific immune polarization to T. muris infection has been linked to variation in sex-specific genes. A significant quantitative trait locus (QTL) gene on chromosome 5 associated with IFN-γ production was found only in male mice. This QTL was in the same location as a QTL for TNF-α and IL-6 production in male mice suggesting a locus of pro-inflammatory cytokines in male mice compared to female mice (Hayes et al., Reference Hayes, Hager and Grencis2014).
Infectious dose can also influence the relative resistance vs susceptibility of mice to T. muris (Klementowicz et al., Reference Klementowicz, Travis and Grencis2012). High dose infection with 200–300 eggs triggers a type-2 immune response and early expulsion of worms from the caecum during acute infection. Acute trichuriasis is further associated with the production of IL-13, which enhances mucus production and epithelial turnover in the caecum (Bancroft et al., Reference Bancroft, McKenzie and Grencis1998; Hasnain et al., Reference Hasnain, Evans, Roy, Gallagher, Kindrachuk, Barron, Dickey, Wilson, Wynn, Grencis and Thornton2011; Klementowicz et al., Reference Klementowicz, Travis and Grencis2012) and IL-9 which induces intestinal hypercontractility (Khan et al., Reference Khan, Richard, Akiho, Blennerhasset, Humphreys, Grencis, Van Snick and Collins2003). Thus, during high burden of infection, IL-13 and IL-9 are both critical for worm eradication from the gastrointestinal track. However, a low-dose infection with 10–25 eggs stimulates a type-1 immune response associated with IFN-γ-dominated CD4+ cells and subsequent chronic infection. As a result the low-dose inoculum is more reflective of natural infection (Bancroft et al., Reference Bancroft, Else, Humphreys and Grencis2001). Despite these differences, both high- and low-dose infection induce immunity mediated by IRF8- and IRF4-dependent dendritic cells and protect against re-infection (Bancroft et al., Reference Bancroft, Else, Humphreys and Grencis2001; Demiri et al., Reference Demiri, Muller-Luda, Agace and Svensson-Frej2017).
Lastly, different T. muris strains or isolates can affect the success of a mouse model for trichuriasis. The Edinburgh, Japan and Sobreda T. muris isolates consist of different molecular components in their ES products (Wakelin et al., Reference Wakelin, Farias and Bradley2002) and thus induce different host immune responses affecting their susceptibility (Koyama and Ito, Reference Koyama and Ito1996). B10.BR, CBA and C57BL/10 mice are usually resistant to both the Edinburgh and Japan isolates, but can develop chronic infection with the Sobreda isolate. The increased infectivity of the Sobreda isolate is related to its ability to increase type-1 associated high levels of IFN-γ and Th1-associated IgG2a production while inhibiting type-2 immune responses in HR mouse strains (Bellaby et al., Reference Bellaby, Robinson and Wakelin1996). The Sobreda isolate is also capable of inducing high concentrations of Tregs in the mouse gut potentially inhibiting the Th2-related protective immunity and promoting chronic infection (D'Elia et al., Reference D'Elia, Behnke, Bradley and Else2009).
Identification of Trichuris vaccine candidates
Development of Trichuris vaccines requires the identification of antigens that induce protective immunity. However, vaccine development against Trichuris infection remains in the early stages without many vaccine candidate antigens identified. Most of the successes with T. muris have been associated with adult-stage worm extracts and stichosome-derived proteins (Table 1).
Table 1. Major Trichuris vaccine candidates discovered to date

a add % after 99.01.
Adult worm extracts
Vaccination with whole worm extracts of T. muris induces a high degree of protective immunity in mice as assessed by reduction in larval worm burden (92%) (Wakelin and Selby, Reference Wakelin and Selby1973). Extract antigens from the anterior region of the adult worms that contain a parasite organ known as the stichosome induce higher protection than antigens prepared from the posterior region, indicating antigens released by stichocytes in the secretory glands in the anterior head elicit higher protective immunity (Wakelin and Selby, Reference Wakelin and Selby1973). Another study for mice vaccinated with whole worm extracts and stichosome extracts of adult T. muris also induce a high degree of protective immunity as assessed by reduction in larval worm burden further suggesting that the proteins originating from the stichosome may be strong immunogens (Jenkins and Wakelin, Reference Jenkins and Wakelin1977).
Oral vaccinations with T. muris adult worm extracts formulated with cholera toxin adjuvant induced significant protection in both HR BALB/c and LR C57BL/10. This response was associated with T. muris-specific intestinal IgA expression in these mice, but was not effective in the LR B10.BR mice (Robinson et al., Reference Robinson, Bellaby and Wakelin1995). Likewise, subcutaneous immunization with T. muris worm extracts formulated with Freund's adjuvant induced high level of circulating IgG1 and significant protection against subsequent T. muris egg challenge (Robinson et al., Reference Robinson, Bellaby and Wakelin1995). Further proteomic and immunological analysis of T. trichiura adult worm extract fractions identified that a homologue of macrophage migration inhibitory factor and heat-shock protein 70 could contribute to the immunomodulatory effects on host immune responses and may be related to the protective immunity (Santos et al., Reference Santos, Gallo, Silva, Figueiredo, Cooper, Barreto, Loureiro, Pontes-de-Carvalho and Alcantara-Neves2013).
Interestingly, Trichinella spiralis and T. muris that are genetically related nematodes, share cross-reactive antigen. Mice infected with each nematode or immunized with each soluble crude worm extracts elicited protective immunity against heterologous challenge infections with accelerated worm expulsion. This cross protection could also be achieved by adoptive transfer of mesenteric lymph node cells taken from mice infected with the heterologous parasite, indicating that there is a specific cross-immunity between T. spiralis and T. muris due to shared antigens (Lee et al., Reference Lee, Grencis and Wakelin1982). Exploring this cross-reactivity in vaccine development may shed light on the development of a pan-helminthic vaccine strategy.
Stichosome and excretory-secretory products
Trichuroidea superfamily nematodes, including Trichuris sp and Trichinella sp, possess a unique structure of stichosome at the anterior portion of the worms, which is a longitudinally arranged cell layer called stichocytes around the oesophagus. The stichosomes or stichocytes contain secretory granules that can be secreted or released as ES products through the anterior ends of the adult worms into the colonic mucosa (Trichuris sp) to facilitate their parasitism in the host (Despommier and Muller, Reference Despommier and Muller1976; Lee et al., Reference Lee, Grencis and Wakelin1982). Mice immunized subcutaneously with secretory exo-antigen extracted from stichosome of T. muris formulated with Freunds’ adjuvant induced high levels of immunity to T. muris challenge (Dixon et al., Reference Dixon, Little and Else2010; Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). The protective immunity was dose-dependent with 1 μg of stichosome extracts reducing worm burden by 50% and 10 μg reducing by 80–90%. The major protective antigen was a protein of 30 kDa (Jenkins and Wakelin, Reference Jenkins and Wakelin1983).
Trichuris adult worm secreted ES product contains a range of proteins with prominent components at 52–54, 35–45 and 17 kDa, with the most abundant protein at 43 kDa in T. muris or 47 kDa in T. trichiura (Lillywhite et al., Reference Lillywhite, Cooper, Needham, Venugopal, Bundy and Bianco1995). Immunological fluorescent assay showed that ES antigens were distributed in a patchy fashion throughout the cytoplasm of the stichocytes (Despommier and Muller, Reference Despommier and Muller1976; Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). Subcutaneous immunization of ES product induced nearly complete protective immunity against challenge infection of T. muris infective eggs, with recovered worms significantly smaller than those from controls indicating their impact on worm maturation (Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018; Leroux et al., Reference Leroux, Nasr, Valanparambil, Tam, Rosa, Siciliani, Hill, Zarlenga, Jaramillo, Weinstock, Geary, Stevenson, Urban, Mitreva and Jardim2018). The ES induced protection was associated with strong type-2 immune response (Dixon et al., Reference Dixon, Johnston and Else2008, Reference Dixon, Little and Else2010), enhanced intestinal goblet cell hyperplasia and proliferation of M2 macrophages. However, unlike natural infection, vaccine-induced immunity did not enhance epithelial turnover rate (Dixon et al., Reference Dixon, Little and Else2010). The use of ES products to induce immunity is not developmental stage dependent, however, L3 ES may contain unique antigens required for early stage maturation, could potentially aid in earlier parasitic eradication (Dixon et al., Reference Dixon, Johnston and Else2008).
Identification of specific antigens in ES products that induce protective immunity against Trichuris challenge is crucial to develop vaccines against whipworm infection. Some high molecular weight antigens (80–85, 90–95, 105–110 kDa) are related to protective immunity with induction of IgG1 (Else and Wakelin, Reference Else and Wakelin1990). A total of 11 immunogenic proteins with vaccine potential were identified in T. muris ES products after selective depletion of the protein with molecular mass of 43 kDa (known as TM43) (Shears et al., Reference Shears, Bancroft, Hughes, Grencis and Thornton2018). Using gel filtration chromatography and mass spectrometry analysis, the 11 selected proteins were able to induce antigen-specific IL-13 and IL-9 production (Shears et al., Reference Shears, Bancroft, Hughes, Grencis and Thornton2018). These proteins, including independent phosphoglycerate mutase (iPGM), serpin and translationally controlled tumour protein (TCTP), are present in T. trichiura but also have been reported in vaccine studies against other parasites (Rao et al., Reference Rao, Chen, Gnanasekar and Ramaswamy2002; Singh et al., Reference Singh, Kushwaha, Rana and Misra-Bhattacharya2014), indicating their potential as vaccine candidates (Shears et al., Reference Shears, Bancroft, Hughes, Grencis and Thornton2018). In addition to the potent protective immunity induced by Trichuris ES product proteins, the extracellular vehicles (EVs) isolated from T. muris ES are also immunogens. C57BL/6 mice vaccinated subcutaneously with EVs isolated from T. muris ES without adjuvant produced more than 50% worm reduction against a low dose of T. muris egg (25 embryonated eggs) challenge, associated with strong IgG1 response. The protection is dependent on intact vesicles. Several immunodominant proteins within EVs, including VWD, vitellogenin N and DUF1943-domain-containing protein, vacuolar protein sorting-associated protein 52 and TSP-1 domain-containing protein, are recognized by immune sera after vaccination and subsequent infection with T. muris. These EV proteins have homologues in other parasites of medical and veterinary importance (Shears et al., Reference Shears, Bancroft, Hughes, Grencis and Thornton2018). Trichuris worms interact with host cells and immune system through stichocytes secreted proteins, non-protein small polar metabolites or miRNA in the form of EVs, therefore these products may be important targets for developing new vaccine (Hansen et al., Reference Hansen, Kringel, Williams and Nejsum2015; Entwistle and Wilson, Reference Entwistle and Wilson2017; Eichenberger et al., Reference Eichenberger, Talukder, Field, Wangchuk, Giacomin, Loukas and Sotillo2018; Shears et al., Reference Shears, Bancroft, Hughes, Grencis and Thornton2018). However, it is not feasible to directly use ES and related products as vaccine to prevent Trichuris infection due to their difficulty in scale-up manufacture, high cost to produce enough worms in animal hosts and safety issue with complex of ES proteins. Identification of specific antigens in ES products that induce protective immunity, and large-scale production of these protective antigens as recombinant proteins or epitope-based vaccine will be an important strategy to develop vaccine against trichuriasis.
Trichuris proteins of vaccine interest
Tm43 and TT47
The most abundant component of T. muris adult ES is protein 43 kDa (TM43) (Drake et al., Reference Drake, Korchev, Bashford, Djamgoz, Wakelin, Ashall and Bundy1994) and 47 kDa in T. trichiura (TT47) (Lillywhite et al., Reference Lillywhite, Cooper, Needham, Venugopal, Bundy and Bianco1995). TM43 is secreted by L3 and adult T. muris stages and detected in the mucus surrounding the worm head. Surprisingly, TM43 is produced by muscle cells beneath the cuticle in adult worms and is not a secretory product of stichocytes (Lillywhite et al., Reference Lillywhite, Cooper, Needham, Venugopal, Bundy and Bianco1995; Bancroft et al., Reference Bancroft, Levy, Jowitt, Hayes, Thompson, McKenzie, Ball, Dubaissi, France, Bellina, Sharpe, Mironov, Brown, Cook, MacDonald, Thornton and Grencis2019). Further molecular cloning and genomic analysis have uncovered that TM43 is a poly-cysteine and histidine tailed protein with 36 cysteine residues, and a histidine-rich C-terminal region (Bancroft et al., Reference Bancroft, Levy, Jowitt, Hayes, Thompson, McKenzie, Ball, Dubaissi, France, Bellina, Sharpe, Mironov, Brown, Cook, MacDonald, Thornton and Grencis2019). Similar to the T. spiralis homologue, TM43 may play a role in metal storage and as a transporter (Radoslavov et al., Reference Radoslavov, Jordanova, Teofanova, Georgieva, Hristov, Salomone-Stagni, Liebau and Bankov2010). Biophysical function assay have shown that TM43 protein can induce pore formation in planar phospholipid bilayers, potentially playing an additional functional role in facilitating worm to invade the host gut, establishing a syncytial environment in host caecal mucosa and promoting plasma protein leakage across the gut mucosal surface in disease pathogenesis of trichuriasis (Drake et al., Reference Drake, Korchev, Bashford, Djamgoz, Wakelin, Ashall and Bundy1994, Reference Drake, Barker, Korchev, Lab, Brooks and Bundy1998). In silico docking analysis provides further insight into how this protein may interact with the host immune response. TM43 has a subdomain homologous to IL-13 receptor α2 suggesting its binding ability to IL-13 and inhibiting IL-13 function (Bancroft et al., Reference Bancroft, Levy, Jowitt, Hayes, Thompson, McKenzie, Ball, Dubaissi, France, Bellina, Sharpe, Mironov, Brown, Cook, MacDonald, Thornton and Grencis2019). As IL-13 is a key effector cytokine during T. muris acute infection, this inhibitory binding activity to IL-13 suggests TM43 plays an important role in host immunomodulation during Trichuris infections, making it a potential target for vaccine development (Bancroft et al., Reference Bancroft, Levy, Jowitt, Hayes, Thompson, McKenzie, Ball, Dubaissi, France, Bellina, Sharpe, Mironov, Brown, Cook, MacDonald, Thornton and Grencis2019). Mice immunized with HPLC-purified TM43 from ES product have comparable protective immunity to mice immunized with worm extracts (Drake et al., Reference Drake, Korchev, Bashford, Djamgoz, Wakelin, Ashall and Bundy1994). Limitations for the use of the 43 kDa protein as a vaccine target centre around the possibility that TM43 is an IFN-γ homologue (Grencis and Entwistle, Reference Grencis and Entwistle1997), raising a concern for the potential production of auto-antibodies against host IFN-γ (Dixon et al., Reference Dixon, Johnston and Else2008). Additionally, it was found that TM43 may not be produced by the early stages of larvae (L1 or L2) limiting its use to prevent early infection. It was also found that TM43 had overall low immunogenicity. These limitations down play this protein as an effective vaccine target (Dixon et al., Reference Dixon, Johnston and Else2008).
Whey acidic protein
An immunodominant T. trichiura antigen with 50 kDa (TT50) was identified by immunological screening of a T. trichiura cDNA library with T. trichiura infection sera. Similar to TM43 or TT47, TT50 also induced pore formation in lipid bilayers, but in contrast to TM43 or TT47, TT50 contains repetitive nine four-disulphide-bonded core domains (Drake et al., Reference Drake, Barker, Korchev, Lab, Brooks and Bundy1998). The four-disulphide-bonded core domain contains 50–51 amino acids with six highly conserved cysteine residues. Probing with anti-TT50 antibody recognized many bands in T. trichiura with high degree of cross-reactivity. Southern blot using TT55 DNA fragment recognized more than nine bands including a large gene product consisting of 904 aa and 95 kDa (TT95). Further analysis suggests TT95 may be a fusion product of two TT50 genes, therefore TT50 and TT95 are believed to be part of a four-disulphide-bonded core domain multigene family (Barker and Bundy, Reference Barker and Bundy1999). A multi-copy expression of this gene family and secreted property may reflect an adaptive and evolutionary response to the need for rapid synthesis of this essential protein (Barker and Bundy, Reference Barker and Bundy1999).
Mice immunized with T. muris adult worm ES products produced more than 90% worm reduction against T. muris infection (Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). The immune sera from the protected mice immunized with T. muris ES products were used to immunoscreen the cDNA library of T. muris adult worms. A total of 102 positive clones were obtained, 63 of them encode different sizes of a four-disulphide-bonded core domain protein. A protein with 49 kDa containing seven four-disulphide-bonded core domains was cloned and expressed as a recombinant protein in yeast (Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). Further structural and functional analysis identified this T. muris four-disulphide-bonded core domain belongs to a whey acid protein (WAP) family (Seki et al., Reference Seki, Matsura, Iwamori, Nukumi, Yamanouchi, Kano, Naito and Tojo2012; Foth et al., Reference Foth, Tsai, Reid, Bancroft, Nichol, Tracey, Holroyd, Cotton, Stanley, Zarowiecki, Liu, Huckvale, Cooper, Grencis and Berriman2014). This 49 kDa WAP protein was named as Tm-WAP49 that shares 54% and 47% amino acid sequence identity with TT95 and TT50, respectively. Native Tm-WAP49 is located in stichocytes as granules and secreted around the head of the worm embedded in caecal mucosa, assuming its potential functions via porin formation in the caecal epithelium (Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018) (Fig. 4). Tm-WAP49 likely plays an important role in the interaction with host cells and facilitates the establishment of parasitism in the host caecum. Mice immunized with yeast-expressed recombinant Tm-WAP49 protein formulated with ISA720 adjuvant produces protection of up to 48% against T. muris challenge infection and the protection is associated with a strong type-2 immune response including high levels of IL-4, IL-9, IL-13 and Th2-related IgG1 production. Mice immunized with only one four-disulphide-bonded core domain (50 amino acid) of Tm-WAP49 fused with Na-GST-1, a leading vaccine candidate for hookworm N. americanus (Zhan et al., Reference Zhan, Perally, Brophy, Xue, Goud, Liu, Deumic, de Oliveira, Bethony, Bottazzi, Jiang, Gillespie, Xiao, Gupta, Loukas, Ranjit, Lustigman, Oksov and Hotez2010), also produces 33% protection from Trichuris challenge (Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). Fused with Na-GST-1 is to increase the immunogenicity of the 50 amino acid WAP domain, also to make the fusion possible as a bivalent vaccine against both trichuriasis and hookworm infection. Tm-WAP49 is highly immunodominant and homologues are found in T. trichiura (TT50 or TT95), making Tm-WAP49 a leading vaccine candidate against whipworm infection.

Fig. 4. Tm-WAP is located in stichosome (red arrow) of T. muris and secreted into the caecal lumen (white arrow) (adapted from Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018).
Protein serine/threonine protein phosphatase 2a (PP2A)
PP2A catalyses the dephosphorylation of phosphoserine/phosphothreonine side chains of proteins involved in many biochemical and cellular processes such as cell motility, embryogenesis, and differentiation and is highly conserved in nematodes (Janssens and Goris, Reference Janssens and Goris2001; Gomez-Samblas et al., Reference Gomez-Samblas, Garcia-Rodriguez, Trelis, Bernal, Lopez-Jaramillo, Santoyo-Gonzalez, Vilchez, Espino, Bolas-Fernandez and Osuna2017; Briggs et al., Reference Briggs, Wei, Versteeg, Zhan, Keegan, Damania, Pollet, Hayes, Beaumier, Seid, Leong, Grencis, Bottazzi, Sastry and Hotez2018). Immunoscreening a cDNA library of Angiostrongylus costaricensis adult worms with a pool of sera from patients with abdominal angiostrongylosis identified a positive clone encoding for the catalytic subunit of the serine/threonine protein phosphatase 2A (AcPP2A). Mice immunized with the recombinant catalytic region of AcPP2A provided complete protection against A. costaricensis challenge associated with increased levels of IFN-γ and IL-17 (Solano-Parada et al., Reference Solano-Parada, Gonzalez-Gonzalez, Torro, dos Santos, Espino, Burgos and Osuna2010). Importantly, significant cross protection was induced in lambs immunized intranasally with AcPP2A against two other intestinal nematodes, Haemonchus contortus and Teladorsagia circumcincta, challenge infections (Mohamed Fawzi et al., Reference Mohamed Fawzi, Cruz Bustos, Gomez Samblas, Gonzalez-Gonzalez, Solano, Gonzalez-Sanchez, De Pablos, Corral-Caridad, Cuquerella, Osuna and Alunda2013), suggesting mucosal immunization in nasal synergistically stimulates protective immunity in intestinal mucosa (Rhee et al., Reference Rhee, Lee and Kim2012; Mohamed Fawzi et al., Reference Mohamed Fawzi, Cruz Bustos, Gomez Samblas, Gonzalez-Gonzalez, Solano, Gonzalez-Sanchez, De Pablos, Corral-Caridad, Cuquerella, Osuna and Alunda2013).
Amino acid sequence alignment confirms the catalytic region of PP2A from A. costaricensis (AcPP2A) shares high homology with those from whipworms (T. trichiura, T. suis and T. muris) (Gomez-Samblas et al., Reference Gomez-Samblas, Garcia-Rodriguez, Trelis, Bernal, Lopez-Jaramillo, Santoyo-Gonzalez, Vilchez, Espino, Bolas-Fernandez and Osuna2017). AKR mice intranasally immunized with recombinant AcPP2A formulated with synthetic self-adjuvant oleic-vinyl sulphone (OVS) or with bacterial walls (BW) resulted in high faecal egg reduction (99.01% and 99.85%) and high reduction in adult worms collected from caecum (97.90% and 59.88%) against subsequent T. muris challenge. This protection was associated with the stimulation of a Th17/Th9 response and high levels of mucus secretion (Gomez-Samblas et al., Reference Gomez-Samblas, Garcia-Rodriguez, Trelis, Bernal, Lopez-Jaramillo, Santoyo-Gonzalez, Vilchez, Espino, Bolas-Fernandez and Osuna2017). CCL20 and CCL11 chemokines are also highly elevated and serve as potent chemoattractants of effector immune cells and stimulators of goblet cells hyperplasia (Williams, Reference Williams2006).
Macrophage migration inhibitory factor
Trichuris secreted ES products contain MIF that inhibits the migration of lymphocytes to tissue in a dose-dependent manner (Gaherwal and Prakash, Reference Gaherwal and Prakash2011). Worm-secreted MIF is one of the immunomodulatory proteins that inhibits macrophage or other effector immune cells from migrating to the site of parasite infection (James and Nacy, Reference James and Nacy1993). Additionally, other parasitic MIF such as TsMIF cloned from T. spiralis, have structural, catalytic and cell-migration-inhibitory properties similar to mammalian MIF (Tan et al., Reference Tan, Edgerton, Kumari, McAlister, Roe, Nagl, Pearl, Selkirk, Bianco, Totty, Engwerda, Gray and Meyer2001). Mice immunized with a DNA vaccine with co-expression of TsMIF and T. spiralis cystatin-like domain protein (TsMCD-1) elicited 37.9% reduction of worm burden against T. spiralis larval challenge associated with specific type-1 immune responses such as increased IFN-γ and CD4+ and CD8+ T cells (Tang et al., Reference Tang, Xu, Yan, Song and Li2012, Reference Tang, Xu, Yan, Song and Li2013). The homologue of MIF was also identified in human T. trichiura with 46% sequence identical to human MIT (Tan et al., Reference Tan, Edgerton, Kumari, McAlister, Roe, Nagl, Pearl, Selkirk, Bianco, Totty, Engwerda, Gray and Meyer2001). Due to the inhibition function for host immune effector cells and the vaccine efficacy in genetically related T. spiralis, MIF in Trichuris may likely be a good vaccine target.
T-cell epitope/VLP vaccine
Histocompatibility complex class II (MHC-II) T-cell epitopes were predicted from predicted ORFs in the Trichuris genome using in silico prediction tools. The coding proteins containing strong MHC-II T-cell epitopes were down-selected using criteria of containing signal peptide but without transmembrane domain, no mouse or human homology, no allergenic potential and with highest predicted solubility in virus-like particles (VLPs). Based on these criteria total four MHC-II T-cell epitopes with potential as vaccine candidates were selected from chymotrypsin-like serine protease and chitin-binding domain containing protein. These epitopes were incorporated into Hepatitis B core antigen virus-like particles (VLPs), which can be taken up and processed by antigen-presenting cells such as macrophages and dendritic cells. Mice immunized with pre-mixed four VLPs expressing each Trichuris T-cell epitope subcutaneously without adjuvant elicited significant protection against T. muris challenge (about 50% adult worm reduction). Additionally vaccinated mice had heightened production of type-2 cytokines produced by mesenteric lymph node, goblet cell hyperplasia, as well as high titres of serological IgM and IgG2c (Zawawi et al., Reference Zawawi, Forman, Smith, Mair, Jibril, Albaqshi, Brass, Derrick and Else2020). The epitope/VLP vaccine-based novel genomic and bioinformatic technologies provide a new era for vaccine design with integration of multiple vaccine candidates.
Tm16
Tm16 is one of 20 immunodominant antigens identified in T. muris adult worm ES products using 2D-gel/Western blot and mass spectrometry screening protocols (Liu et al., Reference Liu, Kelleher, Tabb, Wei, Pollet, Hotez, Bottazzi, Zhan and Asojo2017). The crystal structure of Tm16 indicates Tm16 belongs to the phosphatidylethanolamine-binding-like protein family, possibly involved in regulatory functions (He et al., Reference He, Liu, Lin, Jiang, Ying, Chun, Deng, Zaia, Wen and Luo2016). Given that Tm16 is one of the immunodominant T. muris secreted proteins that induce protective immunity in immunized mice, and a homolog exists in human T. trichiura, Tm16 is putative vaccine candidate for preventing Trichuris infection; however more data are needed to determine the in vivo outcomes of Tm16 vaccines.
Future directions: from antigens to vaccines
Despite the promise of existing antigens so far, reverse vaccinology approaches have not been rigorously applied to Trichuris parasites, so that a multicentred bioinformatics initiative in this regard could potentially identify more suitable antigens in terms of their protective efficacy or durability of protection (Hotez, Reference Hotez2018b). Further, upon selection of antigens that are most effective in mouse or other animal model preclinical studies there are multiple steps required before they can be considered suitable for clinical trials. Some of these critical pathways were outlined earlier, but there still is a lack of consensus of what is the ideal product development strategy and a minimal or preferred target product profile (Diemert et al., Reference Diemert, Bottazzi, Plieskatt, Hotez and Bethony2018). For example, within the product strategy, an important gap is the selection of suitable adjuvants. Because many of the protective immune responses to selected antigens rely on Th2 humoral immunity, it is likely that alum formulations may be suitable as they have been for experimental human hookworm vaccines (Adegnika et al., Reference Adegnika, de Vries, Zinsou, Honkepehedji, Dejon Agobe, Vodonou, Bikangui, Bouyoukou Hounkpatin, Bache, Massinga Loembe, van Leeuwen, Molemans, Kremsner, Yazdanbakhsh, Hotez, Bottazzi, Li, Bethony, Diemert, Grobusch and HookVac2021). Based on the hookworm experience, second immunostimulants will likely also be required, such as synthetic Toll-like receptor-4 (TLR-4) agonists or oligonucleotide CpG molecules (Bottazzi, Reference Bottazzi2015). The epidemiologic modelling assessments are also needed to determine how a trichuriasis vaccine will fit into ongoing deworming and preventive chemotherapy programmes, whether a vaccine specific for trichuriasis will be cost effective when linked to deworming or whether it will be necessary to combine Trichuris antigens with hookworm and Ascaris antigens in a pan-anthelminthic approach as outlined previously (Zhan et al., Reference Zhan, Beaumier, Briggs, Jones, Keegan, Bottazzi and Hotez2014; Bartsch et al., Reference Bartsch, Hotez, Hertenstein, Diemert, Zapf, Bottazzi, Bethony, Brown and Lee2016). Still another consideration is whether recombinant proteins are best suited for polyvalent vaccine approaches given the cost and complexity of testing multiple candidates, vs employing one of the newer mRNA vaccine platform approaches that are evaluated for some parasitic infections (Versteeg et al., Reference Versteeg, Almutairi, Hotez and Pollet2019). The concurrent success of COVID-19 mRNA vaccine has provided proof-of-concept for the suitability of this approach in North America and Europe (Polack et al., Reference Polack, Thomas, Kitchin, Absalon, Gurtman, Lockhart, Perez, Perez Marc, Moreira, Zerbini, Bailey, Swanson, Roychoudhury, Koury, Li, Kalina, Cooper, Frenck, Hammitt, Tureci, Nell, Schaefer, Unal, Tresnan, Mather, Dormitzer, Sahin, Jansen and Gruber2020), but this approach has not yet been shown to be suitable to be applied widely in low- and middle-income countries (LMICs). Finally, there is an urgent need to shape sustainable financing for the advancement of anthelminthic vaccines and these mechanisms do not yet exist.
Conclusions and challenges
Trichuriasis remains a global threat in poverty-stricken areas around the world, leading to significant morbidity and long-term social and economic consequences. Despite targeted efforts at disease control through mass drug administration policies, elimination remains elusive due to high rates of re-infection and poor efficacy of anthelminthic drugs. As a result, there is significant urgency to develop a preventive and/or therapeutic vaccine to aid Trichuris control efforts.
A trichuriasis vaccine and related technologies offers the potential promise of parasite elimination. However, as outlined above, future directions for Trichuris vaccine development will need to focus on (1) identifying homologous vaccine targets across helminth species for the development of panhelminthic vaccines, (2) imploring new technologies, including mRNA approaches, to advance vaccine development for trichuriasis, (3) epidemiologic models to confirm the benefit of vaccines linked to preventive chemotherapy and (4) reaching a clear consensus of what is a suitable product development strategy, what is the preferred target product profile and what would be the financing mechanisms to bring such a vaccine towards licensure.
The global policymakers have not widely accepted the urgency or potential benefits of anti-parasitic disease vaccines in lieu of less expensive but not necessarily cost-effective mass treatment interventions. This situation partly stems from first-generation efforts to develop anti-parasitic disease vaccines that did not benefit from safer and more effective adjuvants and immunostimulants, nor increasing sophistications in epidemiologic modelling. Since then, both fields have advanced and could be leveraged for a new generation of vaccines for parasitic infections, which are sometimes referred to as ‘antipoverty’ vaccines for their potential impact on promoting both public health and economic development (Hotez, Reference Hotez2018a).
For this to occur the essence of global health policy must shift as it pertains to neglected tropical diseases (NTDs). There must be recognition that trichuriasis and related STH infections or NTDs would benefit from vaccines, as well as new drugs and diagnostics, just as HIV/AIDS, malaria, and tuberculosis (Hotez, Reference Hotez2018a). With regards to the STH infections, such recognition should not have to await the results of a Deworm3 initiative that seeks to determine the feasibility of helminth elimination through community-based preventive chemotherapy. In our view, doing so represents a double standard that we would never consider for populations in North America and Europe. The world's poorest people in LMICs deserve the fundamental right of access to innovation as represented by vaccines to combat trichuriasis and other helminth infections (Hotez, Reference Hotez2019).
Author contribution
BZ conceived the review and drafted the manuscript. JH searched the literature and critically revised the manuscript. JW, MEB and PJH revised and edited the manuscript. All authors edited and reviewed the final manuscript.
Financial support
This research was supported by the Michelson Medical Research Foundation.
Conflict of interest
The authors have declared no competing interests.
Ethical standards
There is no ethical issue involved in this review article.