Introduction
Severe hearing loss restricts individuals’ social activity and makes survival in modern society challenging. Cochlear implantation has been an important adjunct in hearing rehabilitation strategies worldwide since its introduction in the 1970s. It is used to restore hearing and quality of life in patients with severe sensorineural hearing loss (SNHL) whose hearing cannot satisfactorily be improved by optimised hearing aid fitting.Reference Sonnet, Montaut-Verient, Niemier, Hoen, Ribeyre and Parietti-Winkler1
In cochlear implantation, the surgical route to the patient's ear goes through mastoid cells, and between the chorda tympani and facial nerve. The electrode array is inserted to the cochlea through the round window or via cochleostomy. The external speech processor is programmed, and the implant is activated on a follow-up visit. The speech processor transmits sound information through the intact skin to the implant by electromagnetic induction, and the implant stimulates the cochlear nerve endings. The perception of pitch is based on tonotopy; the high frequency sounds stimulate the hair cells in the base of the cochlea, and the low frequencies are detected in the apex. An intact auditory pathway is considered a prerequisite for patients to benefit from cochlear implantation.
A patient's suitability for cochlear implantation is carefully evaluated pre-operatively. Magnetic resonance imaging (MRI) is used to inspect inner-ear, cochlear nerve and central structures. Computed tomography (CT) shows bony structures, and gives topographic information to the surgeon. Neural responses of the cochlear nerve to electrical stimulation of the ear have historically been used to evaluate the function of a neural pathway from the cochlea, but unfortunately there is no good method to measure the function of the more central auditory neural tracts in a patient with a deaf ear.
Multiple sclerosis is characterised by focal immune-mediated demyelination of the central nervous system, including the spinal cord and white and grey matter of the brain. Although progress has been made in the development of immunomodulatory therapies for multiple sclerosis, no curative treatment is available as yet. Multiple sclerosis plaques can occur anywhere along the auditory pathway, including the brainstem, auditory cortex and acoustic nerve. Thus, chronic SNHL seems to be common in patients with multiple sclerosis.Reference Lewis, Lilly, Hutter, Bourdette, McMillan and Fitzpatrick2,Reference Daugherty, Lederman, Nodar and Conomy3 In addition, sudden SNHL has been described in several case reports, and is considered an important presentation of multiple sclerosis.Reference Tekin, Acar, Cam and Hanege4–Reference Franklin, Coker and Jenkins7
Herein, we describe a patient whose hearing was successfully restored by cochlear implantation even though his brainstem was affected by several multiple sclerosis lesions. The pertinent clinical literature is systematically reviewed.
Materials and methods
Case report
A patient with severe hearing loss and multiple sclerosis was referred to the tertiary referral centre of Turku University Hospital for evaluation. Hearing was assessed using standard pure tone audiometry and speech recognition testing using bisyllabic words. Pre-operative CT and MRI studies were conducted. After informed consent, a cochlear implant was placed under general anaesthesia.
After surgery, hearing was evaluated using speech discrimination scores. A post-operative follow-up CT study was performed. The patient's electronic medical records were accessed, and relevant data were extracted.
Systematic review of literature
Pubmed/Medline and Web of Science databases were systematically searched in February 2021 for papers concerning cochlear implantation in patients with demyelinative neuropathies or multiple sclerosis using the following search term construct: (multiple sclerosis OR demyelinat*) AND (cochlear implant*). Abstracts were screened for relevance and pertinent studies were evaluated.
Results
Case report findings
A 58-year-old male with a history of multiple sclerosis for over 20 years was referred to the tertiary referral centre with severe hearing impairment and a slight vertigo complaint. The patient's hearing loss was estimated to have a significant neural component associated with the multiple sclerosis, and he was initially not considered to be a suitable candidate for cochlear implantation. After two years’ follow up, however, his speech recognition score in a sound field with bisyllabic words was only 20 per cent with the best possible amplification, and there was no usable hearing above 1 kHz (Figure 1a).

Fig. 1. (a) Audiogram conducted prior to cochlear implantation, demonstrating profound bilateral sensorineural hearing loss, especially in the higher frequencies. (b) Results of video head impulse testing for horizontal semicircular canals, demonstrating bilateral loss of vestibular function. (△ = aided hearing threshold in soundfield (red: right; blue: left); ○ = air conduction masked (right); ] = bone conduction (mastoid) masked (left); [ = bone conduction (mastoid) masked (right); × = air conduction masked (left); downward arrows = no response)
Pre-operative CT and MRI studies were performed. Multiple sclerosis plaques were found to affect the brain stem bilaterally. There were multiple sclerosis plaques located in the vicinity of the cochlear nucleus on the right side, and higher in the brainstem with a left-sided preponderance (Figure 2a, b). In addition, a small incidental vestibular schwannoma with a diameter of 4 mm was found in the left internal auditory meatus (Figure 2c).
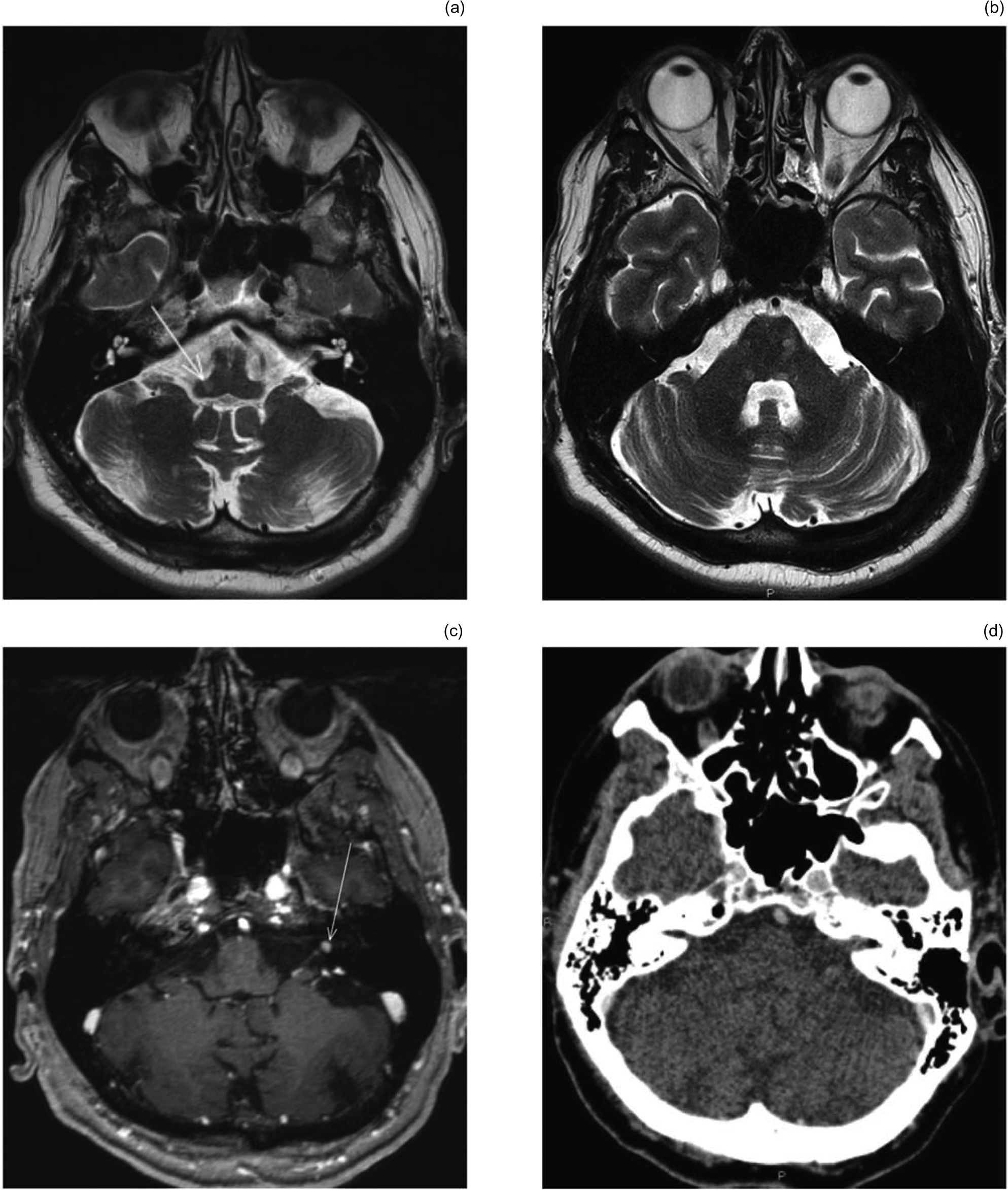
Fig. 2. (a) Axial T2-weighted magnetic resonance imaging (MRI) scan through the upper medulla oblongata shows demyelinating plaque (arrow) located in the area of the cochlear nucleus. (b) Axial T2-weighted MRI scan through the brainstem shows multiple demyelinating plaques on the mid pontine level, predominantly on the left side. (c) Axial, contrast-enhanced, T1-weighted, thin-slice MRI section through the left internal acoustic canal shows a small gadolinium-enhancing mass of 4 mm diameter (arrow) corresponding to a vestibulocochlear nerve schwannoma. (d) A follow-up axial, contrast-enhanced, thin-slice computed tomography image through the left internal acoustic canal and cerebellum shows no evidence of vestibulocochlear nerve schwannoma growth six years after cochlear implantation.
The left ear was selected for cochlear implantation given the presence of several multiple sclerosis lesions along the ascending auditory pathways of the right ear, even though hearing was still somewhat better preserved in the right ear.
Sinistrolateral cochlear implantation using a Cochlear™ CI24RE implant was carried out through cochleostomy. Intra-operative impedance and neural response telemetry measurements were promising. The implant was activated at the one-month follow-up visit. At the time of activation, hearing was already restored to a comfortable level. The patient's residual hearing was measured 10 months after cochlear implantation and was found to be non-existent. The patient still used a hearing aid in the right ear, and found it helpful.
After eight years, the patient's hearing level with the cochlear implant is excellent. His speech discrimination score has stayed consistently at over 90 per cent. His vestibular schwannoma has not demonstrated any tendency for growth on follow-up imaging six years after cochlear implantation (Figure 2d). The sensation of vertigo has remained unchanged. The video head impulse test was performed at the six year follow-up visit and demonstrated bilateral loss of vestibular function (Figure 1b).
Cochlear implants in demyelinating disease
The literature review revealed 30 papers, the abstracts of which were screened for relevance (Figure 3). Altogether, seven papers were reviewed for this article.

Fig. 3. Flow chart of the literature review. PubMed/Medline and Web of Science databases were searched using the search term construct: (multiple sclerosis OR demyelinat*) AND (cochlear implant*). Altogether, seven relevant publications were identified and reviewed for this paper. ADHD = Attention deficit hyperactivity disorder
Only one of these papers concerned cochlear implantation in a multiple sclerosis patient.Reference Shanbhag and Vaid8 Cochlear implantation was successful in the described patient; however, he suffered from long-lasting post-lingual deafness that was unrelated to multiple sclerosis. One further report discussed cochlear implantation in a patient with chronic demyelinating inflammatory polyneuropathy, which is a rare cause of neural hearing loss.Reference Mowry and King9 In this particular setting, cochlear implantation was not very successful in restoring hearing, as speech discrimination reached only 40 per cent at one year after implantation.
Three papers described a total of four patients with Charcot–Marie–Tooth disease, with variable results in terms of speech discrimination levels.Reference Goswamy, Bruce, Green and O'Driscoll10–Reference Matsuda and Kaga12 A 67-year-old patient reached 54 per cent speech discrimination with unilateral implantation, whereas two younger patients were bilaterally implanted with resulting speech discrimination levels of 5 per cent and 45 per cent, respectively. A fourth, 41-year-old male received a cochlear implant and had a significant improvement in hearing during the 8 years of follow up.
The sixth paper discusses the rehabilitation of psychiatric problems in two leukodystrophy patients, with supposedly unrelated profound hearing loss and previous cochlear implants, although no audiometric data are reported.Reference Pundir, Nagarkar and Panda13 The final paper compared the outcomes of cochlear implantation in 30 children with demyelinating lesions in the brain versus children with no demyelination on MRI.Reference Barot, Vishwakarma, Mehta, Patel, Darji and Vishwakarma14 While the audiometric results were positive, the correlation between demyelination site and hearing loss were not reported.
In summary, there are currently no data on cochlear implantation in multiple sclerosis patients with demyelinating plaques along the auditory pathways.
Discussion
Multiple sclerosis is characterised by focal immune-mediated demyelination of the central nervous system, including the spinal cord and white and grey matter of the brain. The pathogenesis of multiple sclerosis is considered to be based on local inflammation, possibly mediated by autoreactive cluster of differentiation 4+ T helper cells. Chronic inflammation leads to demyelination, gliosis, and loss of both oligodendrocytes and neurons.Reference Dendrou, Fugger and Friese15
Despite the high incidence of hearing loss in multiple sclerosis patients,Reference Furst and Levine16 data on cochlear implantation in patients with multiple sclerosis and other demyelinating conditions are lacking. Cochlear implants have, however, been successfully applied to patients with, for example, auditory neuropathy, while results in patients with adult-onset auditory neuropathies have been variable.Reference Shallop, Peterson, Facer, Fabry and Driscoll17–Reference De Leenheer, Dhooge, Veuillet, Lina-Granade and Truy20
Our experience warrants a certain optimism regarding cochlear implantation in patients with auditory pathway lesions. The underlying pathology of our patient's hearing loss remains unknown, as it is impossible to definitely determine whether the hearing loss originated solely from an inner-ear lesion or whether the brain stem lesions associated with multiple sclerosis contributed to the aetiology. In the latter case, the hearing restoration could be due to a more robust neural signal of the cochlear implant.Reference Lundin, Stillesjö and Rask-Andersen21
• Multiple sclerosis is commonly associated with hearing loss
• There exists scarce literature on multiple sclerosis and cochlear implantation
• Our patient with multiple sclerosis had profound hearing loss, probably related to multiple sclerosis
• Cochlear implantation was successful in restoring hearing during a long-term follow up
• A small vestibular schwannoma without a growth tendency should not exclude cochlear implantation
Vestibular schwannoma are benign tumours of the acoustic nerve sheath located in the cerebellopontine angle or internal acoustic canal, arising from vestibular nerves, or sometimes from the cochlea or facial nerve. Symptoms caused by pressure effects on individual nerves of the internal acoustic canal include SNHL, vertigo, facial paralysis and tinnitus. Wider use of high-resolution MRI has resulted in increased detection of vestibular schwannoma in elderly patients too. The growth rate of vestibular schwannoma is usually slow, and surgery is reserved for large or rapidly growing tumours.Reference Stangerup and Caye-Thomasen22
In some countries, vestibular schwannoma is considered an absolute contraindication to cochlear implantation. However, previous studies support cochlear implantation in patients with small, non-growing vestibular schwannoma and especially neurofibromatosis-associated vestibular schwannoma.Reference Harris, Tysome, Donnelly, Durie-Gair, Crundwell and Tam23,Reference Arístegui and Denia24 As exemplified by our case, a small vestibular schwannoma without a growth tendency should not exclude cochlear implantation in an otherwise suitable candidate. However, it should be emphasised that the usual follow up with MRI is limited by cochlear implantation. In our patient, follow up with contrast-enhanced CT was deemed sufficient, as a significant growth tendency had been excluded prior to cochlear implantation.
Conclusion
Our study adds to the scarce literature on cochlear implantation in patients with multiple sclerosis. Our results support cochlear implantation even in the presence of demyelinating plaques along the auditory pathways. Further, it is concluded that a small vestibular schwannoma without a growth tendency should not be considered a contraindication to cochlear implantation.
Competing interests
None declared





