INTRODUCTION
The rapid spread of drug-resistant malaria at the end of the last century extinguished the hope for a near eradication of the disease and highlighted the exigency for new control strategies. These dramatic conditions emphasized the need for an understanding of the sexual phase of the malaria parasite Plasmodium, which represents an essential step during parasite transfer to the mosquito vector and which is thus considered an important target for transmission-blocking strategies.
The sexual phase of Plasmodium begins with the differentiation of gametocytes in the human host, which are taken up by the female anopheline mosquito during a bloodmeal. In the mosquito midgut, the emerged female macrogametes are fertilized by the male microgametes, and the resultant zygote transforms within the next 24 h to a motile tetraploid ookinete. Two decades ago, the first zygote surface proteins were identified in P. falciparum, the causative agent of the deadly malaria tropica (Rener et al. Reference Rener, Graves, Carter, Williams and Burkot1983; Vermeulen et al. Reference Vermeulen, Ponnudurai, Beckers, Verhave, Smits and Meuwissen1985, Reference Vermeulen, Van Deursen, Brakenhoff, Lensen, Ponnudurai and Meuwissen1986; Quakyi et al. Reference Quakyi, Carter, Rener, Kumar, Good and Miller1987; Kaslow et al. Reference Kaslow, Quakyi, Syin, Raum, Keister, Coligan, McCutchan and Miller1988). Immune sera recognizing these antigens, namely Pfs230, Pfs48/45 and Pfs25, were capable of transmission-blocking activity in mosquito membrane feeds, thus demonstrating the feasibility of single or multi-subunit transmission blocking vaccines (TBVs) (Carter et al. Reference Carter, Mendis, Miller, Molineaux and Saul2000; Carter, Reference Carter2001; Stowers and Carter, Reference Carter2001; Kaslow, Reference Kaslow2002). Additional molecules were identified in the following years, including the early gametocyte proteins Pfs16 (Moelans et al. Reference Moelans, Meis, Kocken, Konings and Schoenmakers1991; Baker et al. Reference Baker, Daramola, McCrossan, Harmer and Targett1994; Bruce et al. Reference Bruce, Carter, Nakamura, Aikawa and Carter1994) and Pfg27 (Carter et al. Reference Carter, Graves, Creasey, Byrne, Read, Alano and Fenton1989; Alano et al. Reference Alano, Premawansa, Bruce and Carter1991), as well as the Pfs25 paralogous ookinete protein Pfs28 (Duffy and Kaslow, Reference Duffy and Kaslow1997). Recently, the proteomic information gained following the determination of the P. falciparum genome nucleotide sequence (see Gardner et al. Reference Gardner, Hall, Fung, White, Berriman, Hyman, Carlton, Pain, Nelson, Bowman, Paulsen, James, Eisen, Rutherford, Salzberg, Craig, Kyes, Chan, Nene, Shallom, Suh, Peterson, Angiuoli, Pertea, Allen, Selengut, Haft, Mather, Vaidya, Martin, Fairlamb, Fraunholz, Roos, Ralph, McFadden, Cummings, Subramanian, Mungall, Venter, Carucci, Hoffman, Newbold, Davis, Fraser and Barrell2002), as well as the sequencing of two rodent malaria genomes (Carlton et al. Reference Carlton, Angiuoli, Suh, Kooij, Pertea, Silva, Ermolaeva, Allen, Selengut, Koo, Peterson, Pop, Kosack, Shumway, Bidwell, Shallom, van Aken, Riedmuller, Feldblyum, Cho, Quackenbush, Sedegah, Shoaibi, Cummings, Florens, Yates, Raine, Sinden, Harris, Cunningham, Preiser, Bergman, Vaidya, van Lin, Janse, Waters, Smith, White, Salzberg, Venter, Fraser, Hoffman, Gardner and Carucci2002; Hall et al. Reference Hall, Karras, Raine, Carlton, Kooij, Berriman, Florens, Janssen, Pain, Christophides, James, Rutherford, Harris, Harris, Churcher, Quail, Ormond, Doggett, Trueman, Mendoza, Bidwell, Rajandream, Carucci, Yates, Kafatos, Janse, Barrell, Turner, Waters and Sinden2005), promoted the identification of predicted proteins with cell adhesive motifs. A substantial number of these proteins are expressed in the sexual and mosquito stages of P. falciparum, for example the gametocyte-specific multi-domain adhesion protein family PCCp/PLAP (Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004; Trueman et al. Reference Trueman, Raine, Florens, Dessens, Mendoza, Johnson, Waller, Delrieu, Holders, Langhorne, Carucci, Yates and Sinden2004), and the ookinete adhesive proteins, CTRP (Trottein et al. Reference Trottein, Triglia and Cowman1995; Dessens et al. Reference Dessens, Beetsma, Dimopoulos, Wengelnik, Crisanti, Kafatos and Sinden1999; Yuda et al. Reference Yuda, Sawai and Chinzei1999; Templeton et al. Reference Templeton, Kaslow and Fidock2000) and WARP (Yuda et al. Reference Yuda, Yano, Tsuboi, Torii and Chinzei2001; Li et al. Reference Li, Templeton, Popov, Comer, Tsuboi, Torii and Vinetz2004). Moreover, genome annotation has led to the identification of kinases, which are predominantly expressed in the sexual stages of the parasite, such as PCDPK4 (Billker et al. Reference Billker, Dechamps, Tewari, Wenig, Franke-Fayard and Brinkmann2004) and Pmap-2 (Dorin et al. Reference Dorin, Alano, Boccaccio, Ciceron, Doerig, Sulpice, Parzy and Doerig1999; Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005; Rangarajan et al. Reference Rangarajan, Bei, Jethwaney, Maldonado, Dorin, Sultan and Doerig2005; Tewari et al. Reference Tewari, Dorin, Moon, Doerig and Billker2005), whose functions are involved in microgamete development, or Pnek-4 (Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005; Reininger et al. Reference Reininger, Billker, Tewari, Mukhopadhyay, Fennell, Dorin-Semblat, Doerig, Goldring, Harmse, Ranford-Cartwright, Packer and Doerig2005), which is necessary for zygote formation. These findings have provided important clues on regulatory mechanisms predicted to be used by the parasite for sexual differentiation.
This review summarizes and compares sexual-stage proteins that have hitherto been identified in P. falciparum, and supplements these data with findings gained from observations in the rodent malaria model, P. berghei. The stage-specificity of expression, cellular localization and mode of action of some of these proteins are discussed, and the potential of select proteins for transmission-blocking strategies evaluated. Proteins of the ookinete stage are examined in their relation to transmission-blocking strategies. Additional aspects of cellular and molecular mechanisms during gametocytogenesis and gamete formation can be found in recent key reviews (Talman et al. Reference Talman, Domarle, McKenzie, Ariey and Robert2004; Alano and Billker, Reference Alano, Billker and Sherman2005).
THE MALARIA SEXUAL PHASE – STAGES AND DEVELOPMENT
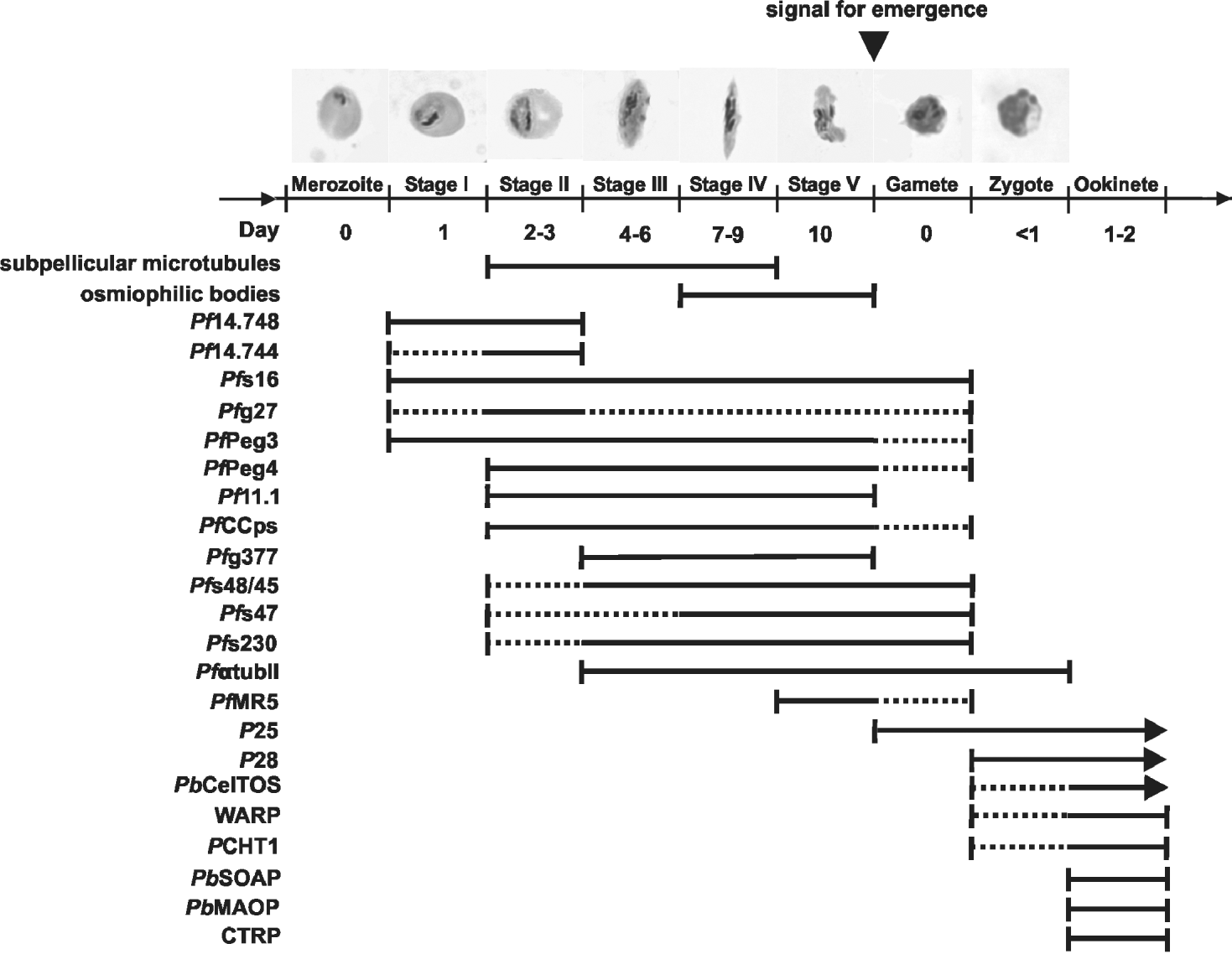
Gametocytes develop in the human host approximately 7–15 days after the appearance of asexual intraerythrocytic parasites in the bloodstream, and about 10 days are required for gametocyte development in P. falciparum (see Thomson and Robertson, Reference Thomson and Robertson1935). Gametocytes are round in most species of Plasmodium, but have a falciform (crescent) shape in P. falciparum, reflected in its species name (Fig. 1C). Development is classified into 5 morphological stages (Hawking et al. Reference Hawking, Wilson and Gammage1971), termed stages I–V (see Fig. 2 for morphological characteristics). While gametocytes of stage I cannot be distinguished morphologically from the asexual trophozoite, morphological differentiation into the crescent gametocytes occurs late in stage II (also termed stage IIB). A typical feature of stage II gametocytes is the presence of a pellicular complex (Fig. 1A), which consists of a subpellicular membrane vacuole subtended by an array of longitudinally-oriented microtubules. The subpellicular microtubules disappear in stage V gametocytes (Sinden, Reference Sinden1982). Other gametocyte-specific features are the osmiophilic bodies, which are membrane-limited, electron-dense organelles beneath the parasite surface (Fig. 1A) and which can be first detected in stage IV gametocytes (Sinden, Reference Sinden1982). The organelles are more abundant in macrogametocytes and disappear within minutes of gametocyte activation, probably releasing their contents into the parasitophorous vacuole (Sinden et al. Reference Sinden, Canning and Spain1976). The host erythrocyte retains a round appearance in gametocytes of stage I and stage II, but later conforms to the crescent shape of the parasite and is adjacent to the outer side of the gametocyte 3-layer pellicle. In the mature gametocyte, the erythrocyte is reduced to a small cytoplasmic hem (Fig. 1A), sometimes referred to as ‘Laveran's bib’.

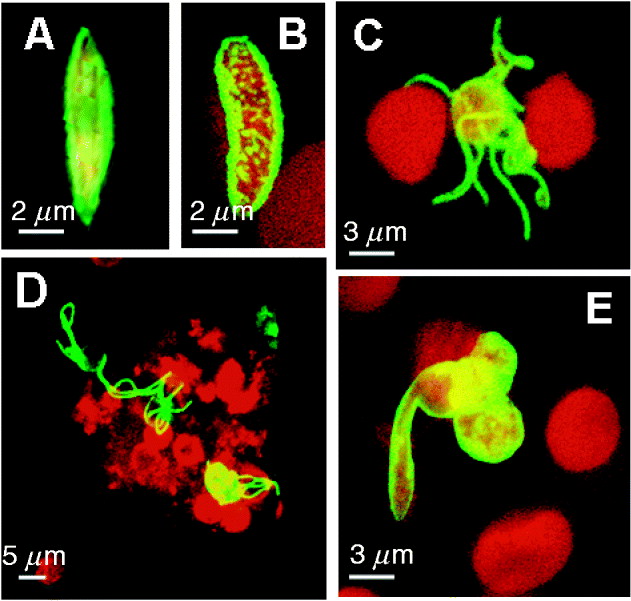
Fig. 1. Cellular structure of Plasmodium falciparum sexual stages. (A) Transmission electron micrograph of a mature gametocyte. The erythrocyte is reduced to an electron-light hem, the parasitophorous vacuole membrane is not discernable at this stage. Inset shows the pellicular membrane complex. (B) Ultrastructure of an exflagellating microgametocyte. The emerging microgametocyte is partly still covered with remnants of the enveloping PVM. Several microgametes have formed, one microgamete is adhering to an erythrocyte (white arrowhead). Inset shows the typical 2+9 structure of axonemal microtubule. (C) Scanning electron micrograph of a mature gametocyte. (D) Ultrastructure of a female gamete after emergence. (E) Light microscopical image of an exflagellation centre, consisting of erythrocytes adhering to a central exflagellating microgametocyte. A, axoneme; E, erythrocyte; ER, endoplasmic reticulum; FV, food vacuole; MG, microgamete; N, nucleus; OB, osmiophilic body; PM, pellicular membrane; PV, parasitophorous vacuole; PVM, parasitophorous vacuole membrane.
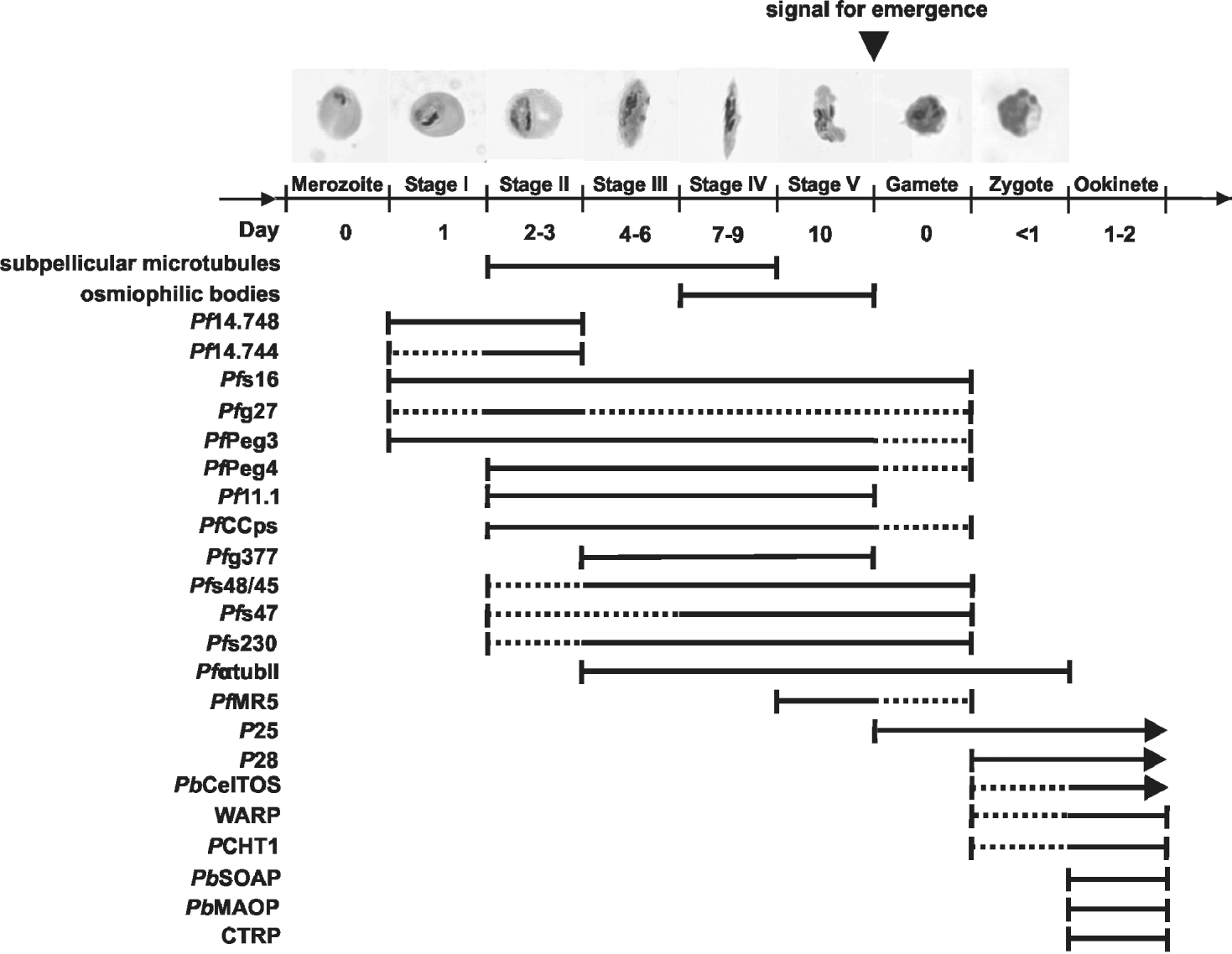
Fig. 2. Diagram depicting stages of expression for sexual and mosquito stage-specific proteins and organelles. Full line indicates high-level expression, dotted line indicates low-level expression. Only gene products, for which detailed protein expression analysis had been available, were included into this figure.
Gametocytes develop along 2 pathways, micro- and macrogametocytes, that upon emergence from erythrocytes within the mosquito midgut form 8 microgametes versus 1 macrogamete, respectively. The sex of gametocytes is established in the committed schizont (Silvestrini et al. Reference Silvestrini, Alano and Williams2000; Smith et al. Reference Smith, Lourenco, Carter, Walliker and Ranford-Cartwright2000), and the gender ratio is female-biased with one male for about five female gametocytes. Ultrastructurally, macrogametocytes harbour more endoplasmic reticulum and reveal larger hemozoin particles.
Gametocyte stages I to IV have been reported to be sequestered in the bone marrow and spleen, whereas stage V gametocytes are released in the peripheral blood system (Thomson and Robertson, Reference Thomson and Robertson1935; Smalley et al. Reference Smalley, Abdalla and Brown1980; Rogers et al. Reference Rogers, Hall, Obiero, Targets and Sutherland2000) and only become infectious to mosquitoes after a further 2 or 3 days of circulation (Smalley and Sinden, Reference Smalley and Sinden1977; Lensen et al. Reference Lensen, Bril, van de Vegte, van Gemert, Eling and Sauerwein1999). During the bloodmeal, the mature gametocytes are taken up and enter the midgut of the feeding mosquito. There, the gametocytes receive environmental cues signalling the switch from the warm-blooded to the insect host and initiating the development to gametes. Within 10 min both male and female gametocytes have escaped from the enveloping erythrocytes (Fig. 1D). In this period, the microgametocyte replicates its genome 3 times, progressing from haploid to octaploid (Janse et al. Reference Janse, van der Klooster, van der Kaay, van der Ploeg and Overdulve1986, Reference Janse, Ponnudurai, Lensen, Meuwissen, Ramesar, van der Ploeg and Overdulve1988), and then produces 8 flagellar microgametes in the process termed exflagellation (Fig. 1B). Exflagellating microgametes typically adhere avidly to neighbouring erythrocytes and are hidden within rosettes, which are termed exflagellation centers (Fig. 1E). Binding of microgametes to erythrocytes involves interactions with sialic acids and glycophorin A on the erythrocyte surface (Templeton et al. Reference Templeton, Keister, Muratova, Procter and Kaslow1998). No mitochondria or apicoplasts have been detected in the microgamete (Sinden et al. Reference Sinden, Canning, Bray and Smalley1978), indicating that these organelles and their genomes are maternally inherited (Vaidya et al. Reference Vaidya, Morrisey, Plowe, Kaslow and Wellems1993; Creasey et al. Reference Creasey, Mendis, Carlton, Williamson, Wilson and Carter1994).
Several environmental signals have been identified that are necessary for the induction of gametocyte emergence, including temperature, pH, CO2 tension, and mosquito midgut factors. A decrease of temperature by around 5°C is required for gametogenesis, as well as a pH shift from 7·2 to ∼8 (Billker et al. Reference Billker, Shaw, Margos and Sinden1997). The involvement of a mosquito-derived factor was first investigated in the avian parasite P. gallinaceum (see Nijhout and Carter, Reference Nijhout and Carter1978; Nijhout, Reference Nijhout1979). Two decades later, the substance was identified as xanthurenic acid, a by-product of eye pigment synthesis, which is present in the mosquito midgut (Billker et al. Reference Billker, Lindo, Panico, Etienne, Paxton, Dell, Rogers, Sinden and Morris1998; Garcia et al. Reference Garcia, Wirtz, Barr, Woolfitt and Rosenberg1998).
After emergence, the microgamete detaches from the residual body and is freely motile in search of a macrogamete. Once encountering and adhering to a macrogamete, fertilization begins by the fusion of the gamete plasma membranes, and the male nucleus enters the macrogamete cytoplasm (Sinden et al. Reference Sinden, Canning and Spain1976). Nuclear fusion is followed by meiosis, and the zygote becomes tetraploid (Janse et al. Reference Janse, van der Klooster, van der Kaay, van der Ploeg and Overdulve1986). During the following 24 h, the zygote transforms into the infective ookinete stage. The ookinete is motile and possesses an apical complex, which enables it to disrupt and traverse the midgut epithelium, before settling down between epithelium and basal lamina. Tetraploidy persists throughout the ookinete stage until sporozoite budding in the oocyst restores the haploid state (Janse et al. Reference Janse, van der Klooster, van der Kaay, van der Ploeg and Overdulve1986). The ookinete is considered a ‘bottleneck stage’ of the parasite's life-cycle and it is estimated that there is up to a 300-fold loss in the abundance during transmission from macrogametocytes to ookinetes for P. falciparum, and an additional 100-fold loss during transmission from ookinete to oocyst (Vaughan et al. Reference Vaughan, Noden and Beier1994).
SEXUAL-STAGE SURFACE ANTIGENS
The first sexual-stage antigenic proteins were discovered in 1983 via the surface radio-isotope labelling of zygotes, which resulted in the identification of 4 proteins, Pfs25, Pfs41, Pfs48/45 and Pfs230 (Rener et al. Reference Rener, Graves, Carter, Williams and Burkot1983; Vermeulen et al. Reference Vermeulen, Ponnudurai, Beckers, Verhave, Smits and Meuwissen1985, Reference Vermeulen, Van Deursen, Brakenhoff, Lensen, Ponnudurai and Meuwissen1986; Quakyi et al. Reference Quakyi, Carter, Rener, Kumar, Good and Miller1987; Kaslow et al. Reference Kaslow, Quakyi, Syin, Raum, Keister, Coligan, McCutchan and Miller1988). While protein Pfs25 was expressed on the surface of gametes after their emergence from the enveloping erythrocytes and continued to be expressed until ookinete formation, proteins Pfs230 and Pfs48/45 were shown to be co-expressed in association with the surface of gametocytes, starting at late stage II until fertilization is completed (Figs 2, 3A and 5; Table 1; Williamson et al. Reference Williamson, Criscio and Kaslow1993, Reference Williamson, Keister, Muratova and Kaslow1995). Proteins Pfs230 and Pfs48/45 contain 14 and 3 copies, respectively, of a motif that has a characteristic pattern of conserved cysteines (Williamson et al. Reference Williamson, Criscio and Kaslow1993; Carter et al. Reference Carter, Coulson, Bhatti, Taylor and Elliott1995; Templeton and Kaslow, Reference Templeton and Kaslow1999; Gerloff et al. Reference Gerloff, Creasey, Maslau and Carter2005). With the completion of the genome sequencing project, additional members of the cysteine motif superfamily were identified and a total of 10 members have been identified in P. falciparum (Fig. 4B; Templeton and Kaslow, Reference Templeton and Kaslow1999; van Dijk et al. Reference van Dijk, Janse, Thompson, Waters, Braks, Dodemont, Stunnenberg, van Gemert, Sauerwein and Eling2001, Reference van Dijk, Douradinha, Franke-Fayard, Heussler, van Dooren, van Schaijk, van Gemert, Sauerwein, Mota, Waters and Janse2005; Williamson, Reference Williamson2003; Gerloff et al. Reference Gerloff, Creasey, Maslau and Carter2005; van Schaijk et al. Reference van Schaijk, van Dijk, van de Vegte-Bolmer, van Gemert, van Dooren, Eksi, Roeffen, Janse, Waters and Sauerwein2006), including the microgametocyte-specific Pfs230 paralogue PfMR5 (Fig. 2; Table 1; Eksi and Williamson, Reference Eksi and Williamson2002). Interestingly, 9 of the genes are typically arranged on the chromosomes in pairs or arrays; the genes encoding Pfs230 and PfMR5 are located in tandem orientation on chromosome 2, whereas those encoding Pfs48/45 and Pfs47 are arranged in tandems on chromosome 13, Pf12 and Pf12p on chromosome 6, and Pf36 and Pf36p on chromosome 4 in a tandem array, which also includes Pf41 (Williamson, Reference Williamson2003).
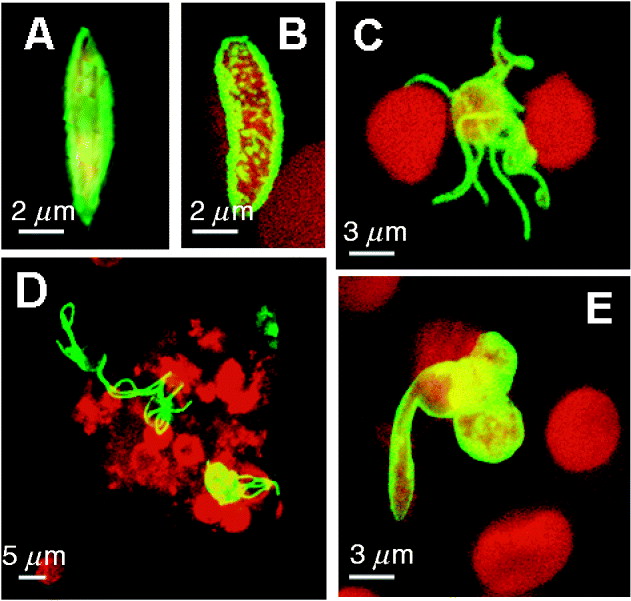
Fig. 3. Expression pattern for select sexual stage-specific proteins in Plasmodium falciparum. (A) Stage IV gametocyte showing surface-associated Pfs230 expression (shown in green). (B) Punctuate expression of PfCCp1 associated with the gametocyte stage V surface (PfCCp1 labelling shown in green, erythrocyte shown in red). (C) Exflagellating microgametocyte with 8 microgametes expressing alpha-tubulin II (alpha-tubulin II labelling shown in green, erythrocytes shown in red). (D) Exflagellation centre labelled with anti-PfCCp3 antibodies (shown in red). The protein is partly associated with the surface of emerged gametes and partly released extracellularly surrounding the exflagellation centre. Male gametes were highlighted by alpha-tubulin II labelling (shown in green). (E) A cluster of 3 zygotes, one of which is transforming into an ookinete, expressing Pfs25 on the surface (Pfs25 labelling shown in green, erythrocytes shown in red). Erythrocytes are highlighted via Evans Blue-labelling (shown in red) in A-C and E.

Fig. 4. Schematic of domain structure of select Plasmodium falciparum sexual and mosquito stage proteins containing adhesive motifs. (A) Structures of Pfs25 and Pfs28. (B) The cysteine-motif superfamily (modified from Templeton and Kaslow, Reference Templeton and Kaslow1999, and Gerloff et al. Reference Gerloff, Creasey, Maslau and Carter2005); arrowhead indicates cleavage site. (Please note that PFD0215c was previously termed PFD0220c, and PFD0215c termed PFD0210c; Williamson, Reference Williamson2003). (C) The PfCCp/PfLAP multi-domain adhesion protein family. (D) Ookinete-specific proteins with adhesive motifs. A, ApicA domain; AD, anchor domain; Anth, anthrax toxin N-terminal region domain; CD, cysteine-rich domain; CM, 6-cysteine motif; Disc, discoidin domain; E, glutamate-rich region; EGF, epidermal growth factor-like domain; FN2, fibronectin type II domain; IR, intervening region; LCCL, Limulus coagulation factor C domain; Lev, levanase domain; LH, lipoxygenase domain; MACPF, membrane-attack complex and perforin domain; NEC, neurexins and collagens domain; PTX, pentraxin; Ric, ricin domain; S, signal peptide; SR, scavenger receptor domain; TM, transmembrane domain; TSP1, thrombospondin type1-like domain; VWA, von Willebrand factor A domain.
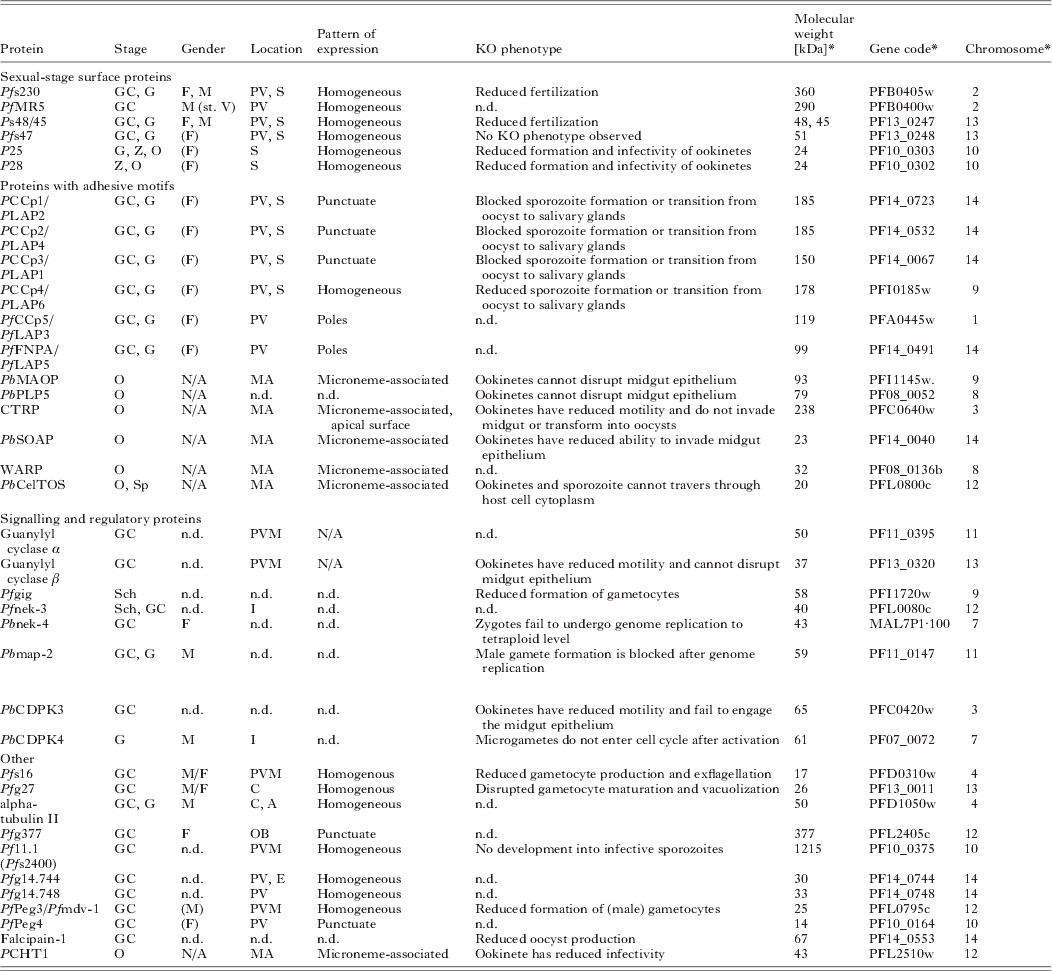
Table 1. Functional characterization of sexual and mosquito stage proteins

* For Plasmodium falciparum. Only proteins, for which either protein expression studies or gene disruption data had been available, were included into this table. A, axoneme-associated; C, cytoplasmic; E, erythrocyte; F, female; G, gamete; GC, gametocyte; M, male; n.d., not determined; MA, microneme-associated; N/A, not applicable; O, ookinete; OB, osmiophilic bodies; PV, parasitophorous vacuole; PMV, parasitophorous vacuole membrane; S, surface; Sch, schizont; Sp, sporozoite. ( ), presumed gender-specificity.
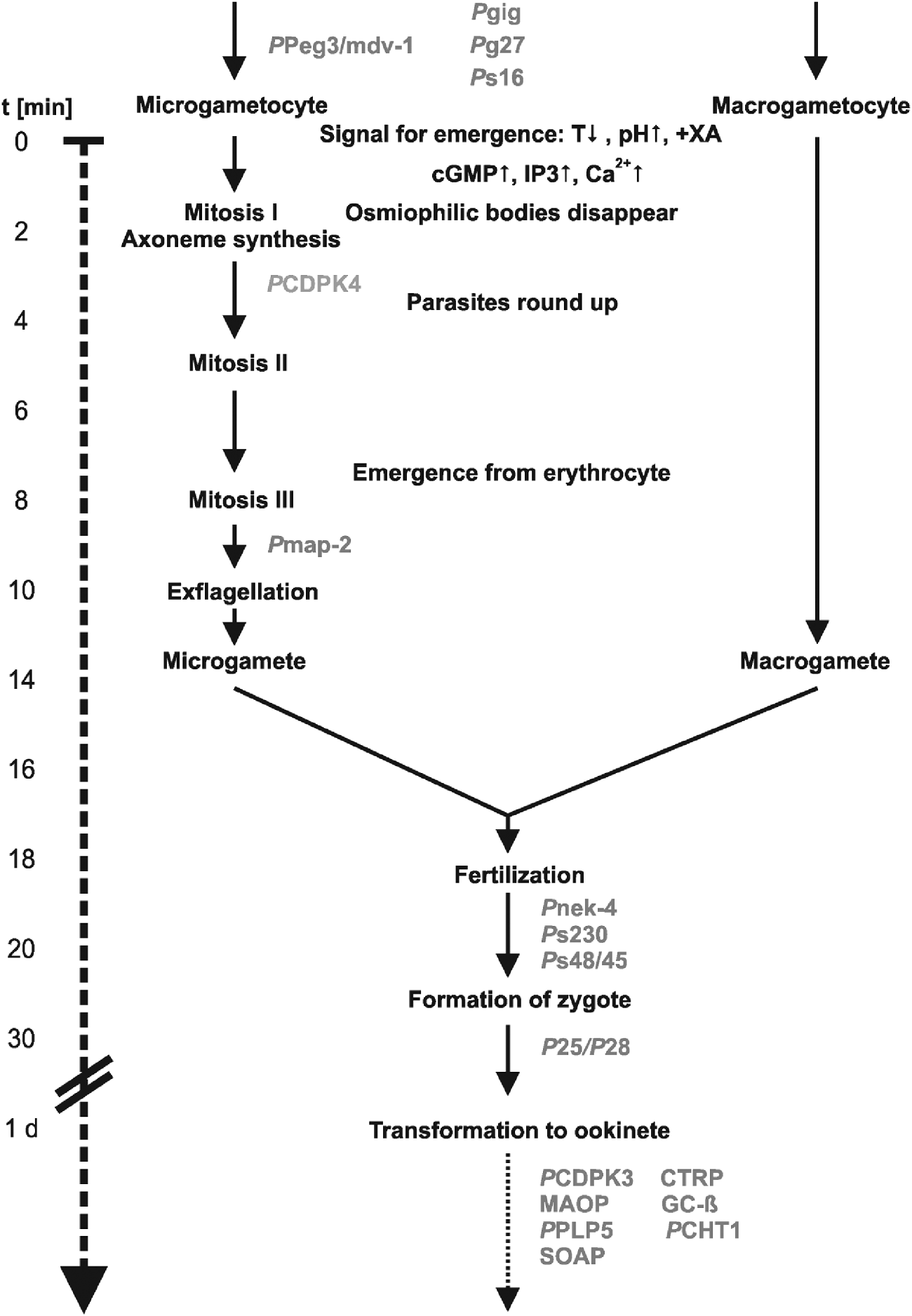
Protein Pfs230 has been studied in detail and is considered to be a promising candidate for TBVs. Antibodies raised against Pfs230 were shown to reduce transmission to the mosquito (Williamson et al. Reference Williamson, Keister, Muratova and Kaslow1995), and a disruption of the gene leads to reduced fertilization and oocyst formation (Fig. 5; Table 1; Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2006). The secreted Pfs230 is associated with the parasite plasma membrane, where it forms a stable membrane-bound complex with the GPI-anchored Pfs48/45 (Kumar, Reference Kumar1987; Kumar and Wizel, Reference Kumar and Wizel1992). However, parasites whose Pfs230 gene is disrupted still express Pfs48/45 as well as other surface proteins, such as Pfs47, PfMR5, Pfs16 and Pfs25 (Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2006). The full-length Pfs230 protein has a molecular weight of ∼360 kDa and is processed during emergence to expose proteins with a molecular weight of ∼307 and 300 kDa on the gamete surface (Williamson et al. Reference Williamson, Fujioka, Aikawa and Kaslow1996). The full-length protein is cleaved between the glutamate-rich motifs and the first cysteine motif domain (Fig. 4B), thereby releasing 2 peptides with a molecular weight of ∼47 and 35 kDa. The membrane-permeable cysteine protease inhibitor E64d blocks the production of the 300 and 35 kDa forms, but not the 307 and 47 kDa forms, whereas the membrane-permissive inhibitor E64 has no effect (Brooks and Williamson, Reference Brooks and Williamson2000). The cleavage of both protein fragments is sensitive to the metalloprotease inhibitor 1,10-phenanthroline. Protein Pfs230 also mediates the binding of male emerging gametocytes to erythrocytes during exflagellation centre formation (Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2006).

Fig. 5. Schematic showing course of gametogenesis and fertilization. Involvement of sexual-stage proteins with functions according to their knock-out phenotypes are indicated in grey (modified from Sinden et al. Reference Sinden, Canning, Bray and Smalley1978, and Alano and Billker, Reference Alano, Billker and Sherman2005).
The cysteine motif superfamily member Pfs48/45 is a second candidate for TBVs which is homogenously expressed on the surface of mature gametocytes and gametes (Fig. 2; Table 1). The distruption of genes encoding Pfs48/45 or the P. berghei orthologue Pbs48/45 result in a failed penetration of macrogametes by microgametes, thereby reducing the formation of zygotes (Fig. 5; van Dijk et al. Reference van Dijk, Janse, Thompson, Waters, Braks, Dodemont, Stunnenberg, van Gemert, Sauerwein and Eling2001). Pfs48/45 gene disruptant gametes do not retain Pfs48/45 or Pfs230 on their surface in P. falciparum (Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2006). The Pfs48/45 paralogue, Pfs47, on the other hand, is expressed specifically in female gametocytes and gametes, and the disruption of the gene encoding Pfs47 does not reveal any essential function in female fertility (Fig. 2; Table 1; van Schaijk et al. Reference van Schaijk, van Dijk, van de Vegte-Bolmer, van Gemert, van Dooren, Eksi, Roeffen, Janse, Waters and Sauerwein2006).
Perhaps the most promising transmission blocking candidates investigated are the zygote and ookinete surface proteins Pfs25 and Pfs28 (Figs 3E and 4A; Table 1). In contrast to the expression of proteins Pfs230 and Pfs48/45, Pfs25 is expressed on the surface of gametes only during the initiation of emergence, and its expression persists until the ookinete has penetrated the midgut epithelium (Fig. 2; Table 1). Protein Pfs28 (encoded by a paralogue of the gene encoding Pfs25) has also been identified (Duffy and Kaslow, Reference Duffy and Kaslow1997); it shares a similar expression pattern (Fig. 2; Table 1) and is adjacent to Pfs25 in the genome (Duffy and Kaslow, Reference Duffy and Kaslow1997). Due to their prominent expression in the zygote and ookinete mosquito stages, the two proteins are also referred to as P25 and P28 (see Tomas et al. Reference Tomas, Margos, Dimopoulos, van Lin, de Koning-Ward, Sinha, Lupetti, Beetsma, Rodriguez, Karras, Hager, Mendoza, Butcher, Kafatos, Janse, Waters and Sinden2001; Mair et al. Reference Mair, Braks, Garver, Wiegant, Hall, Dirks, Khan, Dimopoulos, Janse and Waters2006). Both proteins have a GPI-anchor sequence and 4 epidermal growth factor-like domains (Fig. 4A). Parasite lines harbouring gene disruptions for both Pbs25 and Pbs28 exhibit reduced formation and infectivity of ookinetes for P. berghei, whereas ookinete formation is not significantly reduced in parasites lacking only one of the proteins (Fig. 5; Table 1; Tomas et al. Reference Tomas, Margos, Dimopoulos, van Lin, de Koning-Ward, Sinha, Lupetti, Beetsma, Rodriguez, Karras, Hager, Mendoza, Butcher, Kafatos, Janse, Waters and Sinden2001). A striking feature of both proteins is their regulated expression at the onset of emergence, due to the removal of a translational block of their gene transcripts during macrogametocyte activation independent of fertilization (Billker et al. Reference Billker, Shaw, Margos and Sinden1997). In P. berghei, the expression of proteins Pbs25 and Pbs28 was shown recently to be regulated by the RNA helicase DOZI (development of zygotes inhibited), which represses mRNA translation in gametocytes by forming a complex (Mair et al. Reference Mair, Braks, Garver, Wiegant, Hall, Dirks, Khan, Dimopoulos, Janse and Waters2006).
PROTEINS WITH ADHESIVE MOTIFS
Annotation of predicted adhesive proteins following the completion of the genome sequences of both P. falciparum and P. berghei revealed a family of 6 secreted proteins consisting of multiple predicted adhesive ‘animal-like’ domains, including a common Limulus coagulation factor C (LCCL)-like domain for 5 of the 6 genes (Figs 2, 3B, D and 4C; Table 1; Delrieu et al. Reference Delrieu, Waller, Mota, Grainger, Langhorne and Holder2001; Claudianos et al. Reference Claudianos, Dessens, Trueman, Arai, Mendoza, Butcher, Crompton and Sinden2002; Lasonder et al. Reference Lasonder, Ishihama, Andersen, Vermunt, Pain, Sauerwein, Eling, Hall, Waters, Stunnenberg and Mann2002; Dessens et al. Reference Dessens, Sinden and Claudianos2004; Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004, Reference Pradel, Wagner, Mejia and Templeton2006; Templeton et al. Reference Templeton, Iyer, Anantharaman, Enomoto, Abrahante, Subramanian, Hoffman, Abrahamsen and Aravind2004; Trueman et al. Reference Trueman, Raine, Florens, Dessens, Mendoza, Johnson, Waller, Delrieu, Holders, Langhorne, Carucci, Yates and Sinden2004). The protein family was studied independently by 2 groups and termed PfCCp proteins in P. falciparum (Limulus coagulation factor C domain-containing proteins; Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004) and PbLAP proteins in P. berghei (LCCL-lectin adhesive-like proteins; Trueman et al. Reference Trueman, Raine, Florens, Dessens, Mendoza, Johnson, Waller, Delrieu, Holders, Langhorne, Carucci, Yates and Sinden2004; Raine et al. Reference Raine, Ecker, Mendoza, Tewari, Stanway and Sinden2007). All 6 genes were predicted to possess signal peptides but no transmembrane domains or GPI-anchor sequences. The paralogues PfCCp1 and PfCCp2 have similar architectures, due to relatively recent gene duplication after domain accretion (Fig. 4C). The CCp proteins are conserved across apicomplexans studied to date. Orthologues of PfCCp1, PfCCp2 and PfCCp3 have been identified in Cryptosporidium parvum and Theileria annulata (see Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004; Templeton et al. Reference Templeton, Iyer, Anantharaman, Enomoto, Abrahante, Subramanian, Hoffman, Abrahamsen and Aravind2004; Tosini et al. Reference Tosini, Agnoli, Mele, Moralez and Pozio2004; Pradel and Templeton, Reference Pradel, Templeton, Dobrindt and Hacker2005), and there is evidence for them in Toxoplasma gondii (F. Spano, unpublished observations). An orthologuous gene encoding PfFNPA, the family member lacking the LCCL-domain, was also identified in C. parvum. The proteins are not found in the alveolate Tetrahymena or in other protozoans, suggesting that the CCp proteins originated in the Apicomplexa and likely have an important, relatively conserved function in this group of protists.
All 6 PfCCp proteins are abundantly expressed in gametocytes, with expression commencing at stage II and ceasing after fertilization (Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004, Reference Pradel, Wagner, Mejia and Templeton2006; G. Pradel, unpublished observations). A detailed characterization of the expression of proteins PfCCp1, PfCCp2, and PfCCp3 revealed co-dependent expression and cellular co-localization within the gametocyte parasitophorous vacuole associated with the parasite plasma membrane (Fig. 3B; Pradel et al. Reference Pradel, Hayton, Aravind, Iyer, Abrahamsen, Bonawitz, Mejia and Templeton2004, Reference Pradel, Wagner, Mejia and Templeton2006), and there is first evidence for direct interactions among these 3 proteins during gametocyte differentiation and gametogenesis (Wagner et al. Reference Wagner, Scholz, Abreu, Frank, Templeton and Pradel2006). The 3 proteins are partly released during gametocyte emergence and then localize extracellularly, surrounding exflagellation centres which consist of emerged gametes, gametocytes and uninfected erythrocytes (Fig. 3D). Following targeted disruption of either of the gene loci PfCCp2 or PfCCp3, P. falciparum develops normally to the oocyst stage, but is subsequently blocked in the transition of oocyst sporozoites to the salivary glands, indicating an essential role of these proteins in parasite development within the mosquito vector (Table 1). The disruption of the gene encoding PfCCp4 revealed no non-wild-type phenotype, indicating that this protein might have a redundant or non-essential function in the parasite's life-cycle (S. M. Scholz and G. Pradel, unpublished observations).
Recently, also the PCCp/PLAP proteins have been studied in detail in the rodent P. berghei system (Raine et al. Reference Raine, Ecker, Mendoza, Tewari, Stanway and Sinden2007). Loss of function mutants were generated for PbCCp1/PbLAP2, PbCCp2/PbLAP4, PbCCp3/PbLAP1 and PbCCp4/PbLAP6. For all gene disruptants, a reduced formation of sporozoites in the midgut oocysts has been observed (Raine et al. Reference Raine, Ecker, Mendoza, Tewari, Stanway and Sinden2007). Genetic-cross studies between the abovementioned mutants and female-deficient or male-deficient parasites also revealed that the functional genes have to be inherited from female gametocytes (Raine et al. Reference Raine, Ecker, Mendoza, Tewari, Stanway and Sinden2007). This finding is in accordance with a macrogametocyte-specific expression of a GFP-reporter protein driven by either PbCCp1/PbLAP2 or PbCCp3/PbLAP1 promoters in P. berghei (see Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005). However, no functional characterization is hitherto available for PCCp5/PLAP3 and PFNPA/PLAP5 in P. falciparum or P. berghei.
In recent years, several ookinete-expressed proteins have been identified that exhibit signal peptides and adhesive motifs and thus might have potential as transmission blocking candidates. Best known is the circumsporozoite protein and thrombospondin-related adhesive protein (TRAP)-related protein CTRP (Figs 2, 4D and 5; Table 1; Trottein et al. Reference Trottein, Triglia and Cowman1995). This is the second of 3 identified members of a protein family of adhesive proteins comprising von Willebrand factor-like A domains and thrombospondin type1-like domains. Protein CTRP is expressed in the ookinete micronemes and later relocates to the apical surface and has also been shown to bind to basement membrane components (Li et al. Reference Li, Templeton, Popov, Comer, Tsuboi, Torii and Vinetz2004). After gene disruption in both P. falciparum (Templeton et al. Reference Templeton, Kaslow and Fidock2000) and P. berghei (Dessens et al. Reference Dessens, Beetsma, Dimopoulos, Wengelnik, Crisanti, Kafatos and Sinden1999; Yuda et al. Reference Yuda, Sawai and Chinzei1999), ookinetes develop, but they exhibit a reduced motility and fail to invade the midgut epithelium, indicating that the protein is necessary for ookinete infectivity.
A similar function is assigned to 4 other adhesive proteins of ookinete micronemes, the perforin-like membrane attack ookinete proteins MAOP/PPLP3 (Figs 2, 4D and 5; Table 1; Kadota et al. Reference Kadota, Ishino, Matsuyama, Chinzei and Yuda2004) and PPLP5 (Figs 2 and 5; Table 1; Ecker et al. Reference Ecker, Pinto, Baker, Kafatos and Sinden2007), the secreted ookinete adhesive protein SOAP (Figs 2, 4D and 5; Table 1; Dessens et al. Reference Dessens, Siden-Kiamos, Mendoza, Mahairaki, Khater, Vlachou, Xu, Kafatos, Louis, Dimopoulos and Sinden2003) and the von Willebrand factor A domain-related protein WARP (Figs 2, 4D and 5; Table 1; Yuda et al. Reference Yuda, Yano, Tsuboi, Torii and Chinzei2001; Li et al. Reference Li, Templeton, Popov, Comer, Tsuboi, Torii and Vinetz2004). Gene disruption of MAOP and PPLP5 in P. berghei halts the ookinetes at the point of contact with the mosquito epithelium (Kadota et al. Reference Kadota, Ishino, Matsuyama, Chinzei and Yuda2004; Ecker et al. Reference Ecker, Pinto, Baker, Kafatos and Sinden2007), suggesting that the proteins are essential for breaching the host midgut cell membrane. The SOAP protein, which has 2 cysteine-rich domains and forms high molecular mass complexes via disulphide bonds (Fig. 4D), is proposed to interact with mosquito laminin (Dessens et al. Reference Dessens, Siden-Kiamos, Mendoza, Mahairaki, Khater, Vlachou, Xu, Kafatos, Louis, Dimopoulos and Sinden2003). Gene disruption of SOAP in P. berghei leads to an impaired ability of the ookinete to invade the midgut epithelium. Furthermore, a recently identified secreted micronemal protein, CelTOS (cell-traversal protein for ookinetes and sporozoites), is required for host cell migration after breaching of the cell membrane in both P. berghei ookinetes and sporozoites (Fig. 2; Table 1; Kariu et al. Reference Kariu, Ishino, Yano, Chinzei and Yuda2006).
In this context, an ookinete-specific enzyme plays an important role in invasion of the midgut. Soon after ingestion of the bloodmeal, the mosquito forms an acellular chitin-based peritrophic membrane around its midgut epithelium to protect itself from pathogen invasion. The motile ookinete secretes a hydrolytic chitinase in order to aid in traversing this membrane and to gain access to the midgut epithelium (Vinetz et al. Reference Vinetz, Dave, Specht, Brameld, Xu, Hayward and Fidock1999, Reference Vinetz, Dave, Specht, Brameld, Xu, Hayward and Fidock2000; Langer et al. Reference Langer, Hayward, Tsuboi, Tachibana, Torii and Vinetz2000). Two chitinase genes encoding proteins PgCHT1 and PgCHT2 have been identified in the avian malaria parasite, P. gallinaceum. Gene disruption of PCHT1 led to a reduced ookinete infection of the midgut in both P. falciparum (Table 1; Tsai et al. Reference Tsai, Hayward, Langer, Fidock and Vinetz2001) and P. berghei (Dessens et al. Reference Dessens, Mendoza, Claudianos, Vinetz, Khater, Hassard, Ranawaka and Sinden2001). An additional, unknown function has been assigned to the enzyme, because reduced infectivity has also been observed using artificial ookinete feeds in mosquitoes, in which no peritrophic membrane has formed (Dessens et al. Reference Dessens, Mendoza, Claudianos, Vinetz, Khater, Hassard, Ranawaka and Sinden2001).
SEXUAL-STAGE PROTEINS INVOLVED IN SIGNALLING AND REGULATION
Until recently, the stimuli and mechanisms that trigger sexual-stage transformation events, such as gametocyte development, gametogenesis and fertilization, have been largely unexplored. Several new studies have shed light on these events. In this context, 2 regulatory mechanisms, thought to be involved in initiation of the parasite sexual phase, have been identified. For example, the conserved nuclear protein SET accumulates in male gametocytes (Pace et al. Reference Pace, Olivieri, Sanchez, Albanesi, Picci, Siden Kiamos, Janse, Waters, Pizzi and Ponzi2006). The protein exhibits 2 promoters; one active in all blood stages and the other active in gametocytes and a fraction of schizonts. The 2 promoters are under transcriptional control and might contribute to a prompt entry in S/M phase of male gametes within the mosquito midgut. Furthermore, a gene, Pfgig, was identified recently, which is located on the right arm of chromosome 9 (Fig. 5; Table 1; Gardiner et al. Reference Gardiner, Dixon, Spielmann, Skinner-Adams, Hawthorne, Ortega, Kemp and Trenholme2005). Subtelomeric deletion of this chromosomal region was reported previously to reduce levels of gametocytes in parasite cultures (Day et al. Reference Day, Karamalis, Thompson, Barnes, Peterson, Brown, Brown and Kemp1993; Barnes et al. Reference Barnes, Thompson, Triglia, Day and Kemp1994). Cohesively, gene disruption of Pfgig led to reduced gametocyte production. When the functional gene was inserted into a parasite clone lacking the right arm of chromosome 9, gametocyte-specific transcript expression, particularly of the Pfs16 transcript, was up-regulated (Gardiner et al. Reference Gardiner, Dixon, Spielmann, Skinner-Adams, Hawthorne, Ortega, Kemp and Trenholme2005).
An initial step in elucidating signalling was the identification of 2 guanylyl cyclases, GC-α and GC-β, as integral membrane proteins in P. falciparum (Fig. 5; Table 1; Carucci et al. Reference Carucci, Witney, Muhia, Warhurst, Schaap, Meima, Li, Taylor, Kelly and Baker2000), and both proteins appear to be expressed in gametocytes. Recently, the gene encoding GC-β was disrupted in P. berghei, and resultant ookinetes revealed a reduced motility and failed to invade the midgut epithelium (Hirai et al. Reference Hirai, Arai, Kawai and Matsuoka2006). Induction of exflagellation was also shown to involve an increase in Ca2+ and cGMP (Kawamoto et al. Reference Kawamoto, Alejo-Blanco, Fleck, Kawamoto and Sinden1990, Reference Kawamoto, Fujioka, Murakami, Syafruddin, Hagiwara, Ishikawa and Hidaka1993) and an activation of the guanylyl cyclases (Muhia et al. Reference Muhia, Swales, Deng, Kelly and Baker2001). Gametocyte activation further triggers a phosphoinositide-specific phospholipase C activity that generates the second messengers diacylglycerol and inositol triphosphate IP3 (Martin et al. Reference Martin, Jett and Schneider1994), leading to an increase of intracellular calcium (Kawamoto et al. Reference Kawamoto, Alejo-Blanco, Fleck, Kawamoto and Sinden1990, Reference Kawamoto, Fujioka, Murakami, Syafruddin, Hagiwara, Ishikawa and Hidaka1993).
Recent findings have indicated that sexual stage-specific kinases are involved in gametogenesis and fertilization (see Doerig et al. Reference Doerig, Billker, Pratt and Endicott2005). Genome annotation has revealed an extensive catalogue of parasite-encoded kinases (the ‘kinome’), with at least 86 hypothetical kinases having been identified in P. falciparum (Ward et al. Reference Ward, Equinet, Packer and Doerig2004; Anamika et al. Reference Anamika, Srinivasan and Krupa2005). Gene orthologues of these kinases have been disrupted in P. berghei. The calcium-dependent protein kinase PbCDPK4 is activated by an increase of intracellular Ca2+, which was shown to be triggered by exposing the gametocytes to xanthurenic acid. The kinase then regulates genome replication in microgametocytes, and mutants do not form microgametes and fail to infect mosquitoes (Fig. 5; Table 1; Billker et al. Reference Billker, Dechamps, Tewari, Wenig, Franke-Fayard and Brinkmann2004). In a subsequent step, the mitogen-activated protein kinase homologue Pbmap-2 controls the formation of male gametes at the stage of cytokinesis (Fig. 5; Table 1; Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005; Rangarajan et al. Reference Rangarajan, Bei, Jethwaney, Maldonado, Dorin, Sultan and Doerig2005; Tewari et al. Reference Tewari, Dorin, Moon, Doerig and Billker2005). Microgametes lacking Pbmap-2 progress through DNA replication but are blocked from forming motile axonemes (Tewari et al. Reference Tewari, Dorin, Moon, Doerig and Billker2005). Recently, the NIMA-related protein kinase Pfnek-3 was shown to be expressed in late asexual and gametocyte stages (Table 1; Lye et al. Reference Lye, Chan and Sim2006). This kinase, as well as Pfnek-1 (Dorin et al. Reference Dorin, Le Roch, Sallicandro, Alano, Parzy, Poullet, Meijer and Doerig2001), can phosphorylate Pfmap-2 (Dorin et al. Reference Dorin, Alano, Boccaccio, Ciceron, Doerig, Sulpice, Parzy and Doerig1999) in vitro; however, their endogenous functions remain unknown. Downstream of these events, Pbnek-4 triggers genome replication to the tetraploid level in the zygote stage (Fig. 5; Table 1; Khan et al. Reference Khan, Franke-Fayard, Mair, Lasonder, Janse, Mann and Waters2005; Reininger et al. Reference Reininger, Billker, Tewari, Mukhopadhyay, Fennell, Dorin-Semblat, Doerig, Goldring, Harmse, Ranford-Cartwright, Packer and Doerig2005), whereas PbCDPK3 is required for ookinete motility and engagement with the mosquito midgut epithelium (Fig. 5; Table 1; Ishino et al. Reference Ishino, Orito, Chinzei and Yuda2006; Siden-Kiamos et al. Reference Siden-Kiamos, Ecker, Nyback, Louis, Sinden and Billker2006). While some aspects have been explored, future work is required to elucidate the functions of these kinases in P. falciparum.
OTHER SEXUAL-STAGE PROTEINS
A number of sexual-stage proteins with diverse functions and patterns of expression have been identified during the last 2 decades. This includes the recent discovery of a subtelomeric gene family of 36 members (Eksi et al. Reference Eksi, Haile, Furuya, Ma, Su and Williamson2005). Most of these genes possess signal peptide sequences, pexel motifs, and share a domain termed PHIST (Plasmodium helical interspersed subtelomeric family), indicating that they are likely exported into the erythrocyte cytoplasm (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006). Six of the genes are expressed early in gametocyte differentiation, including those encoding Pfg14.744, Pfg14.745, Pfg14.748, Pfg14.763, Pfg14.752, and Pfg6.21. The two proteins Pfg14.744 and Pfg14.748 both localize to the parasitophorous vacuole of committed ring stages (Fig. 2; Table 1; Eksi et al. Reference Eksi, Haile, Furuya, Ma, Su and Williamson2005). Pfg14.748 stays in the parasitophorous vacuole, whereas Pfg14.744 was subsequently detected in the erythrocyte (Eksi et al. Reference Eksi, Haile, Furuya, Ma, Su and Williamson2005). Although the protein function is hitherto unknown, the fact that GFP expression driven by the Pfg14.748 promoter region was detected in a fraction of asexual schizonts putatively committed to sexual differentiation leads to the assumption that the proteins are involved in parasite commitment to gametocytes (Eksi et al. Reference Eksi, Haile, Furuya, Ma, Su and Williamson2005).
While proteomic studies have mainly focussed on the mature gametocyte stage (see Florens et al. Reference Florens, Washburn, Raine, Anthony, Grainger, Haynes, Moch, Muster, Sacci, Tabb, Witney, Wolters, Wu, Gardner, Holder, Sinden, Yates and Carucci2002; Lasonder et al. Reference Lasonder, Ishihama, Andersen, Vermunt, Pain, Sauerwein, Eling, Hall, Waters, Stunnenberg and Mann2002), a recent study investigated proteins which are upregulated in young gametocytes by comparing transcript levels of the gametocyte-producing P. falciparum isolate 3D7 with the gametocyte-negative strain F12 (Silvestrini et al. Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, deRisi, Pizzi and Alano2005). Two novel proteins of early gametocytes, PfPeg3 and PfPeg4 were identified (Figs 2 and 5, Table 1; Silvestrini et al. Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, deRisi, Pizzi and Alano2005). While PfPeg4 is expressed on the parasite surface starting in stage II gametocytes, expression of PfPeg3 is initiated in stage I gametocytes in association with all membranous structures of the parasitophorous vacuole membrane (Furuya et al. Reference Furuya, Mu, Hayton, Liu, Duan, Nkrumah, Joy, Fidock, Fujioka, Vaidya, Wellems and Su2005; Lanfrancotti et al. Reference Lanfrancotti, Bertuccini, Silvestrini and Alano2007). The protein further co-localizes with Pfs16. PfPeg3 was also termed Pfmdv-1 and gene disruption resulted in a reduced formation of particularly male gametocytes (Furuya et al. Reference Furuya, Mu, Hayton, Liu, Duan, Nkrumah, Joy, Fidock, Fujioka, Vaidya, Wellems and Su2005).
Two gametocyte proteins are considered to be markers of early gametocytogenesis. The first, Pfs16, is initially expressed in stage I gametocytes (Figs 2 and 5; Table 1; Moelans et al. Reference Moelans, Meis, Kocken, Konings and Schoenmakers1991; Baker et al. Reference Baker, Daramola, McCrossan, Harmer and Targett1994; Bruce et al. Reference Bruce, Carter, Nakamura, Aikawa and Carter1994), and localizes in the parasitophorous vacuole membrane. Gene disruption resulted in reduced gametocyte production and an impaired ability of male gametocytes to exflagellate (Kongkasuriyachai et al. Reference Kongkasuriyachai, Fujioka and Kumar2004). Synthesis of Pfs16 is followed by pronounced production of the cytoplasmic phosphoprotein Pfg27 (Figs 2 and 5; Table 1; Carter et al. Reference Carter, Graves, Creasey, Byrne, Read, Alano and Fenton1989; Alano et al. Reference Alano, Premawansa, Bruce and Carter1991; Lobo et al. Reference Lobo, Konings and Kumar1994) in early stage II gametocytes. Pfg27 is phosphorylated (Kumar, Reference Kumar1997) and has been shown to form a homodimer that is able to bind single-stranded RNA and SH3 protein motifs in vitro (Sharma et al. Reference Sharma, Sharma, Kogkasuriyachai and Kumar2003). Disruption of the Pfg27 gene resulted in complete abolishment of gametocyte maturation and the formation of large vacuoles in abortive gametocytes (Lobo et al. Reference Lobo, Fujioka, Aikawa and Kumar1999).
Following the expression of Pfs16 and Pfg27, sexual dimorphism occurs morphologically at the transition between gametocyte stages IIB and III. This is accompanied by expression of the male-specific alpha-tubulin isoform alpha-tubulin II, which is subsequently incorporated into the axonemes of the emerging microgamete (Figs 2 and 3C; Table 1; Rawlings et al. Reference Rawlings, Fujioka, Fried, Keister, Aikawa and Kaslow1992). The electron-dense osmiophilic bodies can be observed subsequently at stage IV of gametocytogenesis and are mainly present in female gametocytes. Osmiophilic bodies contain another gametocyte-specific and highly hydrophilic protein, Pfg377, which is localized in these organelles until maturation of gametocytes (Fig. 2; Table 1; Alano et al. Reference Alano, Read, Bruce, Aikawa, Kaido, Tegoshi, Bhatti, Smith, Luo, Hansra, Carter and Elliott1995; Severini et al. Reference Severini, Silvestrini, Sannella, Barca, Gradoni and Alano1999). The function of Pfg377 has yet to be investigated.
Also discovered at the end of last century was the 1 MDa repeat-containing protein, Pf11-1 (Fig. 2; Table 1; Scherf et al. Reference Scherf, Carter, Petersen, Alano, Nelson, Aikawa, Mattei, Pereira da Silva and Leech1992). The protein is present in granules adjacent to the gametocyte membrane, and after stimulation of gametocyte emergence, was found in the membrane of lysed erythrocytes, suggesting a role in erythrocyte rupture. The Pf11-1 gene locus represents a ‘hyper-fragile’ chromosome region, and deletion of the gene occurs commonly in subpopulations of laboratory isolates. Parasites lacking the gene were reported to not develop into infective sporozoites (Scherf et al. Reference Scherf, Carter, Petersen, Alano, Nelson, Aikawa, Mattei, Pereira da Silva and Leech1992). Antibodies against the gametocyte antigen Pfs2400, which is considered to be the Pf11-1 protein product, were subsequently shown to reduce infection to the mosquito (Feng et al. Reference Feng, Hoffmann, Nussenzweig, Tsuji, Fujioka, Aikawa, Lensen, Ponnudurai and Pologe1993).
PROSPECTIVE CANDIDATES FOR TRANSMISSION-BLOCKING STRATEGIES
Currently, there is no vaccine available for either the prevention of malaria or lessening of morbidity or mortality. Moreover, pharmaceutical approaches for combating the disease are increasingly encountering parasite drug resistance. Consequently, there is an urgent need for novel anti-malarials and effective vaccine regimens. Ideally, malaria control measures are designed to target multiple metabolic pathways in combination therapies, in concert with a vaccine that attenuates morbidity and mortality. If the vaccine or drug were to have a component that targets transmission of the disease, then this would also serve to lessen the burden of infection rate and reduce the chance of spreading drug resistance. Transmission-blocking strategies, both on the level of either drugs or vaccines, are aimed at disrupting development of mosquito midgut stages, specifically gametes, zygotes, and ookinetes, thereby preventing the spread of malaria. While there is no compound available to specifically target parasite transmission, the possibility of TBVs has been explored for more than 2 decades.
Transmission-blocking vaccines (TBVs)
TBVs target parasite antigens exposed in the midgut of the mosquito, thereby relying on human antibodies to inhibit parasite development within this insect host, with conceivable additional involvement of complement or cell-mediated destruction of parasites (Carter et al. Reference Carter, Mendis, Miller, Molineaux and Saul2000; Carter, Reference Carter2001; Stowers and Carter, Reference Carter2001; Kaslow, Reference Kaslow2002). The induction of transmission-blocking immunity as a potential tool in malaria control was first reported for the avian malaria parasite, P. gallinaceum (Carter and Chen, Reference Carter and Chen1976; Gwadz, Reference Gwadz1976). Main TBV candidates explored thus far can be divided into 2 categories i.e. (1) antigens expressed in gametocytes and gametes, such as Pfs48/45 and Pfs230, immunity against which will be naturally boosted by infection, and (2) antigens expressed solely in the gamete, zygote and ookinete stages of the mosquito vector, including Pfs25 and Pfs28, which are therefore never expressed within the human host.
The feasibility of a TBV has been demonstrated for a number of candidate antigens. Protein Pfs230 has been shown as a major target of complement-fixing antibodies (Quakyi et al. Reference Quakyi, Carter, Rener, Kumar, Good and Miller1987; Read et al. Reference Read, Lensen, Begarnie, Haley, Raza and Carter1994), which might be important for antibody-mediated, transmission-blocking immunity (Healer et al. Reference Healer, McGuinness, Carter and Riley1999). The immunogenic region C of Pfs230, which spans the first cysteine-rich motif as well as the first intervening region (Fig. 4A; Bustamante et al. Reference Bustamante, Woodruff, Oh, Keister, Muratova and Williamson2000), induces higher IgG titres via DNA vaccination in mice when an additional GPI-anchor sequence was included (Fanning et al. Reference Fanning, Czesny, Sedegah, Carucci, van Gemert, Eling and Williamson2003). Similarly, infection with P. berghei boosts the antibody response after priming with a DNA vaccine coding for Pbs48/45 (Haddad et al. Reference Haddad, Maciel and Kumar2006). A distinct conformer of 10 cysteines within Pfs48/45 elicits major antibody response in mice, showing transmission-blocking activity (Outchkourov et al. Reference Outchkourov, Vermunt, Jansen, Kaan, Roeffen, Teelen, Lasonder, Braks, van de Vegte-Bolmer, Qiu, Sauerwein and Stunnenberg2007). Nasal immunization with protein Pfs25 induces complete protective immunity in mice against field isolates of P. falciparum (see Arakawa et al. Reference Arakawa, Komesu, Otsuki, Sattabongkot, Udomsangpetch, Matsumoto, Tsuji, Wu, Torii and Tsuboi2005). Also, serum from mice immunized with the transmission-blocking candidates Pvs25 and Pvs28 from P. vivax inhibited parasite development in the mosquito (Hisaeda et al. Reference Hisaeda, Stowers, Tsuboi, Collins, Sattabongkot, Suwanabun, Torii and Kaslow2000). One TBV candidate, the recombinant protein Pvs25H from P. vivax, was expressed in Saccharomyces cerevisiae and has exited phase I clinical trials (Malkin et al. Reference Malkin, Durbin, Diemert, Sattabongkot, Wu, Miura, Long, Lambert, Miles, Wang, Stowers, Miller and Saul2005). Antibodies recognizing Pvs25H were shown to be active in transmission-blocking assays (Malkin et al. Reference Malkin, Durbin, Diemert, Sattabongkot, Wu, Miura, Long, Lambert, Miles, Wang, Stowers, Miller and Saul2005).
Other antigens are currently under investigation for their potency as transmission-blocking targets. A promising candidate is the parasite chitinase PfCHT1. A single-chain antibody directed against PfCHT1 reduced parasite transmission to the mosquito (Li et al. Reference Li, Patra and Vinetz2005). Additional potential candidates with relevant functions for the parasite development in the mosquito vector include CTRP, as well as the PfCCp protein family members, PfCCp2 and PfCCp3. Initial experiments, in which antibodies raised against select PfCCp proteins were used in exflagellation assays, indicated that protection might be complement-mediated (N. Simon and G. Pradel, unpublished observations).
Transmission-blocking drugs (TBDs)
Selected parasite kinases represent promising targets for TBDs, given their function in key steps of parasite development in the mosquito, as discovered in P. berghei (see Doerig, Reference Doerig2004, Doerig et al. Reference Doerig, Billker, Pratt and Endicott2005; Ward et al. Reference Ward, Equinet, Packer and Doerig2004). Importantly, structures of several malaria kinases are unique and do not have orthologues in the human host. For example, the Pmap-2 kinase displays a divergent putative activation site with the amino acid sequence Thr-Ser-His instead of the Thr-X-Tyr in conserved MAP-kinases (Dorin et al. Reference Dorin, Alano, Boccaccio, Ciceron, Doerig, Sulpice, Parzy and Doerig1999). These features make them excellent targets for anti-malarial drugs. However, the majority of functional studies of Plasmodium kinases have been conducted using the rodent malaria parasite, P. berghei. Therefore, the characterization of these kinases is necessary in P. falciparum as a prelude for consideration in TBD design.
Almost unexplored is the possible use of protease inhibitors for TBDs. Parasite proteases are essential for several events during gametogenesis and fertilization, such as parasite egress from the erythrocyte during emergence, exflagellation of the microgametocyte and cell contact between male and female gamete prior to cell fusion. Genome annotation has identified 92 predicted proteases in P. falciparum (see Wu et al. Reference Wu, Wang, Liu and Wang2003). While research has largely focussed on the involvement of proteases during merozoite invasion (O'Donnell and Blackman, Reference O'Donnell and Blackman2005) and food digestion (Rosenthal, Reference Rosenthal2002; Linares and Rodriguez, Reference Linares and Rodriguez2007), a number of protease transcripts have been shown to be specific for the parasite sexual stages e.g., the cysteine protease metacaspase II, the aspartate protease plasmepsin 6 and the serine protease PfSub3 (Wu et al. Reference Wu, Wang, Liu and Wang2003; Rosenthal, Reference Rosenthal2004). In this context, a recent study showed that exflagellation in P. berghei can be blocked by the cysteine/serine inhibitors TPCK and TLCK as well as by the metalloprotease inhibitor 1,10-phenanthroline, indicating that select proteases are involved in exflagellation (Torres et al. Reference Torres, Rodriguez, Rodriguez and de la Cruz Hernandez-Hernandez2005); a similar inhibition of exflagellation has now been confirmed in P. falciparum (I. Rupp and G. Pradel, unpublished observations). Also, gene disruption of the malaria cysteine protease falcipain-1 in P. falciparum has resulted in normal exflagellation, but reduced oocyst production (Table 1; Eksi et al. Reference Eksi, Czesny, Greenbaum, Bogyo and Williamson2004). In agreement with these findings, the membrane permeable cysteine protease inhibitor E64d blocks oocyst formation but not exflagellation (Eksi et al. Reference Eksi, Czesny, van Gemert, Sauerwein, Eling and Williamson2007), providing a first foundation for the development of parasite-specific protease TVD inhibitors.
Several studies have addressed the role of the mosquito vector in host-parasite interactions and the identification of mosquito molecular targets for the disruption of transmission (see Catteruccia, Reference Catteruccia2007). An excellent example of such avenues of research was the demonstration that mosquito xanthurenic acid plays a role in the induction of gametogenesis (Billker et al. Reference Billker, Lindo, Panico, Etienne, Paxton, Dell, Rogers, Sinden and Morris1998; Garcia et al. Reference Garcia, Wirtz, Barr, Woolfitt and Rosenberg1998). A second example is a study reporting that the midgut carboxypeptidase gene CpdAg1 of Anopheles gambiae was upregulated during parasite infection, which resulted in higher activity of carboxypeptidase B-Ag1 (Lavazec et al. Reference Lavazec, Bonnet, Thiery, Boisson and Bourgouin2005, Reference Lavazec, Boudin, Lacroix, Bonnet, Diop, Thiberge, Boisson, Tahar and Bourgouin2007). Antibodies against this protein blocked parasite development in the midgut of this mosquito (Lavazec et al. Reference Lavazec, Boudin, Lacroix, Bonnet, Diop, Thiberge, Boisson, Tahar and Bourgouin2007). Only recently was binding of the protein Pvs25 from P. vivax to the mosquito-specific molecule calreticulin described (Rodriguez et al. Reference Rodriguez, Martinez-Barnetche, Alvarado-Delgado, Batista, Argotte-Ramos, Hernandez-Martinez, Gonzalez Ceron, Torres, Margos and Rodriguez2007). Calreticulin is present in midgut membranes of the mosquito and might therefore represent a novel target for transmission-blocking strategies (Rodriguez et al. Reference Rodriguez, Martinez-Barnetche, Alvarado-Delgado, Batista, Argotte-Ramos, Hernandez-Martinez, Gonzalez Ceron, Torres, Margos and Rodriguez2007).
FUTURE PERSPECTIVES
In recent years, research on the molecular and cellular biology of the malaria sexual stages has benefited from a substantial resurgence provided by proteomic, microarray and bioinformatic projects, following the genome sequencing projects for multiple species and isolates of Plasmodium. New surface-associated proteins with adhesive functions have been identified in sexual and mosquito stages, and significant progress has been made in the identification and functional characterization of enzymes and regulatory proteins which are intimately involved in gametocytogenesis and fertilization. Studies focusing on parasite kinases should unveil the signalling cascades involved in gametocyte differentiation and emergence, and we can expect to learn much more about gametocyte proteases and their functions in the near future. Taken together, research on the malaria sexual stages is experiencing a renaissance that is greatly illuminating the transmission phase of the parasite's life-cycle.
I want to thank Sabrina Scholz, Marc Kirschner and Tom Templeton for carefully reviewing the manuscript, and Kim Williamson, Christian Doerig and Pietro Alano for fruitful discussions. Images C and E in Fig. 1 were kindly provided by my co-workers Ingrid Rupp and Roland Frank. The work in my laboratory is funded by an Emmy-Noether grant as well as the SFB479 of the Deutsche Forschungsgemeinschaft (DFG).