Introduction
Mustard seed meal (MSM) is the solid residue of rape (Brassica napus L.), brown mustard [B. juncea (L.) Czern] or white mustard (Sinapis alba L.) seeds after they have been pressed for oil. When MSM is incorporated into soil that is subsequently hydrated, MSM releases chemicals that have microbicidal, insecticidal (Brown et al. Reference Brown, Morra, McCaffrey, Auld and Williams1991; Delaquis and Sholberg Reference Delaquis and Sholberg1997; Dufour et al. Reference Dufour, Stahl and Baysse2015; Sarwar et al. Reference Sarwar, Kirkegaard, Wong and Desmarchelier1998; Smolińska et al. Reference Smolińska, Morra, Knudsen and Brown1997), herbicidal (Boydston et al. Reference Boydston, Morra, Borek, Clayton and Vaughn2011; Rice et al. Reference Rice, Johnson-Maynard, Thill and Morra2007; Vaughn et al. Reference Vaughn, Palmquist, Duval and Berhow2006), and nematicidal (Donkin et al. Reference Donkin, Eiteman and Williams1995) properties. Thus, MSM is a promising tool to simultaneously manage weeds, insect pests, and soilborne diseases that adversely affect crop production.
Pest suppression from MSM is caused by the enzymatic breakdown of glucosinolates, which are a class of secondary metabolites found in most plants in the Brassicaceae (Rask et al. Reference Rask, Andréasson, Ekbom, Eriksson, Pontoppidan and Meijer2000). Hydrolysis of glucosinolates by the enzyme myrosinase (thioglucoside glucohydrolase E.C. 3.2.3.1) releases pesticidal, volatile chemicals, including isothiocyanates and thiocyanates. Enzymatic catalysis of glucosinolates is greater in a waterlogged soil (i.e., soil with a thin film of water on the surface) compared with soil hydrated to field capacity (at water potential of −0.03 MPa) (Morra and Kirkegaard Reference Morra and Kirkegaard2002). Although waterlogging promotes glucosinolate hydrolysis, the pesticidal efficacy of MSM may not be greater in waterlogged soils than in soils at lower levels of hydration. This is because for MSM to control pests effectively, hydrolysis of glucosinolates must take place when pests are at life stages susceptible to glucosinolate breakdown products (De Cauwer et al. Reference De Cauwer, Vanbesien, De Ryck and Reheul2019).
Seed dormancy is a critical stage in the life cycle of many weeds because it inhibits germination under conditions otherwise suitable for germination and promotes seed persistence in soil (Vleeshouwers et al. Reference Vleeshouwers, Bouwmeester and Karssen1995). For small-seeded weeds, susceptibility to MSM increases as the percentage of dormant seeds in a population decreases (Lefebvre et al. Reference Lefebvre, Leblanc and Watson2018). This is consistent with previous research that indicated glucosinolate degradation products at low concentrations prevent radicle protrusion but do not directly kill seeds (Angelini et al. Reference Angelini, Lazzeri, Galletti, Cozzani, Macchia and Palmieri1998; Leblová-Svobodová and Koštíř Reference Leblová-Svobodová and Koštíř1962; Peterson et al. Reference Peterson, Belz, Walker and Hurle2001). If dormant or nongerminable seeds are not susceptible to glucosinolate degradation products, soil moisture conditions that inhibit germination but promote enzymatic hydrolysis of glucosinolates would negate the efficacy of MSM. Although this hypothesis is compelling, glucosinolate hydrolysis and MSM pesticidal activity have not been studied across soil moisture levels that differ in their ability to stimulate or inhibit weed seed germination, to our knowledge. Clarifying relationships among soil moisture levels, rates of glucosinolate hydrolysis, and MSM pesticidal activity will support the development of sustainable strategies for irrigations that occur immediately after the incorporation of MSM into soil.
Chile pepper is an important, irrigated crop in the U.S. Southwest (Bosland Reference Bosland2015). However, chile pepper can be difficult to grow because it is susceptible to weeds (Amador-Ramirez Reference Amador-Ramirez2002) and soilborne diseases (Sanogo Reference Sanogo2003). Consequently, chile pepper production in the U.S. Southwest will benefit from novel management tactics that simultaneously target weeds and soilborne diseases.
Palmer amaranth is a common, summer annual weed in chile pepper fields in the U.S. Southwest (Skaggs et al Reference Skaggs, Decker and VanLeeuwen2000) and can substantially reduce chile pepper yield (Schroeder Reference Schroeder1992). It can grow 5 to 7 cm d−1, making Palmer amaranth an aggressive competitor for light, water, and nutrient resources (Ehleringer Reference Ehleringer1983). Palmer amaranth seeds are small (1 to 2 mm diameter) and capable of persistence under waterlogged conditions (Schutte et al. Reference Schutte, Klypin and Shukla2016). In the absence of competition, Palmer amaranth plants can produce 200,000 to 600,000 seeds plant−1 (Keeley et al. Reference Keeley, Carter and Thullen1987), and plants growing in competition with crops can produce between 1,800 and 91,000 seeds plant−1 (Massinga et al. Reference Massinga, Currie, Horak and Boyer2001). Although Palmer amaranth is a highly competitive weed with prolific reproductive capacity, its emergence is suppressed by products of glucosinolate hydrolysis (Norsworthy and Meehan Reference Norsworthy and Meehan2005).
Verticillium wilt (caused by Verticillium dahliae) is a common soilborne disease in chile pepper in the U.S. Southwest (Sanogo and Carpenter Reference Sanogo and Carpenter2006; Skaggs et al. Reference Skaggs, Decker and VanLeeuwen2000). Current strategies for managing soilborne diseases include the use of chemical soil fumigants and biofungicides in combination with cultural tactics including crop rotation and soil-water management to avoid prolonged saturation. These strategies do not always adequately reduce soilborne diseases, and as a result, Verticillium wilt can cause substantial yield loss in chile pepper fields. MSM treatments inhibit V. dahliae growth and reduce Verticillium wilt disease incidence (Neubauer et al. Reference Neubauer, Hüntemann, Heitmann and Müller2015; Smolińska et al. Reference Smolińska, Kowalska, Kowalczyk and Horbowicz2010). Thus, MSM amendments may be an additional effective tool for managing Verticillium wilt in chile pepper.
Seeking information to guide irrigation after MSM amendments for chile pepper, we conducted experiments to determine if waterlogged soils lead to ineffective MSM applications by inhibiting weed seed germination but promoting glucosinolate hydrolysis. We also conducted experiments to determine if decreasing soil moisture inhibits herbicidal and fungicidal activities of MSM. The objectives of this study were to (1) determine the effects of MSM on Palmer amaranth soil seedbanks under high soil moisture, (2) measure glucosinolate degradation in soil hydrated to saturation and field capacity, and (3) determine the effects of decreasing moisture availability on MSM control of Palmer amaranth and V. dahliae.
Materials and Methods
Biological Materials
MSM derived from brown mustard seed was purchased from a commercial supplier in September 2017 (Pescadero Gold; Farm Fuel Inc. Watsonville, CA). Experiments were performed from September 2018 through March 2019. When not in use, MSM was stored in airtight plastic bags at room temperature. The primary glucosinolate in brown mustard seed meal is sinigrin (Rice et al. Reference Rice, Johnson-Maynard, Thill and Morra2007; Rothlisberger et al. Reference Rothlisberger, Hons, Gentry and Senseman2012). Just prior to the beginning of the experiment, the sinigrin concentration of MSM was 168 µmol g−1. This was determined using gas chromatography and mass spectrometry (Heaney et al. Reference Heaney, Spinks and Fenwick1988).
Seeds of Palmer amaranth were collected from the New Mexico State University (NMSU), Leyendecker Plant Science Research Center (32.198°N, 106.748°W) in September 2017. Mature Palmer amaranth inflorescences were clipped from plants, removed from the field, and hand-thrashed with sequential combinations of sieving and forced-air separation. Collected seeds were stored in an airtight container at 4 C.
V. dahliae was isolated from chile pepper plants grown on a farm located in Doña Ana County, New Mexico (32.341°N, 106.824°W). V. dahliae cultures were maintained on Czapek-Dox agar (CDA) at room temperature. CDA was prepared using a method adapted from Hawke and Lazarovits (Reference Hawke and Lazarovits1994). Specifically, 35 g of CDA broth (Sigma-Aldrich, St. Louis, MO) was mixed with 15 g of agar, 1 mL of streptomycin sulfate (50 mg L−1), and 1 L of distilled water. This CDA solution was autoclaved and poured into Petri dishes (100 × 15 mm).
MSM Effects on Palmer Amaranth Soil Seedbanks Under High Moisture
The effects of MSM rate (0 and 4,400 kg ha−1) and soil moisture level (flooded, −0.03 MPa, and saturated, −0.6 MPa) combinations on Palmer amaranth seed survival were examined in a completely randomized, factorially designed experiment with four replicates. This experiment was conducted twice.
Experimental units were seedbank mesocosms designed to maintain a desired soil water potential. Mesocosms (herein called “cores”) were constructed with polyvinyl chloride (PVC) pipes (6-cm diameter, 7-cm height). To one end of each pipe, cheesecloth (grade 40) was attached to hold soil but allow for subirrigation. A Vinton sandy loam soil (Thermic Typic Torrifluvents, pH 7.9, 1.6% organic matter) collected from the NMSU Los Lunas Agricultural Science Center (34.766°N, 106.762°W) was the medium for the experiment. This soil was passed through a 2-mm sieve to remove all rocks and debris before being used to fill the cores. For MSM-treated cores, soil was mixed with MSM at a rate equivalent to 4,400 kg ha−1, which is an application rate recommended by the supplier and suppresses soil pathogens (Wang and Mazzola Reference Wang and Mazzola2019). Control cores contained no MSM. Palmer amaranth was seeded in each core at a depth of 1 cm to create a density of 50 seeds per seedbank.
All cores were subirrigated to saturation. Then, for flooded cores, additional water was added to create 1 cm of standing water on the soil surface. For −0.03 and −0.6 MPa cores, saturated cores were dried to the weight corresponding with the desired soil water potential. Drying requirements for different soil-water potentials were established using a function for the relationship between soil gravimetric moisture content and soil-water potential. This function was developed using a soil pressure-plate extractor to establish a calibration curve for soil-water potential (Dane et al Reference Dane, Hopmans and Topp2002). Once desired moisture levels were reached, the cores were wrapped with a single layer of low-density polyethylene film (Glad Cling Wrap; The Glad Products Company, Oakland, CA). This seedbank study system maintains desired soil moisture levels for prolonged periods (Schutte et al. Reference Schutte, Klypin and Shukla2016).
Hydrated cores were placed in a growth chamber set to 35 C day/25 C night and a 12-hr photoperiod—conditions favorable for germination of Palmer amaranth (Willis et al. Reference Willis, Baskin, Baskin, Auld, Venable, Cavender-Bares, Donohue and Rubio de Casas2014). After 10 d, the cores were unwrapped and all emerged seedlings were counted. The soil from each core was then individually sieved using a 500-µm sieve to collect all nongerminated Palmer amaranth seeds. Recovered seeds were assayed for viability using a 0.6% aqueous solution of 2,3,5-triphenyl-tetrazolium chloride (Peters Reference Peters2000). Viable seeds were classified as “persistent.” Rates of persistence were expressed as percentages of seeds buried.
The number of seeds and/or pre-emergent seedlings terminated by MSM (hereafter, “MSM-induced mortality”) was determined for every replication and moisture level, using Equation 1:
where E crl is the number of emerged seedlings in cores without MSM for replicate r and moisture level l, V crl is the number of viable seeds recovered from cores without MSM for replicate r and moisture level l, E mrl is the number of emerged seedlings in cores with MSM for replicate r and moisture level l, and V mrl is the number of viable seeds recovered from cores with MSM for replicate r and moisture level l.
Data analyses were performed with the statistical software R, version 3.5.2 (The R Foundation for Statistical Computing, http://www.r-project.org). Levene tests for homogeneity of variances, performed with the R library car, indicated equal variances among runs. Accordingly, data from runs were combined. Generalized linear mixed models for the binary response variables MSM-induced mortality and seed persistence were developed using the R library lme4. In these models, fixed effects included moisture level, and random effects included run and replication within each run. The R library multcomp was used to generate Tukey all-pairs comparisons for the factor “moisture level.”
Glucosinolate Degradation in Soil Hydrated to Saturation and Field Capacity
In a completely randomized experiment that was conducted twice, the effect of factorial combinations of three soil moisture levels (i.e., saturated, field capacity [−0.03 MPa], and dry) and two MSM levels (i.e., 0 and 20% [wt/wt]) on glucosinolate degradation was assessed.
Experimental units were cores similar to those described for objective 1. The medium for cores was a Vinton sandy loam soil (Thermic Typic Torrifluvents, pH 7.9, 1.6% organic matter) collected from the NMSU Los Lunas Agricultural Science Center. Soil was passed through a 2-mm sieve; mixed with MSM, if required; and poured into PVC pipes (6-cm diameter, 7-cm tall). Each core contained 100 g of soil and, if needed, 20 g of MSM. Cores were subirrigated to saturation and then dried to the weight corresponding to desired soil-water potential. Dry cores were not subirrigated. Once desired moisture levels were reached, the cores were wrapped with a single layer of low-density polyethylene film and placed in a growth chamber set to 30 C.
Cores were destructively sampled at 0, 3, 6, 9, 12, 24, and 48 h after reaching the desired moisture level. Samples were collected using a method adapted from Doheny-Adams et al. (Reference Doheny-Adams, Redeker, Kittipol, Bancroft and Hartley2017). Specifically, soil was sampled from the middle of each core using a hole borer to extract 1 cm3 of soil. Then, soil samples were placed into 2-mL Eppendorf tubes, immediately flash-frozen in liquid nitrogen, and then mixed with cold (−20 C) 80% methanol. Once the methanol was added, samples were vortexed for 1 min to break up soil pellets. The samples were then placed in a refrigerator at 4 C for 30 min. Afterward, the samples were vortexed for 30 sec and then stored at 4 C for another 30 min. Samples were then centrifuged at 8,000 rpm for 10 min. Supernatants were filtered through 0.20-µm syringe filters and transferred into 2-mL high-performance liquid chromatography (HPLC) vials. These vials were stored at −20 C for 2 to 52 h.
The HPLC analyses were conducted using an Agilent 1100 series (Agilent Technologies, Santa Clara, CA) with a Zorbax column (C18; 4.6 × 100 mm, 3.5 µm). HPLC methods for quantifying sinigrin followed those of Doheny-Adams et al. (Reference Doheny-Adams, Redeker, Kittipol, Bancroft and Hartley2017). Solvents were 0.02 M tetrabutylammonium bromide (A) and a 70:30 mixture of tetrabutylammonium bromide and acetonitrile (B). The initial concentration started at 0% B and increased to 100% B over 30 min, holding for 5 min, and then returning to the initial concentration for another 10 min. Injection volume was 5.0 µL and flow rate was set to 1 mL min−1. The column temperature was maintained at 25 C, and detection was conducted using a photodiode array detector set to record at 229 nm. Agilent software ChemStation (V.B.04.01) was used to record all data. Sinigrin standards (Sigma-Aldrich) of 1.25, 2.5, 5, 10, and 20 mM were prepared in triplicate and analyzed with the extracted samples.
Sinigrin concentrations for each moisture level and run were expressed as proportions of concentrations at 0 h. Mean concentrations were plotted as functions of incubation time, and SigmaPlot (Systat Software, Inc. San Jose, CA) was used to fit two-parameter, single exponential decay curves to the data. To test the null hypotheses that regressions for different runs and moisture levels estimated the same population regression model, we used the following F test (Equation 2) for coincidental regression (Zar Reference Zar1999):
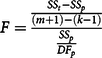 $$F = {\rm{ }}{{{{S{S_t} - S{S_p}} \over {\left( {m + 1} \right) - \left( {k - 1} \right)}}} \over {{{S{S_p}} \over {D{F_p}}}}}$$
$$F = {\rm{ }}{{{{S{S_t} - S{S_p}} \over {\left( {m + 1} \right) - \left( {k - 1} \right)}}} \over {{{S{S_p}} \over {D{F_p}}}}}$$
where SS t is the total residual sums of squares of the combined data sets, SS p is the pooled residual sums of squares, m is the number of independent variables, k is the number of regressions being compared, and DF p is the pooled degrees of freedom of the individual regressions.
Decreasing Moisture Availability Effects on MSM Control of Palmer Amaranth and V. dahlia
In this experiment, we used germination assays conducted in Petri plates (100 × 15 mm). Water potentials for germination assay solutions were manipulated with polyethylene glycol 8000 (PEG) following methods described by Steuter et al. (Reference Steuter, Mozafar and Goodin1981). This experiment was conducted twice; on each occasion, a completely randomized design with four replicates was used. Treatments were factorial combinations of MSM amount (0, 1.2, and 2.4 g MSM, equivalent to 0, 2,200, and 4,400 kg ha−1, respectively) and germination-assay water potential (0, −0.03, −0.6, −1.0, and −2.0 MPa).
Petri plates were first filled with the appropriate amount of MSM. Control dishes contained no MSM. A blotter paper (Anchor Paper, St. Paul MN) was then placed on top of the MSM and saturated with 5 mL of water or PEG solution. Fifty Palmer amaranth seeds were placed on each blotter paper in a grid pattern. Petri dishes were then sealed with Parafilm (Bemis Co., Neenah, WI) and placed in a growth chamber at 30 C. A steady, rather than fluctuating, temperature was used to maintain constant water potential of the PEG solutions (Steuter et al. Reference Steuter, Mozafar and Goodin1981). After 21 d, the Petri dishes were unwrapped and all germinated seeds were counted. All nongerminated seeds were tested for viability using the tetrazolium chloride stain test (Peters Reference Peters2000). Pesticidal activity potential for Palmer amaranth (PAP Palmer ) was determined using Equation 3:
where G crl is the number of germinated seeds in plates without MSM for replicate r and water potential l, G mrl is the number of germinated seeds in plates with MSM for replicate r and water potential l, and G cr0MPA is the number of germinated seeds in plates without MSM and the water potential treatment 0 MPa for replicate r.
Because germination assay solutions of varying water potential were in contact with Palmer amaranth seeds and MSM, PAP Palmer results indicated the effects of moisture availability on both Palmer amaranth seed germination and MSM hydrolysis. To understand how decreasing moisture affects only hydrolysis of MSM, plugs of V. dahlia were plated on CDA and suspended over mixtures of MSM and different PEG solutions. Specifically, CDA plates (described under Biological Materials) were inoculated with a 1-cm diameter plug of V. dahliae and then inverted over a container with MSM and 50 mL of PEG solution or water. Containers were sealed with Parafilm and placed in an incubator at 25 C with no supplemental lighting. This experiment was conducted twice. For each run, the experimental design was a completely randomized design with four replications. Treatments were factorial combinations of MSM amount (0, 1.2, and 2.4 g MSM; the last two equivalent to 2,200 and 4,400 kg MSM ha−1, respectively) and MSM solution water potential (0, −0.03, −0.6, −1.0, and −2.0 MPa).
Mycelial growth of V. dahliae was measured at 14 d after the start of incubation over MSM and treatment solutions. Pesticidal activity potential for V. dahliae (PAP Vert ) was determined by Equation 4:
where D crl is the growth across the diameter of V. dahliae mycelia in Petri plates suspended above solutions without MSM for replicate r and water potential l, and D mrl is the diameter growth of V. dahliae mycelia in Petri plates suspended above solutions with MSM for replicate r and water potential l.
Levene tests for homogeneity of variances indicated equal variances among runs. However, among water potential treatments, PAP Palmer and PAP Vert data severely violated the assumption of variance homogeneity. Data transformations did not sufficiently improve variance homogeneity. Therefore, these data were analyzed with nonparametric procedures using the R library PMCMRPlus. Friedman tests with post hoc Conover tests were conducted to compare PAP Palmer and PAP Vert among moisture levels.
Results and Discussion
MSM Effects on Palmer Amaranth Soil Seedbanks Under High Moisture
MSM-induced mortality in Palmer amaranth seedbanks was 17% greater in soil hydrated to field capacity than in saturated soil and flooded soil (Figure 1). Low rates of MSM-induced mortality in saturated and flooded soils coincided with higher rates of seed persistence, compared with soil at field capacity (Table 1). High rates of persistence in flooded and saturated soils were consistent with previous research that found increased persistence of Palmer amaranth seedbanks under saturated and flooded conditions (Schutte et al. Reference Schutte, Klypin and Shukla2016). MSM did not influence rates of emergence (Table 1), which were conditioned by soil moisture level. Seedbanks hydrated to −0.03 and −0.6 MPa generally produced more seedlings than flooded and saturated seedbanks. Although MSM did not influence emergence, MSM did reduce persistence in seedbanks hydrated to −0.03 MPa and −0.6 MPa.
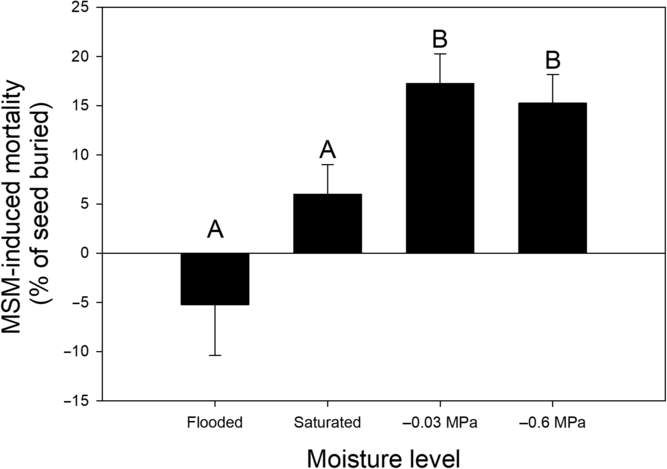
Figure 1. Mustard seed meal (MSM)-induced mortality in Palmer amaranth soil seedbanks hydrated to specific moisture levels and incubated for 14 d at 35 C/25 C day/night cycles with 12-h photoperiods. MSM was added to soil at a rate equivalent to 4,400 kg ha−1. Bars are means with SEs (N = 8). Bars with the same letter are not different at P ≤ 0.05.
Table 1. Emergence and persistence percentages for Palmer amaranth seedbanks hydrated to specific moisture levels and subjected to MSM treatments.
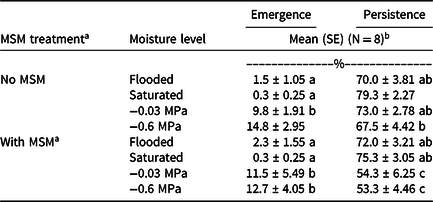
a Abbreviation: MSM, mustard seed meal.
b Within a column, means with the same letter are not significantly different at P ≤ 0.05.
c MSM was added to soil at a rate equivalent to 4,400 kg ha−1.
When saturated or flooded, seedbanks, as used in this study, have reduced levels of oxygen (Schutte et al. Reference Schutte, Klypin and Shukla2016), which can induce secondary dormancy in seeds of many weed species (Baskin and Baskin Reference Baskin and Baskin2014). Dormant seeds are less vulnerable to MSM because germination facilitates MSM-induced mortality (Lefebvre et al. Reference Lefebvre, Leblanc and Watson2018). In our study, low levels of MSM-induced mortality and high levels of persistence in saturated and flooded soil were likely caused by inhibited germination resulting from reduced oxygen availability.
Germination promotes MSM-induced mortality because the final stage of germination (i.e., radicle protrusion) removes the physical barrier of the seed coat and potentially allows isothiocyanate to infiltrate the seed. Isothiocyanates readily react with thiols and amines (Dufour et al. Reference Dufour, Stahl and Baysse2015), as well as cysteine residues to inhibit adenosine triphosphate binding sites (Breier and Ziegelhoffer Reference Breier and Ziegelhoffer2000). High concentrations of isothiocyanates stall the metabolic processes associated with seedling growth (Angelini et al. Reference Angelini, Lazzeri, Galletti, Cozzani, Macchia and Palmieri1998; Leblová-Svobodová and Koštíř Reference Leblová-Svobodová and Koštíř1962), leaving the seedling susceptible to degradation from soil microbes (Dalling et al. Reference Dalling, Davis, Schutte and Elizabeth Arnold2011). Moisture levels that are optimum for germination differ among species (Werle et al. Reference Werle, Sandell, Buhler, Hartzler and Lindquist2014) and can be conditioned by soil temperature (Kebreab and Murdoch Reference Kebreab and Murdoch1999). Accordingly, soil moisture and temperature conditions that promote MSM effects on Palmer amaranth may not be the same for other weed species in the seedbank.
Sinigrin Degradation in Soil Hydrated to Saturation and Field Capacity
Dry soil amended with MSM contained 98% of initial sinigrin content after 48 h of incubation (data not shown), indicating that sinigrin was stable in dry soil. For saturated soil, the F test for coincidental regression indicated that individual regressions for experimental runs estimated the same population (F 2,10 = 1.75; P = 0.223), which meant there was no difference in the sinigrin decay curve between the two runs. Similarly, experimental run did not affect the decay curve for sinigrin in soil hydrated to field capacity (F 2,10 = 0.38; P = 0.693). Accordingly, and for both moisture treatments, data from the two runs were combined.
The F test for coincidental regression indicated that individual regressions for soil moisture treatments estimated different populations (F 2,10 = 28; P < 0.05); thus, decay curves for sinigrin were developed separately for saturated and field capacity soil. Saturated soil had a higher rate of sinigrin hydrolysis than soil at field capacity, as shown by a more rapid decrease in sinigrin concentration in saturated soil (Figure 2). Sinigrin was depleted after 24 h in saturated soil, whereas in field-capacity soil, sinigrin was depleted up to 24 h after that of the waterlogged soil. The breakdown of sinigrin corresponds with isothiocyanate production (Rask et al. Reference Rask, Andréasson, Ekbom, Eriksson, Pontoppidan and Meijer2000). Previous research indicated that isothiocyanate formation from MSM-treated soil was greater in waterlogged soil than in soil at field capacity (Morra and Kirkegaard Reference Morra and Kirkegaard2002). Thus, the results from our study were consistent with those from previous research (Morra and Kirkegaard Reference Morra and Kirkegaard2002).
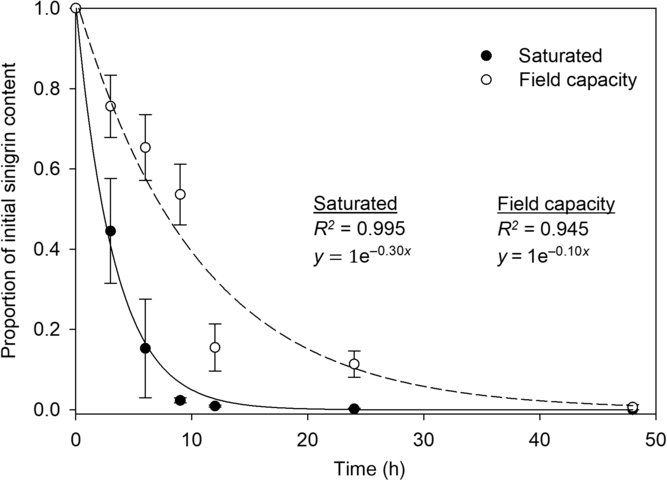
Figure 2. Exponential decay curves for sinigrin derived from mustard seed meal in soil hydrated to saturation (solid line) or field capacity (dashed line). Experimental units were incubated in a growth chamber at 30 C. Data points are means with SEs (N = 6).
Decreasing Moisture-Availability Effects on MSM Control of Palmer Amaranth and V. dahliae
Palmer amaranth seeds incubated in the presence of MSM failed to complete germination, whereas Palmer amaranth seeds without MSM completed germination at rates conditioned by the water potential of the germination assay solution (Table 2). At 0 MPa, 16% of Palmer amaranth seeds germinated, whereas at −1.0 MPa, only 4% of Palmer amaranth seeds germinated. At −2.0 MPa, no seeds germinated. Because fewer seeds germinated as available water diminished, PAP Palmer (Equation 3) decreased with decreasing water potential in the germination assay solution.
Table 2. Germination percentages for Palmer amaranth seeds in Petri plates with MSM and aqueous solutions with different water potentials.
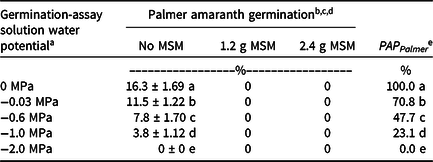
a Water potential treatments were created with polyethylene glycol solutions.
b Data are reported as mean ± SE (N = 8).
c Within a column, means with the same letter are not significantly different according to Conover test (α = 0.05).
d Abbreviations: MSM, mustard seed meal; PAP Palmer, pesticidal activity potential for Palmer amaranth.
e Germination data were used to calculate the pesticidal activity potential for MSM on Palmer amaranth, PAP Palmer (Equation 3).
When incubated in PEG solutions with water potentials of 0 MPa, −0.03 MPa, and −0.6 MPa, both rates of MSM (1.2 g and 2.4 g MSM) inhibited growth of V. dahliae (Table 3; Figure 3). However, when incubated in PEG solutions with water potentials of −1.0 MPa and −2.0 MPa, MSM effects on V. dahliae growth were conditioned by MSM rate. For the PEG solution with the water potential −1.0 MPa, V. dahliae suspended above 1.2 g MSM grew 2.2 cm in diameter after 14 d of incubation, whereas V. dahliae suspended above 2.4 g MSM was unable to grow. For the PEG solution with the water potential of −2.0 MPa, V. dahliae suspended above 1.2 g MSM grew 3.0 cm in diameter, whereas V. dahliae suspended above 2.4 g MSM grew 0.6 cm in diameter. V. dahliae suspended above no MSM grew approximately 5 cm in diameter at all moisture levels. For each MSM rate, PAP Vert decreased with decreased moisture availability. The decrease in PAP Vert (Equation 4) was likely due to decreased sinigrin hydrolysis at lower moisture levels, which reduced isothiocyanate formation and allowed V. dahliae to grow. These results were consistent with previous research that determined isothiocyanate formation from MSM decreased as soil-water level decreased (Wang and Mazzola Reference Wang and Mazzola2019).
Table 3. Mycelial growth of Verticillium dahliae in Czapek-Dox agar after 14 d of incubation above factorial combinations of MSM amounts and MSM-solution water potentials.
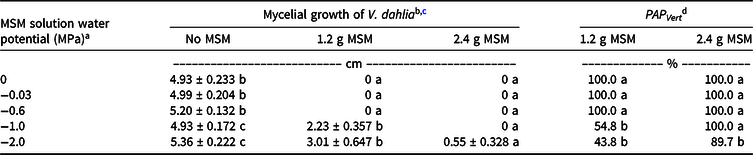
a Abbreviations: MSM, mustard seed meal; PAP Vert, pesticidal activity potential for Verticillium dahlia.
b Data are reported as mean ± SE (N = 8).
c Means within a column that share the same letter are not different at P ≤ 0.05.
d Mycelial growth data were used to calculate the pesticidal activity potential for MSM on V. dahliae, PAP Vert (refer to Equation 4). PAP Vert was separately calculated for 1.2-g and 2.4-g MSM treatments.

Figure 3. Images of Verticillium dahliae cultures after 14 d of incubation above factorial combinations of mustard seed meal (MSM) amounts and MSM-solution water potentials. At the beginning of the incubation period, cultures featured V. dahlia plugs (1-cm diameter) centrally located in Petri plates (inner diameter, 8.8 cm) with Czapek-Dox agar. V. dahliae plugs did not enlarge when incubated above 0, −0.03, and −0.6 MPa solutions containing 1.2 g MSM; and 0, −0.03, −0.6, and −1.0 MPa solutions containing 2.4 g of MSM.
Implications for Management
A previous field study determined preplant incorporated (PPI) applications of MSM reduced Palmer amaranth densities and hand-hoeing requirements during the first 4 wk of the chile pepper season (Wood Reference Wood2019). After this period, there was no effect of PPI applications of MSM on weed densities and hoeing requirements. Accordingly, PPI applications of MSM are potential tactics for managing early-season weeds in chile pepper fields in the U.S. Southwest. However, for MSM to become a sustainable component of cropland pest management programs in this water-limited region, irrigations after MSM amendments must not reduce the pesticidal potential of MSM but maximize MSM-induced control of weeds and soilborne diseases.
Morra and Kirkegaard (Reference Morra and Kirkegaard2002) suggested irrigation as a method to reduce loss of volatile isothiocyanates from soil amended with brassicaceous plant residues. We propose that moisture conditions intended to trap isothiocyanates should also support germination and growth of targeted pests. By irrigating to create conditions that simultaneously enhance pest susceptibility to MSM and capture of pesticidal compounds derived from MSM, farmers can obtain maximum efficacies for MSM applications.
MSM control of Palmer amaranth is greatest in soil hydrated to field capacity and hindered in soil hydrated to only −1.0 MPa and saturated soil. MSM-induced reductions in weed emergence depend on the concentration of isothiocyanate formed during the enzymatic catalysis of glucosinolates (Aliki et al. Reference Aliki, Reade and Back2014). Enzymatic breakdown of glucosinolates requires a sufficient amount of water (Wang and Mazzola Reference Wang and Mazzola2019). The results of this study, specifically the PAP Vert results, indicate a water potential of −1.0 MPa inhibits the enzymatic breakdown of sinigrin and production of pesticidal compounds from MSM. A water potential of −1.0 MPa also inhibits Palmer amaranth germination, thereby reducing the potential for MSM to control this weed species. In saturated soil, the pesticidal properties of MSM are wasted because Palmer amaranth seed germination is inhibited, but gluocosinolates are rapidly and irreversibly degraded. Thus, saturated soils should be avoided after MSM amendments intended to target Palmer amaranth.
Although the results of this study indicated hydration to field capacity is optimal for MSM-induced, PRE control of Palmer amaranth and V. dahliae, these results need to be validated in the field before recommendations can be made. In addition, more research is required to determine the minimum length of time soil needs to hydrate after MSM applications. Previous research determined that pesticidal compounds from degradation of sinigrin in soil reached maximum concentrations within 24 h of hydration, and subsequent declines of sinigrin-derived pesticidal compounds differed among soil types (Borek et al. Reference Borek, Morra, Brown and McCaffrey1994). Accordingly, additional research seeking to clarify minimum durations of hydration after MSM applications will need to account for the effects of soil type, as well as soil pH and soil temperature, which also influence enzymatic hydrolysis of sinigrin (Al-Turki and Dick Reference Al-Turki and Dick2003; Borek et al. Reference Borek, Morra, Brown and McCaffrey1994). Improved understanding of the relationships among soil characteristics, moisture, and persistence of sinigrin-derived pesticidal compounds will facilitate the development of models that allow farmers in the U.S. Southwest to use MSM without unnecessary irrigation, thereby conserving regional water resources.
Acknowledgements
Funding sources for this project included the US Department of Agriculture National Institute of Food and Agriculture Hatch Project (NM-Schutte-12H) and the New Mexico Chile Association. Salaries and research support were provided by state and federal funds appropriated to the New Mexico Agricultural Experiment Station. No conflicts of interest have been declared. We gratefully acknowledge the technical assistance of Israel Marquez, Asmita Nagila, and Dr. Abdur Rashid. We also thank Barry Dungan and Dr. Omar Holguin for their helpful insights on high-performance liquid chromatography operation.








