Introduction
Top-down control can strongly influence community structure and function, including both direct effects of consumption and also trophic cascades. The cascading effects of large-mammalian carnivores (Estes et al. Reference Estes, Tinker, Williams and Doak1998, Ford et al. Reference Ford, Goheen, Otieno, Bidner, Isbell, Palmer, Ward, Woodroffe and Pringle2014, Pace et al. Reference Pace, Cole, Carpenter and Kitchell1999, Shurin et al. Reference Shurin, Borer, Seabloom, Anderson, Blanchette, Broitman, Cooper and Halpern2002) and large-mammalian herbivores (Holdo et al. Reference Holdo, Sinclair, Dobson, Metzger, Bolker, Ritchie and Holt2009, Pringle et al. Reference Pringle, Young, Rubenstein and McCauley2007) on lower trophic levels, via either consumptive or non-consumptive effects, have been documented repeatedly through experimental and observational approaches. Some studies additionally claim that effects of higher trophic levels on lower trophic levels generate highly indirect community-wide responses in a whole suite of guilds, propagated through both trophic and non-trophic interactions (Estes et al. Reference Estes, Peterson, Steneck, Terborgh and Estes2010, Ripple & Beschta Reference Ripple and Beschta2006, Ripple et al. Reference Ripple, Estes, Beschta, Wilmers, Ritchie, Hebblewhite, Berger, Elmhagen, Letnic, Nelson, Schmitz, Smith, Wallach and Wirsing2014). Such phenomena have been coined extended trophic cascades (Goheen et al. Reference Goheen, Augustine, Veblen, Kimuyu, Palmer, Porensky, Pringle, Ratnam, Riginos, Sankaran, Ford, Hassan, Jakopak, Kartzinel, Kurukura, Louthan, Odadi, Otieno, Wambua, Young and Young2018), trophic ricochets (Nuttle et al. Reference Nuttle, Yerger, Stoleson and Ristau2011), or trophic bouncebacks (Terborgh & Feeley Reference Terborgh, Feeley, Terborgh and Estes2010); here, we use the phrase extended trophic cascades. For example, Terborgh & Feeley (Reference Terborgh, Feeley, Terborgh and Estes2010) show that cascading positive effects of howler monkey and iguana folivory on soil nutrients lead to increased tree growth, generating effects on birds, a guild that is not part of the trophic cascade interaction chain (see also Morris & Letnic Reference Morris and Letnic2016).
A variety of studies document extended trophic cascades generated by large-mammalian herbivory (Baines et al. Reference Baines, Sage and Baines1994, McCauley et al. Reference McCauley, Keesing, Young, Allan and Pringle2006, Reference McCauley, Keesing, Young and Dittmar2008; Roberson et al. Reference Roberson, Chips, Carson and Rooney2016, Titcomb et al. Reference Titcomb, Allan, Ainsworth, Henson, Hedlund, Pringle, Njoroge, Campana, Fleischer, Mantas and Young2017, Wardle & Bardgett Reference Wardle and Bardgett2004, Weinstein et al. Reference Weinstein, Titcomb, Agwanda, Riginos and Young2017). These studies show that large-mammalian herbivory can speed up nutrient cycling (Wardle & Bardgett Reference Wardle and Bardgett2004), reduce plant density (Baines et al. Reference Baines, Sage and Baines1994), change vegetation structure (Roberson et al. Reference Roberson, Chips, Carson and Rooney2016), or elicit changes in density of other herbivores (Goheen et al. Reference Goheen, Augustine, Veblen, Kimuyu, Palmer, Porensky, Pringle, Ratnam, Riginos, Sankaran, Ford, Hassan, Jakopak, Kartzinel, Kurukura, Louthan, Odadi, Otieno, Wambua, Young and Young2018) that then lead to additional effects on guilds not involved in the trophic cascade. Due to the strong effects of large-mammalian herbivores on plant density (Goheen et al. Reference Goheen, Palmer, Charles, Helgen, Kinyua, Maclean, Turner, Young and Pringle2013, Jacobs & Naiman Reference Jacobs and Naiman2008) and plant community composition (Augustine & McNaughton Reference Augustine and McNaughton1998, Côté et al. Reference Côté, Rooney, Tremblay, Dussault and Waller2004, Diaz et al. Reference Diaz, Lavorel, Mcintyre, Falczuk, Casanoves, Milchunas, Skarpe, Rusch, Sternberg, Noy-Meir, Landsberg, Zhang, Clark and Campbell2007), extended trophic cascades arising from large-mammalian herbivory could manifest in any of the many guilds that interact strongly with plants.
In this study, we provide experimental evidence for an extended trophic cascade, whereby large-mammalian herbivores affect floral assemblages (namely, the composition of flowers available for floral visitors), which then elicits changes in assemblages of floral visitors, a guild strongly linked to flowering plants. Using experimental manipulations of densities of large-mammalian herbivores (the most rigorous test for extended trophic cascades, Ford & Goheen Reference Ford and Goheen2015), two hypotheses were tested. First, we tested whether large-mammalian herbivores elicited a trophic cascade, affecting assemblages of flowers and floral visitors. Second, we tested whether large-mammalian herbivores elicited an extended trophic cascade on floral-visitor assemblages, whereby floral assemblages, themselves influenced by herbivores, had added effects on assemblages of floral visitors.
Methods
Our fieldwork was conducted at Mpala Ranch in Laikipia County of central Kenya (0°17′N, 37°52′E), in a semi-arid acacia-dominated (Vachellia spp. and Senegalia spp.) savanna exhibiting little seasonality in temperature. Rainfall is weakly bi- or tri-modal across Mpala Ranch; January–February are the driest months of the year, with April–May and October–November the wettest months (Goheen et al. Reference Goheen, Palmer, Charles, Helgen, Kinyua, Maclean, Turner, Young and Pringle2013). This system harbours a diverse large-mammalian herbivore assemblage; common herbivores include elephant (Loxodonta africana), giraffe (Giraffa camelopardalis), eland (Taurotragus oryx), Cape buffalo (Syncerus caffer), common zebra (Equus quagga), impala (Aepyceros melampus), warthog (Phacochoerus africanus) and dik-dik (Madoqua guentheri) (Louthan et al. Reference Louthan, Doak, Goheen, Palmer and Pringle2014). Our work was conducted within the UHURU experiment, a series of large-scale, long-term herbivore exclosures arrayed across a pronounced aridity gradient (Goheen et al. Reference Goheen, Palmer, Charles, Helgen, Kinyua, Maclean, Turner, Young and Pringle2013). UHURU consists of four different herbivore treatments (all large-mammalian herbivores present, megaherbivores absent, meso- and mega-herbivores absent, all large-mammalian herbivores absent), replicated in three blocks at each of three sites spanning an aridity gradient (Goheen et al. Reference Goheen, Palmer, Charles, Helgen, Kinyua, Maclean, Turner, Young and Pringle2013).
The floral and insect assemblages were sampled in the two extreme herbivore treatments (all large-mammalian herbivores present, all absent) twice, once in the dry season (23 January–10 February 2015) and once in the wet season (4–28 May 2015). In the wet season, we also sampled assemblages in the two intermediate herbivore treatments (megaherbivores absent, mega- and mesoherbivores absent).
To sample the insect assemblages, pan traps were used, constructed with 96.1-cm3 white soufflé cups painted white, yellow or blue (silica flat, yellow fluorescent and blue fluorescent from the Guerra Paint and Pigment company; colours attract pollinating insects). Each pan trap was filled with water, to which soap was added to reduce surface tension (Westphal et al. Reference Westphal, Bommarco, Carré, Lamborn, Morison, Petanidou, Potts, Roberts, Szentgyörgyi, Tscheulin, Vaissière, Woyciechowski, Biesmeijer, Kunin, Settele and Steffan-Dewenter2008). In both the wet and dry season, 27 trios of each colour pan trap were placed ~5 m apart (ranging from 3–7 m apart, depending on accessible ground space) in a 70 × 70-m cross pattern in each herbivore treatment × block combination. The 27 pan traps were deployed during a 30-min window between 8h30 and 11h0, and after 24 h, the trapped insects were collected and stored. Insect species were identified to family, with individual species classified into unique but unknown species within each family. To control for phenology and block effects, pan traps were deployed at one site × block combination at a time, rotating among levels on subsequent days after collection at that site × block was finished (collection, sorting, and storage of insects took 2 d per site × block in the dry season, and 4 d in the wet season).
Within 8 h of deployment of the pan traps, we sampled the floral assemblage in the area immediately adjacent to pan traps. We recorded whether each flowering species had <10, 10–50, 50–100, or >100 flowers within 3 m of the pan trap. We used bins because counting flowers individually was time-consuming and prone to error, as one flowering plant could have presented hundreds of flowers. Note that each individual flowering plant could have had either a singular flower, or multiple flowers, and all subsequent analyses were conducted on the number of flowers observed (either total number, or on number of flowers of each species). Thus, we refer to response variables involving flowers as number of flowers or floral assemblage, rather than number of flowering plants, flowering plant assemblage or similar phrasing.
Of the 81 insect species we collected, floral visitors (which may serve as pollinators) were identified using data from a simultaneous study on pollinator service in UHURU (Guy et al. unpubl. data). Because we do not know the efficacy of the pollination services of these floral visitors, we call these species floral visitors throughout this manuscript. This study and our own suffered from uncertainty in the identification of insects in this poorly sampled region, so for subsequent analyses, we classified as floral visitors all insects in the same family as pollinators identified by Guy et al. (unpubl. data; this study identified all pollinators to at least family; many were identified to species).
We tested for effects of herbivory and rainfall on number of flowers and floral visitors, as well as assemblages of flowers and floral visitors. Note that the discrepancy between seasons in which herbivore exclosure treatments were sampled resulted in an unbalanced design; thus, in order to use all of the data, rainfall in the 3 mo prior was used as a predictor variable to capture differences in season and site (following Louthan et al. Reference Louthan, Pringle, Goheen, Palmer, Morris and Doak2018). Similarly, variation among herbivore activity within herbivore treatments was condensed to season × site × herbivore treatment × block-specific dung counts (calculated as in Louthan et al. Reference Louthan, Pringle, Goheen, Palmer, Morris and Doak2018, but using dung counts only from the dung survey immediately prior to our surveys of flowers and floral visitors). Hereafter, we refer to these variables as rainfall and herbivore activity, respectively. We conducted analyses on the total number of floral visitors in each of the 49 season × site × herbivore treatment × block combinations, and similarly, on the total number of flowers in each of these 49 combinations. To summarize data on binned estimates of number of flowers, the mean of the bounds of each flower number category (5, 30, 75, 100) was used.
A model selection approach was used to determine whether rainfall, herbivore activity or their interaction affected numbers of flowers or floral visitors. A mixed model with rainfall, herbivore activity and their interaction as fixed effects was fitted, with square-root transformed total number of flowers in each of the 49 season × site × herbivore treatment × block combinations as a response variable. AICc was used to compare mixed models of all possible subsets of this global mixed model (all with a random block effect, unique to site; note that this random effect controls for site effects as well). We assumed that any fixed effects present in the best-fit model exerted effects on number of flowers. A similar mixed model selection approach was used to assess whether rainfall, herbivore activity, or their interaction affected log-transformed number of floral visitors in each of the 49 season × site × herbivore treatment × block combinations.
We also tested for effects of herbivory and rainfall on assemblages of flowers and floral visitors. To quantify variation in assemblages of flowers and floral visitors, two detrended correspondence analyses (DCA) were conducted on the abundances of flowers of each flowering species and floral visitor species. Abundances of each species were quantified in each of the 49 season × site × herbivore treatment × block combinations; three combinations in which no flowers were found were discarded (dry season × arid site × all herbivores present × block 3, dry season × arid site × all herbivores absent × block 3, dry season × mesic site × all herbivores present × block 1). A mixed model selection approach was used to assess whether rainfall, herbivore activity, and their interaction affected the first and second DCA axes of the floral assemblage and the first and second DCA axes of the floral-visitor assemblage, again using AICc to compare all possible subsets of a global model, where all models included a block effect. Some models of the first DCA axis of the floral assemblage were singular, but the same model selection approach was used to compare linear models and found similar results, so we present our mixed model results here.
Associations between assemblages of flowers and of floral visitors could indicate that these assemblages might impact one another, showing support for an extended trophic cascade. Associations between assemblages of flowers and floral visitors were assessed in three ways; in these analyses, the season × site × herbivore treatment × block combinations were the units of replication. First, a Mantel test on the dissimilarity matrices of these assemblages was conducted. Second, we used these dissimilarity matrices to calculate a Robinson–Foulds distance between floral and floral-visitor assemblages. The observed Robinson–Foulds distance was compared to that of a null distribution. To obtain the null distribution, observed floral-visitor assemblages were randomly assigned 10 000 times to a season × site × herbivore treatment × block combination and the Robinson–Foulds distance between the dissimilarity matrices of floral and floral-visitor assemblages was calculated each time. Finally, we assessed whether assemblages of flowers and floral visitors were associated while controlling for effects of rainfall, herbivore activity and site, which would provide more robust evidence for an extended trophic cascade. We assessed the relationship between residuals of our best-fit mixed models for each DCA axis as a function of rainfall, herbivore activity, and their interaction using two linear models. Specifically, AICc was used to test whether residuals of the floral axes affected the residuals of each floral-visitor DCA axis, comparing all possible subsets of a global model with residuals of each floral-visitor axis, and their interaction, as predictor variables. The analysis was also conducted with opposite causality (residuals of floral-visitor DCA axes affect residuals of the floral axes) to ensure results were robust to the assumption that floral assemblages drive floral-visitor assemblages.
Finally, to test whether an extended trophic cascade was a key driver of floral-visitor assemblages, we fitted structural equation models (SEMs) incorporating effects of rainfall, herbivore activity and floral assemblages on assemblages of floral visitors, and determined whether floral assemblages exerted strong effects using a model selection approach. Specifically, AICc was used to compare an SEM that included effects of rainfall, herbivore activity, and their interaction on both DCA axes of floral visitor and flower assemblages (similar to the structure of the SEM in Byrnes et al. Reference Byrnes, Reed, Cardinale, Cavanaugh, Holbrooks and Schmitts2011), as well as our proposed extended trophic cascade, an effect of floral DCA axes on floral-visitor DCA axes, with one that did not include this effect of floral assemblage on assemblage of floral visitors.
A series of model comparisons were conducted to ensure our SEM was robust to statistical issues and to our assumptions. First, full versus reduced SEMs when outliers were included were compared. Second, full versus reduced SEMs that only included rainfall and herbivore activity effects present in models with AICc weight > 0.2 at the univariate level were compared. For both of these comparisons, we assessed whether the full model (with floral assemblage effects on assemblage of floral visitors) was supported over the reduced model (floral assemblage did not affect assemblage of floral visitors). Finally, in our main SEM comparison, variation in floral assemblages was assumed to generate variation in floral-visitor assemblages (rather than floral-visitor assemblages generating variation in floral assemblages). To test that our results were robust to assuming this directional causality, our SEM was modified to have causality flowing in the other direction (floral-visitor assemblages affected floral assemblages). We then tested whether we still saw support for a full model (with floral-visitor assemblage effects on floral assemblage) versus a reduced model (without these effects).
Results
We observed 59 floral visitor species and 60 species in flower during our study. The total number of floral visitors found in a season × site × herbivore treatment × block combination ranged from 5 to 203, and number of flowers ranged from 0–4805. The majority of floral visitors were from the Apidae, with lower numbers of floral visitors from the Muscidae and Lycaenidae. The largest fraction of flowers were Senegalia brevispica (Harms) Seigler & Ebinger, with sizable numbers of Croton dichogamous Pax and Solanum campylacanthum Hochst. ex A.Rich.
Numbers of floral visitors and flowers were both affected by herbivore activity and rainfall. Rainfall increased and herbivore activity decreased square-root transformed number of flowers (AICc weight = 0.406, Figures 1, 2, Appendix 1; note that correlations among random effect estimates were large). Herbivore activity and rainfall both reduced log-transformed number of floral visitors (AICc weight = 0.528; Figures 1, 2, Appendix 1). There was no relationship between numbers of floral visitors and flowers (AICc weight of model with only block effect = 0.754; Appendix 2; blocks had significantly different variances of residuals), and numbers of floral visitors and flowers were not significantly correlated overall (P = 0.26).

Figure 1. Effects of rainfall and herbivore activity on number of flowers (a), number of floral visitors (b), floral DCA axes (c, e), and floral-visitor DCA axes (d, f) within experimental manipulations of herbivore activity across a rainfall gradient at Mpala Ranch in Kenya. We show the contrast between the mesic versus arid site rainfall values (averaged across seasons), and the presence versus absence of herbivory (difference between extreme herbivore treatments, averaged across blocks, sites and seasons). A lack of bar indicates that the effect was not present in the best-fit model, and error bars show standard error of coefficients. Note that no best-fit models contain an interaction between rainfall and herbivore activity, and note also the change of scale in (a).

Figure 2. Effects of herbivore activity on numbers and assemblages of flowers and floral visitors within experimental manipulations of herbivore activity across a rainfall gradient at Mpala Ranch in Kenya. We show the effect of herbivore activity on number of flowers (a), number of floral visitors (b), floral DCA axes 1 and 2 (c, e) and floral-visitor DCA axes 1 and 2 (d, f). See Appendix 6 for an illustration of effects of rainfall on the most common flowers and floral visitors.
Structures of floral and floral-visitor assemblages were well-described by our DCA axes. The percentages of total variance explained by the first and second DCA axes of floral assemblages were 41% and 27%, respectively, and the percentages of total variance explained by the first and second DCA axes of floral-visitor assemblages were 47% and 20%, respectively. Maerua angolensis DC. and Euphorbia heterospina S.Carter loaded strongly onto floral DCA axis one, and Phyllanthus maderaspatensis L. and Aerva lanata L. loaded strongly onto floral DCA axis two (Appendix 3). A member of the Colletidae and of the Scarabaeidae each loaded strongly onto floral-visitor DCA axis one, and a member of the Crabronidae and of the Sarcophagidae loaded onto floral-visitor DCA axis two (Appendix 3). In our surveys, there were relatively high diversities of both floral visitors and plants, so there was no one species that dominated the DCA loadings, which instead showed relatively weak contributions of multiple species. For example, the most numerous flowers were those of Senegalia brevispica, but flowers of this species did not dominate either DCA floral axis. Numbers of flowers were also not correlated with site scores for floral DCA axes one or two (P > 0.05); numbers of floral visitors were correlated with floral-visitor DCA axis one at a Pearson’s correlation coefficient of −0.38 (P = 0.007), but not correlated with DCA axis two (P > 0.05).
Assemblages of flowers and floral visitors were both affected by herbivore activity and rainfall, meaning that we found support for top-down effects of herbivores on both of these assemblages. Rainfall and herbivore activity affected the first DCA axis of the floral assemblage (AICc weight = 0.427; Figures 1, 2, Appendix 1), while neither rainfall, herbivore activity, nor their interaction affected the second axis (AICc weight = 0.497, Figures 1, 2, Appendix 1). An analogous analysis on assemblages of floral visitors showed that rainfall affected DCA axis one (AICc weight = 0.622, Figures 1, 2, Appendix 1), while both rainfall and herbivore activity affected DCA axis two (AICc weight = 0.734, Figures 1, 2, Appendix 1). All of these models, besides analysis of floral DCA axis two, exhibited unequal variances of residuals across blocks.
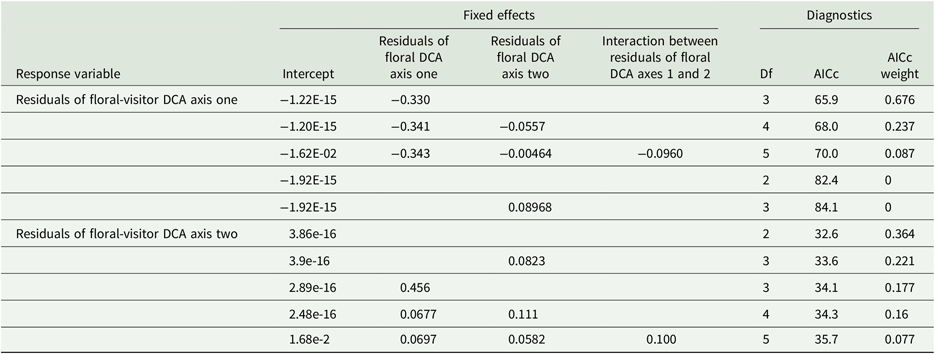
Assemblages of floral visitors and flowers were significantly correlated with one another, even after accounting for herbivore and rainfall effects, showing evidence for an extended trophic cascade. A Mantel test showed a significant correlation between the dissimilarity matrices of floral-visitor and floral assemblages (R = 0.218, P = 0.001). The observed Robinson–Foulds distance between the dissimilarity matrices of floral and floral-visitor assemblages was significantly smaller than the null distribution of the distances (one-sided permutation test, P = 0.0273). Finally, we found a significant relationship between the residuals of our mixed models of rainfall and herbivore activity effects on the floral-visitor DCA axes and the residuals of our mixed models of rainfall and herbivore activity effects on the floral DCA axes. Specifically, we found significant effects of floral DCA axis one residuals on floral-visitor DCA axis one residuals (AICc weight = 0.676, Figure 3, Appendix 4), with no effects of either floral DCA axes’ residuals on floral-visitor DCA axis two residuals (AICc weight = 0.364, Figure 3, Appendix 4). Results were similar when we used floral DCA axes residuals as response variables and floral-visitor DCA axes as predictor variables (the best-fit model for floral DCA axis one contained residuals of floral-visitor DCA axis two, with an AICc weight of 0.683, but the best-fit model for floral DCA axis two contained no effects of floral-visitor axes; AICc weight was 0.392).

Figure 3. The relationship between floral and floral-visitor DCA axes after accounting for rainfall and herbivore activity within experimental manipulations of herbivore activity across a rainfall gradient at Mpala Ranch in Kenya. The effect of residuals of floral DCA axis one (mixed model residuals of DCA axis one scores as a function of rainfall and herbivore activity) on the residuals of floral-visitor DCA axis one (a; mixed model residuals of DCA axis one scores as a function of rainfall) and on the residuals of floral-visitor DCA axis two (b; mixed model residuals of DCA axis two scores as a function of rainfall and herbivore activity) are shown. The relationship between floral DCA axis one residuals and floral-visitor DCA axis one residuals (a) is significant, but the relationship between floral DCA axis one residuals and floral-visitor DCA axis two residuals (b) is not.
We saw strong support for an extended trophic cascade using an SEM approach, with support for floral DCA axes influencing floral-visitor DCA axes. We parameterized an SEM with effects of rainfall and herbivore activity on both floral and floral-visitor DCA axes; these effects represent the trophic cascade. This SEM also had effects of floral DCA axes on floral-visitor DCA axes, representing the hypothesized extended trophic cascade (form of model is shown in Figure 4). We compared its model fit (AICc) to that of an SEM without the effect of floral DCA axes on floral-visitor DCA axes. We found stronger support for our full SEM over our reduced SEM (AICc of full was 1344.4 and of reduced was 1352.6; AICc weight of full was 0.984), supporting an effect of floral assemblages on assemblages of flower visitors. The full SEM was a good fit to the data, with a non-significant chi-square statistic, a root mean square error of approximation (RMSEA) value of 0.052, and a comparative fit index (CFI) of 0.998. This result was robust to including outliers, to including only well-supported univariate effects (though some of the model fits were somewhat poor), and to assuming assemblages of floral visitors affected floral assemblages (Appendix 5). Coefficients for our best-supported SEM with rainfall, herbivore activity, their interaction, and floral DCA axes effects on floral-visitor DCA axes are shown in Figure 4; we also show the unexplained correlation between residuals of floral-visitor DCA axes (double-headed arrow).

Figure 4. Structural equation model containing effects of rainfall, herbivore activity and their interaction on both floral-visitor and floral DCA axes within experimental manipulations of herbivore activity across a rainfall gradient at Mpala Ranch in Kenya. We show the estimate of each standardized coefficient along the corresponding single-headed arrow and the unexplained correlation between floral-visitor DCA axes residuals using the double-headed arrow. For clarity, we do not show fixed parameter estimates. Blue arrows indicate positive effects; green indicate negative effects. Single-headed arrows ending in an asterisk indicate effects that were significant at the univariate level.
Discussion
We found strong effects of herbivores on numbers of floral visitors and flowers, as well as on assemblages of floral visitors and flowers. Effects of herbivores on floral numbers are consistent with work showing that herbivores reduce overall plant biomass or density (Goheen et al. Reference Goheen, Palmer, Charles, Helgen, Kinyua, Maclean, Turner, Young and Pringle2013, Jacobs & Naiman Reference Jacobs and Naiman2008), as well as alter plant allocation to reproductive structures (Goheen et al. Reference Goheen, Young, Keesing and Palmer2007, Koptur et al. Reference Koptur, Smith and Lawton1996, Niesenbaum Reference Niesenbaum1996, Young & Augustine Reference Young and Augustine2007). Similar to our work, a wide variety of past work has found evidence that herbivores alter plant community composition (Augustine & McNaughton Reference Augustine and McNaughton1998, Côté et al. Reference Côté, Rooney, Tremblay, Dussault and Waller2004, Diaz et al. Reference Diaz, Lavorel, Mcintyre, Falczuk, Casanoves, Milchunas, Skarpe, Rusch, Sternberg, Noy-Meir, Landsberg, Zhang, Clark and Campbell2007, Pringle et al. Reference Pringle, Prior, Palmer, Young and Goheen2016). We also found top-down effects of herbivores on both numbers and assemblages of floral visitors. Consistent with these results, a variety of previous studies have found that herbivores affect insect and pollinator abundance and assemblages via top-down effects (Carvell Reference Carvell2002, Warren Reference Warren1993, Yoshihara et al. Reference Yoshihara, Chimeddorj, Buuveibaatar, Lhagvasuren and Takatsuki2008). One study found possible effects of an extended trophic cascade, namely, an impact of changes in the insect community on fruit set (potentially resulting from changes in pollinator service; Mayer Reference Mayer2004). Herbivores could affect assemblages of floral visitors via multiple mechanisms independent of their modification of floral resources, including modifications of soil, vegetative aspects of the plant community, disturbance frequency or intensity, or predation rates (van Klink et al. Reference Van Klink, Van Der Plas, Van Noordwijk, Wallisdevries and Olff2015).
In addition to these potentially direct effects, we found strong support for an extended trophic cascade: herbivore activity affected floral assemblages, which in turn affected assemblages of floral visitors (Figure 4). This conclusion is supported by all our metrics of analysis: Robinson–Foulds distance, correlation of residuals, and SEMs (note that a non-significant chi-square statistic indicates that an SEM is a good fit to the data, RMSEA values ≤ 0.05 are indicative of a good fit, with 0.05–0.08 adequate fit, and CFI values ≥0.97 are indicative of a good fit; Schermelleh-Engel & Moosbrugger Reference Schermelleh-Engel and Moosbrugger2003). These results are consistent with many previous studies showing extended trophic cascades resulting from large-mammal herbivory on other community members (Donihue et al. Reference Donihue, Porensky, Foufopoulos, Riginos and Pringle2013, Nuttle et al. Reference Nuttle, Yerger, Stoleson and Ristau2011, Roberson et al. Reference Roberson, Chips, Carson and Rooney2016, Wardle & Bardgett Reference Wardle and Bardgett2004, Young et al. Reference Young, McCauley, Dirzo, Goheen, Agwanda, Brook, Otarola-Castillo, Ferguson, Kinyua, McDonough, Palmer, Pringle, Young and Helgen2015 and studies reviewed in Goheen et al. Reference Goheen, Augustine, Veblen, Kimuyu, Palmer, Porensky, Pringle, Ratnam, Riginos, Sankaran, Ford, Hassan, Jakopak, Kartzinel, Kurukura, Louthan, Odadi, Otieno, Wambua, Young and Young2018, Pringle et al. Reference Pringle, Palmer, Goheen, McCauley and Keesing2011 and Ripple et al. Reference Ripple, Estes, Beschta, Wilmers, Ritchie, Hebblewhite, Berger, Elmhagen, Letnic, Nelson, Schmitz, Smith, Wallach and Wirsing2014). In our system, the effect of floral assemblages on assemblages of floral visitors was quite strong; for example, the standardized coefficient of floral DCA axis one scores on floral-visitor DCA axis one scores was −0.33, an order of magnitude greater than effects of herbivores on floral-visitor DCA axis one scores (Figure 4). Similarly, the coefficient of floral DCA axis one scores on floral-visitor DCA axis two scores was 0.53, comparable in magnitude to effects of herbivores on the same floral-visitor DCA axis. Our findings of an extended trophic cascade affecting assemblages of floral visitors are consistent with a previous study finding herbivore effects on a single pollinator species, modulated via the effect of herbivores on a flowering plant species (Wilkerson et al. Reference Wilkerson, Roche and Young2013). Our findings are also consistent with studies showing interactions between herbivory and pollinator service: Strauss (Reference Strauss1997) found that herbivory can reduce pollinator visits to flowering plants by changing floral characteristics or abundance. Similarly, Vasquez & Simberloff (Reference Vasquez and Simberloff2004) found that herbivory can alter relative densities of plant species in such a way that pollination success is reduced.
Most studies documenting extended trophic cascades show evidence that a trophic cascade or top-down effect generates effects on guilds that are entirely separate from any direct or indirect top-down effects. However, in our system, herbivores generated apparently direct effects on assemblages of both flowers and floral visitors. The direct effects of herbivores on flowers generally served to amplify the top-down effects of herbivores on floral visitors. For example, herbivore activity increased both floral-visitor DCA axis two scores and floral DCA axis one scores (both direct top-down effects; Figure 4). Positive effects of floral DCA axis one scores on floral-visitor DCA axis two scores resulted in an additional increase in floral-visitor DCA axis two scores, mediated through effects of herbivores on floral DCA axis one scores (an extended trophic cascade; Figure 4). Similar effects occurred for all other impacts of floral DCA axes scores on floral-visitor DCA axes scores, though effects of the interaction between rainfall and herbivore activity acted in the opposite direction, reducing the magnitude of top-down effects of herbivores on floral-visitor DCA axes scores (Figure 4).
This example shows that separate top-down effects can interact to modulate each others’ overall strength via extended trophic cascades, as has been suggested by previous work on multiple pathways of trophic cascades in island systems (Terborgh & Feeley Reference Terborgh, Feeley, Terborgh and Estes2010). In this system, predator loss led to cascading effects on pollinator abundance, seed dispersers and herbivores; clearly these disparate effects of predator loss on multiple guilds could interact in complex ways with each other, potentially exerting interacting effects on plant reproduction, colonization and herbivory. If interactions among trophic cascades generally act to dampen rather than augment the strength of trophic cascades, such effects provide a potential explanation for why trophic cascades might be weaker or less common in strongly connected or diverse ecological communities (Fahimipour et al. Reference Fahimipour, Anderson and Williams2017).
One limitation to our study is that we do not know the mechanistic underpinnings of associations between flowers and floral visitors; it may be that assemblages of floral visitors partly drive floral assemblages, contrary to what we have assumed in our main SEM analyses. We know that the structure of pollinator assemblages is dependent on nectar resource diversity and other components of the flowering plant assemblage (Goulson et al. Reference Goulson, Nicholls, Botías and Rotheray2015, Potts et al. Reference Potts, Vulliamy, Dafni, Ne’eman and Willmer2003), and pollinator visitation rates can also vary with plant community composition (Moeller Reference Moeller2005). At the extreme, plants can suffer extinction if their pollinators go extinct (Biesmeijer et al. Reference Biesmeijer, Roberts, Reemer, Ohlemüller, Edwards, Peeters, Schaffers, Potts, Kleukers, Thomas, Settele and Kunin2006, Memmott et al. Reference Memmott, Craze, Waser and Price2007), particularly if interactions are specialized (Weiner et al. Reference Weiner, Werner, Linsenmair and Blüthgen2014). Specialization of pollinator–plant relationships should be common in this tropical system (Martins & Johnson Reference Martins and Johnson2013), potentially resulting in strong effects of pollinator assemblages on plant populations. Regardless of the causal mechanism, recently observed simultaneous declines in insect-pollinated plants and insects (Biesmeijer et al. Reference Biesmeijer, Roberts, Reemer, Ohlemüller, Edwards, Peeters, Schaffers, Potts, Kleukers, Thomas, Settele and Kunin2006) indicate that plant and pollinator communities are likely to track one another (though see Kaiser-Bunbury et al. Reference Kaiser-Bunbury, Vásquez, Stang and Ghazoul2014).
Our results provide experimental support for an extended trophic cascade, whereby herbivore impacts on floral assemblages affected assemblages of floral visitors. Our work shows that these effects were comparable in strength to top-down effects of herbivores, suggesting that extended trophic cascades can have strong impacts on many components of the ecosystem, even modulating the strength of top-down effects. Similar phenomena are likely in ecosystems where trophic cascades exert strong effects on primary productivity, because primary productivity affects many disparate guilds of organisms, and in ecosystems where guilds interact strongly with each other, such as flowering plant and pollinator assemblages.
Acknowledgements
We acknowledge funding from the P.E.O. Scholar Award, L’Oréal-UNESCO International Fellowship for Women in Science, funds from the University of Colorado-Boulder, and NSF DEB 1311394 (with D. Doak), to A. Louthan, funds from Princeton University, Nature Kenya and the Whitley Fund for Nature to D. Martins, an NSF GRFP to T. Guy (DGE-1315138), NSF DEB 1355122 to Robert Pringle, NSF DEB EAGER 1547679, NSF DEB 1556728 and awards from the University of Wyoming and the University of Wyoming Biodiversity Institute to J. Goheen, NSF DEB 1149980 to T. Palmer, and NSF DEB 1242355 and 1352781 to D. Doak. The UHURU experiment was built with a Natural Sciences and Engineering Council Research Tools and Instruments grant. We gratefully acknowledge research support and intellectual contributions from Robert Pringle.
Appendix 1
AICc comparisons of models of number of floral visitors, flowers, and floral and floral-visitor DCA axes as functions of rainfall and herbivore activity described in the text; note that in the text, we report only the best fit model that enjoys the highest AICc weight. We show response variables, fixed effects, as well as degrees of freedom (df), AICc and AICc weight of each model. For each set of models, we indicate the response variable only for the best fit model; all models without a response variable below the best fit model have the same response variable.

Appendix 2
AICc comparisons of models of number of floral visitors as a function of number of flowers described in the text; note that in the text, we report only the best fit model that enjoys the highest AICc weight. We show response variables, fixed effects, as well as degrees of freedom (df), AICc, and AICc weight of each model. For this set of models, number of floral visitors is always the response variable.

Appendix 3
DCA loadings of species of plants in flower and floral visitors for each of the first four floral and floral-visitor DCA axes within experimental manipulations of herbivore activity across a rainfall gradient at Mpala Ranch in Kenya. Unique unknown floral species are denoted by unknown followed by a number. Unique species of floral visitors in the same family are denoted by unique numbers following the family name of the floral visitor. Families of floral visitors with no numeric suffix represent one (unknown) species within that family of floral visitors.

Appendix 4
AICc comparisons of models of residuals of DCA axes described in the text; note that in the text, we report only the best fit model that enjoys the highest AICc weight. We show response variables, fixed effects, as well as degrees of freedom (df), AICc, and AICc weight of each model. For each set of models, we indicate the response variable only for the best fit model; all models without a response variable below the best fit model have the same response variable.
Appendix 5
Support for full (with effects of floral assemblage on floral-visitor assemblage or vice versa) versus reduced (without these effects) SEMs when SEMs or data were modified in some way. Note that while the full model was always supported over the reduced model, model fit was relatively poor (as indicated by a high RMSEA value).

Appendix 6
Effects of rainfall and herbivore activity on the two most common floral visitors and flowers. A species from the Muscidae was the most common floral visitor, with Apidae 5 (the fifth unique species in the Apidae genus) the second most common. Apidae is likely a frequent floral visitor (Martins Reference Martins2004). Loadings for Muscidae were: DCA axis 1: −0.443; axis 2: 0.204, and for Apidae 5 were: −2.02 and 0.381. The most common flowers were those of Senegalia brevispica, with the second most common flowers those of Croton dichogamous. Loadings for S. brevispica were: DCA axis 1: −0.235; axis 2: 0.0621, and for C. dichogamous loadings were: −1.02 and 0.359.







