Published online by Cambridge University Press: 17 October 2003
In theory, the age at which maturation occurs in parasitic nematodes is inversely related to pre-maturational mortality rate, and cross-species data on mammalian nematodes are consistent with this prediction. Immunity is a major source of parasite mortality and parasites stand to gain sizeable fitness benefits through short-term adjustments of maturation time in response to variation in immune-mediated mortality. The effects of thymus-dependent immune responses on maturation in the nematode parasites Strongyloides ratti and Nippostrongylus brasiliensis were investigated using congenitally thymus-deficient (nude) rats. As compared with worms in normal rats, reproductive maturity of parasites (presence of eggs in utero) in nude rats occurred later in S. ratti but earlier in N. brasiliensis. Immune-mediated differences in maturation time were not associated with differences in worm length. Thymus-dependent immunity had no effect on pre-maturational mortality. Results are discussed in relation to theoretical expectations and possible explanations for the observed patterns in parasite maturation.
For most animals, life begins with a period of pre-maturational somatic growth, the duration of which can have a large impact on fitness. For example, generation time, mortality, body length and fecundity are themselves often functions of maturation time. The age of reproductive maturity is therefore of central biological importance and likely to be a major target of natural selection (Roff, 1992; Stearns, 1992).
For parasitic nematodes, an optimality model of maturation time has been developed based on the relationship of body size and fecundity (Gemmill, Skorping & Read, 1999). According to this model, extended growth before reproduction results in larger size which enhances fecundity, but also entails a heightened risk of death prior to reproduction. Thus, natural selection favours smaller, more rapidly maturing worms when the resulting increase in survival outweighs size-related reductions in fecundity. Conversely, when pre-maturational mortality is sufficiently low, the fecundity benefits of large size tip the balance in favour of delayed maturity. This model explains about half the variation in maturation time across a wide range of mammalian gastrointestinal nematodes (Gemmill et al. 1999). It might also explain variation in maturation time within species of nematodes (Gemmill et al. 1999).
In some free-living species, individuals adjust maturation time adaptively in response to environmental variation. For example, Daphnia (small freshwater crustacea) adjust their age and size at maturity in the presence of a predator, and are even able to discriminate between predators that prey on Daphnia of different sizes (and presumably of different ages). Daphnia exposed to chemicals from predators that prey on small Daphnia mature later and larger, while chemicals from predators that prey on large Daphnia result in earlier maturation at a smaller size (Weider & Pijanowska, 1993; Stibor & Luning, 1994). This is a phenotypic response and the magnitude of the response can differ between Daphnia clones (Weider & Pijanowska, 1993). Theoretical studies show that such a capacity can evolve where environmental variability has large effects on fitness and where individuals have reliable cues that indicate the nature of that variability (e.g. Moran, 1992; Scheiner, 1993).
Within a host population, immunocompetence varies with factors such as nutritional status, age, pregnancy and stress (e.g. Anderson, 1988; Stear & Wakelin, 1998). Consequently, the life-expectancy of a developing nematode is likely to depend very much on the type of host in which it finds itself. A worm that could assess the likely efficacy of the immune system of a host, and vary its life-history accordingly, could maximize its life-time reproduction whatever the immune competence of the host. In principle, components of the host immune system could provide the necessary cues. There is evidence that components of the host immune system might play a role in controlling development in schistosomes (Amiri et al. 1992; Davies et al. 2001; Ravindran, 2001). The nematode Caenorhabditis elegans can enter a non-feeding, stress-resistant stage, called a dauer larva, which can survive long past the typical C. elegans life-span (Golden & Riddle, 1982; Riddle & Albert, 1997, and references therein). Many nematodes of veterinary importance (e.g. Ostertagia spp., Haemonchus contortus, Trichostrongylus spp.), can likewise enter a diapause state, in response to cool temperature or other environmental factors (Anderson, 1992, and references therein).
The thymus-dependent immune system can substantially decrease Strongyloides ratti and Nippostrongylus brasiliensis reproduction and/or survival (Dawkins, Mitchell & Grove, 1982; Kassai, 1982, and references therein; Smith, Ovington & Bryant, 1991; McKay et al. 1995; Gemmill, Viney & Read, 1997). Consequently there should exist strong pressure to optimize life-history parameters in line with parasite mortality resulting from the host immune response. Can nematodes respond to the presence of the host immune system as Daphnia respond to the presence of predators?
Here we investigate whether and how nematode maturation time is dependent on host immune status in S. ratti and N. brasiliensis, two intestinal nematode parasites of rats. We make use of congenitally athymic (nude) rats, which are incapable of mounting thymus-dependent immune responses. We quantify variation in parasite establishment, maturation time and body length to explore the following questions. Do nematode parasites delay maturation in immunodeficient hosts? If so, is there a size benefit associated with the delay?
S. ratti and N. brasiliensis are gastrointestinal nematodes that are natural parasites of rats. Adult parasites of both species inhabit the mucosa of the small intestine. In the intestine, S. ratti are exclusively parthenogenetic females (Viney, 1994), while N. brasiliensis reproduce sexually (Anderson, 1992). Eggs of both species are shed into the intestine and pass with host faeces to the external environment where further development takes place resulting in infective 3rd-stage larvae (iL3s). When an iL3 comes into contact with a rat, it burrows through host skin and migrates to the intestine. The majority of worms have arrived at the gut by 50 h (N. brasiliensis) or 70 h (S. ratti) post-skin penetration (Tindall & Wilson, 1990).
For both S. ratti and N. brasiliensis experiments, 100 iL3s were counted under a dissecting microscope and administered subcutaneously in saline (0·80% w/v NaCl). The syringe and needle were then flushed with saline, and the number of remaining worms counted. In this way, a precise inoculum size could be calculated.
The isofemale line ED5 (Viney, 1996) was used and was maintained by serial passage in Wistar rats (B & K Universal, UK). Experimental animals were female rats, aged 6–10 weeks (Harlan, UK). Fifteen nude (Hsd:rnu/rnu) and 15 heterozygous (Hsd:rnu/+) rats were infected with 100 iL3s as described above. We henceforth refer to the heterozygous rats as ‘normal’ rats, as they have fully functioning immune systems. To sample adult worms in the gut, 5 nude and 5 normal rats were euthanized on each of days 4, 5 and 6 post-infection (p.i.). The experiment was carried out in 2 experimental blocks, the first block consisting of 2 normal and 2 nude rats euthanized on each of days 4, 5, and 6 p.i., and the second block consisting of 3 normal and 3 nude rats euthanized on each of days 4, 5, and 6 p.i.
Parasites were obtained from R. M. Maizels, University of Edinburgh, and maintained in female nude rats (HsdHan:rnu/rnu; Harlan, UK). Experimental animals were female rats, aged 9 weeks (Harlan, UK). Fifteen nude (HsdHan:rnu/rnu) and 15 normal (HsdHan:rnu/+) rats were infected with 100 iL3s as described above. To sample adult worms in the gut, 5 nude and 5 normal rats were euthanized on each of days 5, 6 and 7 p.i. This experiment was not done in blocks.
To measure parasite reproductive output, faeces from the animals euthanized on day 6 p.i. were collected overnight on days 3 and 4 p.i. Faecal cultures were prepared as described by Viney, Mathews & Walliker (1992). After 2 days incubation at 25 °C free-living stages were washed from culture plates, collected and counted under a binocular microscope as described by Gemmill et al. (1997).
To measure parasite reproductive output, faeces were collected from every rat overnight on days 5, 6 and 7 p.i. Egg output per night was estimated using a modified McMaster's technique.
Intestinal parasite numbers (worm burdens) were determined as described by Gemmill, Viney & Read (2000). Briefly, the method is as follows. Immediately after rat death, the small intestine was excised, opened longitudinally and rinsed briefly in tap water. Each small intestine was then divided into 3 approximately equal parts and each part incubated separately in saline (0·8% w/v NaCl solution). After 2 h incubation at 37 °C, each portion of small intestine was vigorously rinsed and backwashed with fresh saline in order to detach any remaining parasites. Recovered parasites were killed and straightened by immersion in boiling formalin solution (10% formal saline). Parasites were then transferred to a droplet of glycerol on a microscope slide, encircled with fine ground glass, covered with a cover-slip and sealed with a polyurethane-based sealant.
Intestinal parasites were recovered as described by Kassai (1982). Briefly, the method is as follows. The small intestine of each rat was removed immediately after death and cut into 3 approximately equal sections. Each section was then cut into 3 pieces, slit along its length and placed in a gauze sack. The sack was suspended in saline in a test-tube and incubated at 37 °C for 2 h. Worms migrated through the gauze and collected at the bottom of the tube. Recovered parasites were killed and fixed by immersion in boiling formalin solution (7% formalin). They were transferred to anhydrous glycerine according to Seinhorst's (1959) method as modified by De Grisse (1969). The worms were then placed in a drop of glycerine on a slide and sealed with wax.
Parasites of both species were examined under a binocular microscope. Sex (for N. brasiliensis) and presence or absence of eggs in utero were recorded for each worm. The body length of each worm was measured using the PC_IMAGE software package (version 2.2.01: Foster Finlay Associates, UK) and a JVC video-camera module (model TK 1270).
Worm measurements were averaged over rat to avoid pseudoreplication. The SAS System: Release 8.0 (SAS Institute) was used for all statistical analyses. Data were analysed using analyses of variance (ANOVAs), except for proportion data (worm sex ratio and the proportion of female worms with eggs in utero) which were analysed using the Wald chi-squared statistic (QW) from the proc logistic command in the SAS system. Host immune status, day p.i., initial innoculum size, gut worm burden and experimental block were included in maximal models where appropriate. Significant effects involving block (replicate) are thus controlled for, but they are of little intrinsic interest in their own right, so that they are reported only where they qualitatively affect the conclusion. Where possible, all 2- and 3-way interactions were also included in maximal models. Non-significant terms (P>0·05) were eliminated from models using backwards elimination (Crawley, 1993).
Inoculum sizes ranged from 88 to 100 larvae with a mean of 94·8 and did not differ with host immune status, day p.i. or the interaction between the two (P>0·05). One of the nude rats scheduled to be euthanized on day 6 p.i. died of unknown causes prior to being euthanized; this rat was excluded from analyses. A total of 499 worms were included in this study. An attempt was made to measure every worm from every rat; however, because some of the worms were broken or too twisted to measure accurately, approximately 45% could not be measured.
Larvae were first detected in the faeces of all rats on day 4 p.i. Worm output on day 4 p.i. was lower in nude rats as compared to normal rats, but this difference was non-significant (F1,7=3·57, P=0·10). The proportion of egg-bearing worms in nude rats was significantly less than that in normal rats, indicating a delay in the onset of reproductive maturity in nude rats (Fig. 1A; QW=5·28, D.F.=1, P=0·022). Nearly all worms (>99%) recovered on days 5 and 6 p.i. had eggs in utero.

Fig. 1. Parasites from nude rats and normal rats. Graphs A and B show the proportion of worms with eggs in utero day 4 (graph A, Strongyloides ratti) or day 5 (graph B, Nippostrongylus brasiliensis) for nude and normal rats (averaged over rat). Graphs C and D show the number of worms per rat on days 4, 5 and 6 p.i. (graph C, S. ratti) or 5, 6 and 7 p.i. (graph D, N. brasiliensis) for nude and normal rats. Graphs E and F show the mean body length (mm) of adult worms on day 4, 5 and 6 p.i. (graph E, S. ratti) or 5, 6 and 7 p.i. (graph F, N. brasiliensis). Each bar in graphs A–F is the mean of n=5 rats, except for S. ratti, nude, day 6 p.i. which is the mean of 4 rats. Errors are ±1 S.E.M.
Worm burdens increased with day p.i. faster in nude rats than in normal rats (Fig. 1C; day p.i.: F2,17=6·04, P=0·010; host immune status: F1,17=0·38, P=0·54; host immune status by day p.i. interaction: F2,17=4·43, P=0·028).
Average worm length increased with day p.i. (Fig. 1E; F2,24=31·92, P<0·0001). Parasite body length was not significantly affected by host immune status (Fig. 1E; host immune status: F1,23=0·50, P=0·49; host immune status by day p.i. interaction: F2,21=0·03, P=0·97).
Inoculum sizes ranged from 80 to 107 larvae with a mean of 97·23 and did not differ with host immune status, day p.i. or the interaction between the two (P>0·05). A total of 1099 worms were included in this study. An attempt was made to measure every worm from every rat; however, because some of the worms were broken or too twisted to measure accurately, approximately 10% could not be measured.
Eggs were first detected in the faeces of all rats on day 6 p.i. Nude and normal rats had similar egg outputs on day 6 p.i. (F1,18=0·00, P=0·97); but nude rats expelled more eggs on day 7 p.i. (F1,7=13·82, P=0·0075). On day 5 p.i., a higher proportion of female worms were gravid in nude rats than normal rats (Fig. 1B; host immune status: QW=5·51, D.F.=1, P=0·019). By day 6 p.i., nearly all female worms (96%) had eggs in utero.
Of all the N. brasiliensis recovered in this study, 85·6% were in the anterior, 13·3% were in the middle and 1·1% were in the posterior third of the small intestine. Fifty-two percent of recovered worms were female. Sex ratios did not vary significantly with day p.i., host immune status or the interaction between the two (P>0·05). There was a slight decrease in gut worm burdens (total number of male and female worms) in normal rats as the infection progressed; this trend was not seen in nude rats (Fig. 1D; host immune status by day p.i. interaction: F2,23=5·93, P=0·0084).
Female worm length increased with day p.i., but was not affected by host immune status (Fig. 1F; day p.i.: F2,26=26·54, P<0·0001; host immune status: F1,25=0·05, P=0·83; host immune status by day p.i. interaction F2,23=1·74, P=0·20). Male worm length increased with day p.i., with those in nude rats increasing in length faster than those in normal rats (Fig. 1F; day p.i.: F2,24=12·60, P=0·0002; host immune status: F1,24=0·10, P=0·75; host immune status by day p.i. interaction F2,24=6·62, P=0·0051).
Gemmill et al. (1999) predicted that individual parasitic nematodes stand to gain sizeable fitness benefits by adjusting maturation time in line with prevailing levels of immune-imposed, pre-maturational mortality. Where such mortality is high, more rapid maturation can enhance fitness by maximizing survival to reproductive age. Where it is low, extended growth will pay off as increased size resulting in increased egg production.
In this study, exposure to the thymus-dependent immune system did indeed affect S. ratti and N. brasiliensis maturation time, but the two species experienced opposite responses. S. ratti matured later in nude rats than in normal rats, as found previously (Dawkins et al. 1982; Gemmill et al. 1997, 2000). Conversely, N. brasiliensis matured later in normal rats. The alterations in the timing of reproduction did not have any subsequent effect on worm length in either species. Thus, our study does not support our hypothesis that parasites experiencing less immune-dependent mortality mature later to increase size and optimize reproduction: N. brasiliensis actually matured earlier and the delay in maturation of S. ratti did not result in larger worms.
The model assumes that negligible worm growth occurs after maturation. We used the length of iL3s and the average female worm length on day 4 p.i. (S. ratti) and day 5 p.i. (N. brasiliensis) to estimate the average increase in length per worm per day before maturation (maturation was defined as occurring on day 5 p.i. for S. ratti, on day 6 p.i. for N. brasiliensis). We then calculated the average increase in length per worm per day post-maturation (growth between days 5 and 6 p.i. for S. ratti, between days 6 and 7 p.i. for N. brasiliensis). We found that the pre-maturational growth (mm/day) was at least 3 (S. ratti) to 10 (N. brasiliensis) times greater than the post-maturational growth, supporting our assumption.
There are many possible reasons why this study did not support our life-history hypothesis, one of which is that the hypothesis is wrong. Alternatively, it might be that the worms are unable to appropriately sense the experimental immune environment we provided or appropriately adjust their life-history in it. Nude rats are presumably extremely rare in nature, indeed, if they occur at all, and it might be that the worms were confused by some novelty of their physiology. This could be tested by using other methods of immunosuppression.
Another possibility is that the worms are actually extremely good at predicting their life-expectancy and that our experimental manipulation does not in fact capture the situation in which alteration in maturation time would be favoured by natural selection. In these experiments, as in a previous experiment using S. ratti (Gemmill et al. 2000), there was no evidence that thymus-deficient hosts had lower pre-maturational mortality, although the increase in worms in nude rats with day p.i. might support this. In S. ratti, adult worm burdens on the first day of sampling were lower in nude rats, and in N. brasiliensis they were similar. Thus, the worms might have been correctly predicting no reduction in mortality in the nude rats. But, if so, it begs the question of why maturation times were nonetheless altered. Moreover, the larval forms of these parasites are highly susceptible to the immune response in hosts with prior exposure (Kassai, Takáts & Redl, 1974, as cited by Kassai, 1982; Gemmill et al. 1997). We believe that the observed lack of immune-mediated, pre-maturational parasite mortality is most likely to result from a lag between parasite invasion and host immune response in naïve hosts, rather than a lack of immune response to larval stages. Under more natural circumstances, a lack of thymus-dependent immunity should predict enhanced larval survival, and worms should delay reproduction accordingly.
Alternatively, the model might be too simple. For example, if post-maturational or size-dependent mortality are themselves important functions of maturation time, this might affect the nature of the optimal phenotypic response to shifts in the prevailing mortality schedule (Skorping & Read, 1998). If so, quantitative success of the Gemmill et al. (1999) model in predicting cross-species variation in age to maturity would presumably be enhanced by taking these complexities into account.
It might be significant that the highest worm counts for both parasite species occurred in nude rats on the last day of the experiment, and day p.i. was correlated with worm number for N. brasiliensis in nude rats (N. brasiliensis: F2,11=6·12, P=0·03; S. ratti: F1,12=2·31, P=0·15). It is possible that the worms in nude rats experienced slowed migration, development and reproduction in the absence of immune cues; this would not be the first such finding in a helminth parasite (Amiri et al. 1992; Davies et al. 2001; Ravindran, 2001). If this is the case, no appreciable size benefit was associated with the delay, and therefore no evidence of an adaptive advantage was detected. The alterations in time of maturity in S. ratti and N. brasiliensis in nude rats could be a pathological consequence of life in an novel environment for which they were unprepared by evolution; alternatively, the alterations might represent adaptive short-term adjustments of the developmental schedule, the fitness consequences of which remain to be determined.
We thank the staff of the March Animal House for superb animal care; Y. Harcus and R. Maizels for advice and Nippostrongylus; S. Harvey and Eyualem Abebe for help and advice; and 2 anonymous reviewers for their helpful comments on a previous version of the manuscript. The work was funded by the BBSRC and Royal Society and a NERC studentship (A. G.) and a National Science Foundation Graduate Research Fellowship (M. G.).
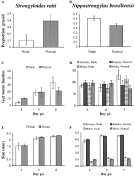
Fig. 1. Parasites from nude rats and normal rats. Graphs A and B show the proportion of worms with eggs in utero day 4 (graph A, Strongyloides ratti) or day 5 (graph B, Nippostrongylus brasiliensis) for nude and normal rats (averaged over rat). Graphs C and D show the number of worms per rat on days 4, 5 and 6 p.i. (graph C, S. ratti) or 5, 6 and 7 p.i. (graph D, N. brasiliensis) for nude and normal rats. Graphs E and F show the mean body length (mm) of adult worms on day 4, 5 and 6 p.i. (graph E, S. ratti) or 5, 6 and 7 p.i. (graph F, N. brasiliensis). Each bar in graphs A–F is the mean of n=5 rats, except for S. ratti, nude, day 6 p.i. which is the mean of 4 rats. Errors are ±1 S.E.M.