Introduction
Sexual interactions between species (reproductive interference) have a strong evolutionary potential, as they might reinforce the evolution of premating barriers (Dobzhansky, Reference Dobzhansky1937; Butlin, Reference Butlin, Otte and Endler1989; Servedio & Noor, Reference Servedio and Noor2003). Moreover, the ecological consequences of reproductive interference may be dramatic, since species sharing similar signal channels could suffer high fitness costs. This may promote habitat partitioning between species (Kawano, Reference Kawano2004; Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007) or lead to demographic displacement of one species (Ribeiro & Spielman, Reference Ribeiro and Spielman1986; Kuno, Reference Kuno1992; Liou & Price, Reference Liou and Price1994; Söderbäck, Reference Söderbäck1994; Rhymer & Simberloff, Reference Rhymer and Simberloff1996; Westman et al., Reference Westman, Savolainen and Julkunen2002; Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007; Liu et al., Reference Liu, De Barro, Xu, Luan, Zang, Ruan and Wan2007). The evolution of specific mate recognition systems is, therefore, of high significance for the coexistence of closely related species (Paterson, Reference Paterson and Vrba1985). Reproductive interference has been examined mainly in the context of hybrid zones (e.g. Butlin & Ritchie, Reference Butlin and Ritchie1991) or invasive species (e.g. Söderbäck, Reference Söderbäck1994; Liu et al., Reference Liu, De Barro, Xu, Luan, Zang, Ruan and Wan2007). Species with a strong overlap in distribution are thought to be unlikely to interact sexually, as their mate recognition system should have evolved in response to such interactions (Paterson, Reference Paterson and Vrba1985). Nevertheless, a high number of closely related species overlap considerably in distribution but rarely co-occur at the same site. Such mosaic distribution patterns often have been explained through competitive interactions; but, if a shared limited resource is missing, reproductive interference might be an appropriate alternative hypothesis (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007).
Reproductive interference is defined as any kind of interspecific interaction during the process of mate acquisition which adversely affects the fitness of at least one of the species involved and is caused by incomplete species recognition (Gröning & Hochkirch, in press). Costly heterospecific interactions are possible principally during all stages of mating, including signal jamming during mate attraction (e.g. Wollerman, Reference Wollerman1999), interspecific rivalry (e.g. Schultz & Switzer, Reference Schultz and Switzer2001), misdirected courtship (e.g. Verrell, Reference Verrell1994), erroneous female choice (e.g. Gray, Reference Gray2005), interspecific mating attempts (e.g. Andrews et al., Reference Andrews, Petney and Bull1982), interspecific mating (e.g. Ribeiro & Spielman, Reference Ribeiro and Spielman1986) and hybridization (reviewed in Mallet, Reference Mallet2005). All of these types of sexual interactions may be associated with fitness costs, regardless of the fertilization of eggs (Gröning & Hochkirch, in press). These costs can be dramatic, including not only a wastage of time and gametes, but also a decreased reproductive success or even survival of individuals (Ribeiro & Spielman, Reference Ribeiro and Spielman1986; Sota & Kubota, Reference Sota and Kubota1998).
Previously, we have documented reproductive interference between the two ground-hopper species T. ceperoi and T. subulata, which strongly overlap in their habitat preferences but rarely co-occur at the same site (Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007; Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). In this species pair, heterospecific mating attempts occurred and males of both species preferred females of T. subulata in laboratory experiments. Although heterospecific matings rarely were observed, the reproductive success was reduced in mixed enclosures, which might be explained by the different courtship displays of these two species (Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006). In contrast to the former species pair, T. subulata and Tetrix undulata are sister species and perform very similar visual courtship displays (Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006). They occur sympatrically in large parts of Europe, covering nearly the complete range of T. undulata (Kleukers et al., Reference Kleukers, Van Nieukerken, Odé and Van Wingerden1997). Moreover, these two species overlap in their habitat preferences, but T. undulata seems to have a broader niche (Detzel, Reference Detzel1998). On sites where both species are found, T. undulata inhabits drier zones, whereas T. subulata can be found in damp areas (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005). It has been hypothesized that reproductive interference might account for this pattern (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005; Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006). Here, we test whether two ground-hopper species with overlapping ranges but different habitats interact sexually. We conducted a laboratory experiment to test whether reproductive interference occurs between both species. We were particularly interested in the influence of the presence of heterospecifics on mating behaviour (including directed approaches, mating attempts, defensive reactions and matings).
Methods
The study objects
Ground-hoppers (Tetrigidae) are a basal group of Orthoptera which mainly inhabit damp, open habitats (Paranjape et al., Reference Paranjape, Bhalerao, Naidu and Baccetti1987; Rowell & Flook, Reference Rowell and Flook1998). Tetrix undulata (Sowerby, 1806) occurs in western and Central Europe, whereas Tetrix subulata (Linnaeus, 1758) has a holarctic distribution. The area of overlap comprises nearly the whole range of Tetrix undulata, including northern Spain, France, England, Ireland, Central Europe, southern Scandinavia and the Baltic states (Kleukers et al., Reference Kleukers, Van Nieukerken, Odé and Van Wingerden1997). Both species are terricolous and confined to damp, open habitats, but T. undulata has a broader ecological niche and also occurs at forest edges, on clearings, in peat bogs and in heathland (Detzel, Reference Detzel1998). Both species have frequently been found in sand pits but segregate spatially when they co-occur, with T. undulata inhabiting drier zones (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005). Tetrigidae feed on a variety of algae, mosses, small plants and detritus (Hochkirch et al., Reference Hochkirch, Gröning, Loos, Metzing and Reichelt2000). Adults of both species reproduce in May and June and do not differ in their daily or seasonal activity patterns (Kleukers et al., Reference Kleukers, Van Nieukerken, Odé and Van Wingerden1997). Females oviposit in moist soil and nymphs hatch in June and July. They either become adult in autumn or hibernate during a late nymphal instar (Sicker, Reference Sicker1964). Both species utilise similar visual cues in their courtship displays; they stretch their fore and mid legs and swing their body horizontally (Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006). In all Tetrigidae, sexual size dimorphism is distinct. Females are substantially larger than males, as they pass through one additional nymphal instar (Ingrisch & Köhler, Reference Ingrisch and Köhler1998). Similar to other European Tetrigidae, the two species occur in two wing morphs. T. undulata has an elevated pronotum and is usually flightless. The fully winged morph of this species has been found only occasionally (Groenendijk & Groenendijk, Reference Groenendijk and Groenendijk1998). In T. subulata, the long-winged morph is more common than the short-winged morph. In contrast to T. undulata, the species has a flat pronotum. The study was conducted in Lower Saxony (Germany), where T. subulata is listed as ‘vulnerable’, while T. undulata is common (Grein, Reference Grein2005).
Experimental design
To analyse the effect of species co-occurrence on mating frequencies, we conducted a substitutive behavioural experiment in a greenhouse of the University of Osnabrück. The experiment was performed at a temperature of 23–26°C and a relative humidity of 50–55% during the reproductive period of both species (from 23 April to 05 June 2003). For each replicate, we transferred two males and two females into a 15×26×19 cm plastic enclosure with a sand-covered floor and fresh food, which was placed under an Osram HQIT 250-W bulb (white daylight). We conducted five different treatments (two conspecific, two heterospecific and one mixed treatment). In conspecific treatments, four individuals of one species were observed. These treatments were performed as control samples to get an overview of the natural behaviour patterns of each species. In heterospecific treatments, we confronted two males of one species with two heterospecific females (no-choice treatments). These treatments were conducted to test whether these insects can be ‘forced’ to perform interspecific matings. In the mixed treatment, one male and one female of each species were kept together (choice treatments). This treatment was conducted to test whether heterospecifics may interact and influence the mating success of one species. The animals were allowed to habituate for five minutes and then were observed for 30 min. We noted the behaviour of each specimen every 30 s, including the direction of courtship displays, mating attempts (mounts) or defensive behaviour (leg movements and body shaking) for later analyses of mate preferences. We replicated each treatment 30 times in an alternating order, starting each day with another treatment to avoid effects of diurnal activity patterns. In order to minimize the impact on the natural populations of the threatened species, we did not collect the large number of insects required to run completely independent replicates. Instead, individuals were randomly drawn from a pool of 160 specimens (40 individuals per species and sex), which were sampled from three sites near Osnabrück (Germany), where both species co-occurred at different relative abundance. Specimens were collected as early in the year as possible (14–15 April 2003). In order to increase their mating motivation, the sexes and species were kept separately in plastic containers (15×26×19 cm) with a moist sand-covered floor and fresh food (mosses and soil algae). The housing conditions were similar to the experimental conditions. We selected individuals randomly to represent natural encounters. After each copulation, females were kept in isolation for at least three days until they were receptive again, to avoid confounding effects from previous mating experience. Female Tetrigidae are highly polyandrous (Caesar et al., Reference Caesar, Ahnesjö and Forsman2007). They oviposit every three to four days and have a maximum receptivity soon after oviposition (Hochkirch & Beichler, unpublished results).
Data analysis
For each replicate, we calculated the relative frequencies of behaviour types for each sex and species. To test for general changes in the behaviour patterns, we first calculated MANOVAs, followed by three-way ANOVAs using the time spent with a special behaviour as response variable and species, sex and treatment as explanatory variables. We simplified our models, by removing stepwise non-significant three-way or two-way interactions (Crawley, Reference Crawley2005). Pairwise t-tests with Bonferroni correction were used to identify the differing treatments. Since ANOVA assumes normally distributed residuals and homogenous variances, it was necessary to transform our data. We applied Box-Cox-transformation using Venables and Ripley's MASS library for R (Venables & Ripley, Reference Venables and Ripley2002), which reveals the optimal power transformation (λ) to fit the data to meet the model assumptions. If it was not possible to transform our data to comply with the models assumptions, we conducted non-parametric Kruskal-Wallis rank sum tests. Since our main interest was the number of copulations (and not the time invested in copulations), we also compared the number of intraspecific copulations between the conspecific and mixed treatments using Pearson's χ2 test (Crawley, Reference Crawley2005). To analyse the mate preferences in the mixed treatment, we calculated the relative frequencies of approaches towards the con- and heterospecific female for each male, including directed locomotion, courtship and mating attempts. Data were included only if males exhibited approaches (N=20 for each species). These data were analysed with Kruskal-Wallis rank sum tests. The females' reaction was examined by calculating the proportions of repelled approaches of hetero- and conspecific males for each female, which was approached by a male (T. subulata, N=21; T. undulata, N=31). This rejection frequency was afterwards analysed with a Kruskal-Wallis rank sum test. All statistical analyses were carried out with ‘R 2.5.1’ (R Development Core Team, 2007).
Results
The mean time spent with copulations was significantly higher in the conspecific treatment than in the mixed or heterospecific treatments (Kruskal-Wallis test: N=30, χ22=14.14, P<0.001), whereas the species differed only marginally in this regard (Kruskal-Wallis test: N=30, χ12=3.29, P=0.069). When only intraspecific copulations were considered, T. undulata spent significantly more time copulating than T. subulata (Kruskal-Wallis test: N=30, χ12=4.03, P=0.045). A similar pattern was found when the number of intraspecific copulations in the mixed and in the conspecific treatment was compared instead of the time spent with mating (fig. 1). Five male T. subulata copulated in the conspecific treatment, whereas no intraspecific copulation occurred in the mixed treatment (Chi-square test: χ12=5.45, P=0.020). Instead, one interspecific copulation, between a T. subulata male and a T. undulata female, was observed in the mixed treatment. The number of conspecific copulations in T. undulata did not differ significantly between the conspecific (seven copulations) and the mixed treatment (three conspecific copulations, Chi-square test: χ12=1.92, P=0.17), but four T. undulata males copulated with T. subulata females in the heterospecific treatment (fig. 1). The time spent with mating attempts differed significantly between treatments, species and sexes (table 1). Generally, the temporal investment in mating attempts was higher in the heterospecific and mixed treatments than in the conspecific treatment; and males were engaged in mating attempts for a longer time than females, mainly due to homosexual mounts. T. undulata, generally, invested more time in mating attempts than T. subulata (fig. 2).
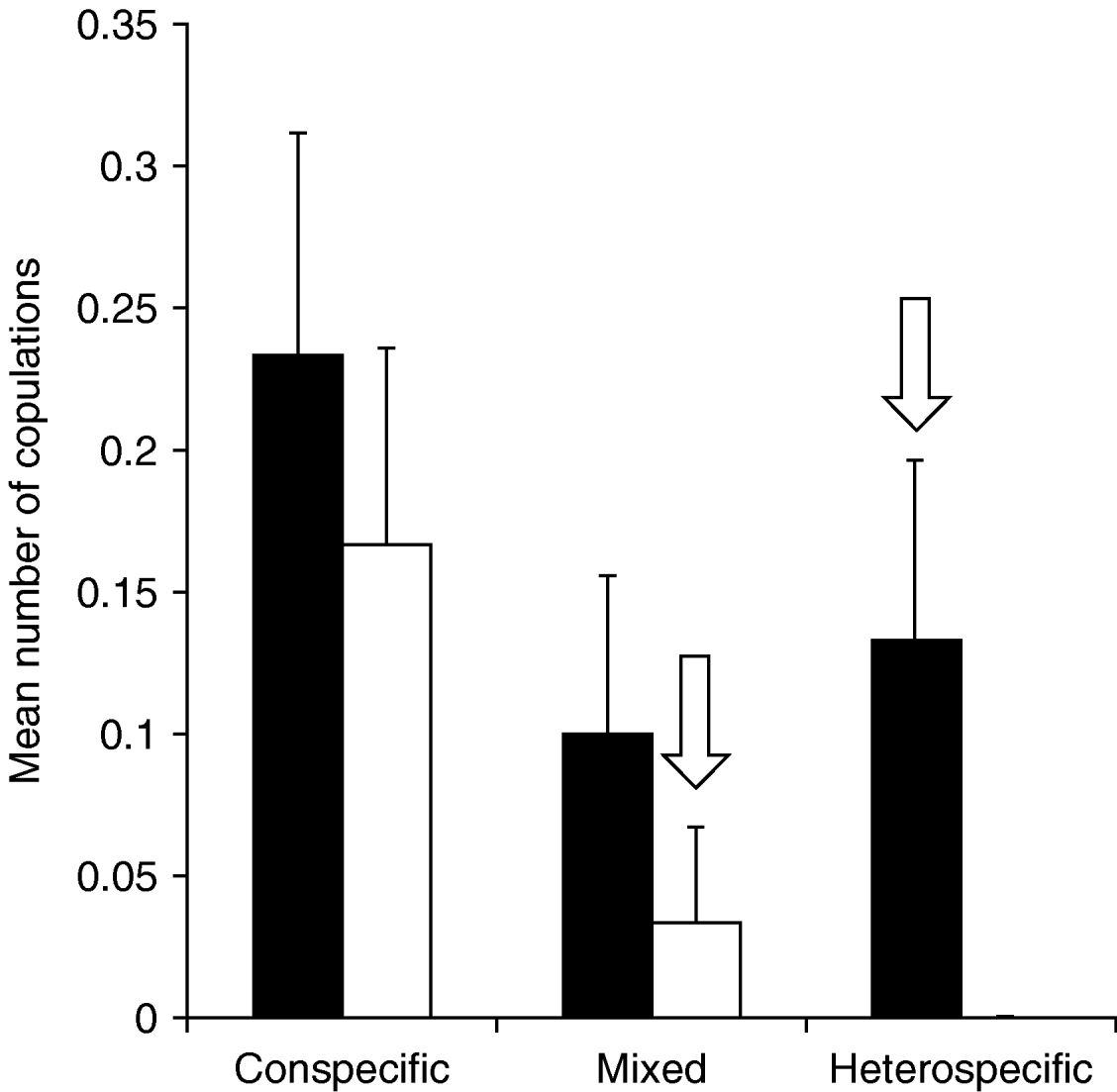
Fig. 1. Average number of copulations per male for Tetrix undulata (■) and Tetrix subulata (□) in the conspecific, mixed and heterospecific treatments (N=30). Interspecific matings are marked with an arrow. The number of intraspecific copulations decreased significantly in T. subulata in the mixed treatment, while it did not differ significantly in T. undulata. Error bars are standard errors.
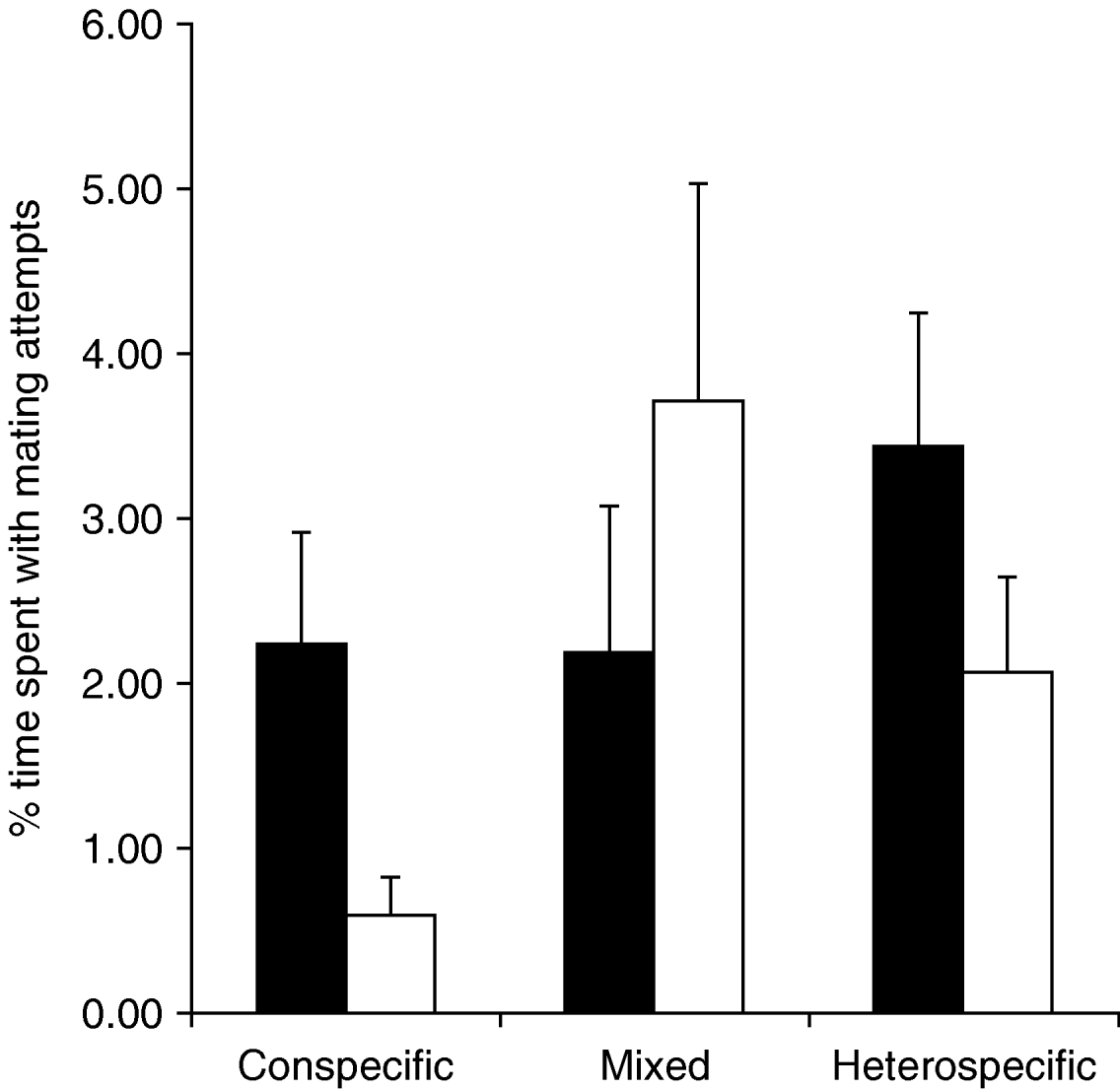
Fig. 2. Average time, which males invested in mating attempts in the conspecific, mixed and heterospecific treatments (N=30). Error bars are standard errors (■, Tetrix undulata; □, Tetrix subulata).
Table 1. Simplified models of ANOVAs, explaining differences in the time spent with mating attempts, courtship and defence, using ‘Species’, ‘Sex’ and ‘Treatment’ as explanatory variables. The data were Box-Cox-transformed (λ) using Venables and Ripley's (2002) MASS library for R.
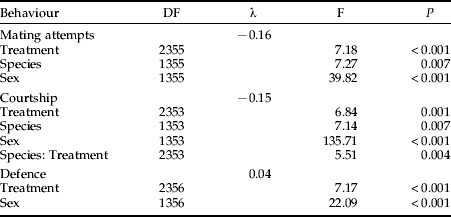
In addition to heterospecific matings and mating attempts, the time spent with courtship behaviour was significantly influenced by treatment, species and sex (table 1). T. subulata males invested more time in courtship displays than T. undulata males. While T. subulata courtship took more time in the mixed and in the conspecific treatments than in the heterospecific treatment, T. undulata did not differ in this regard. The direction of male approaches in the mixed treatment differed significantly between the two species (Kruskal-Wallis test: N=20, χ12=4.55, P=0.033, fig. 3). T. undulata males were more attracted by females of their own species (on average 64.2% of a male's approaches), whereas T. subulata preferred heterospecific females (approaches to conspecific females: 32.7%).
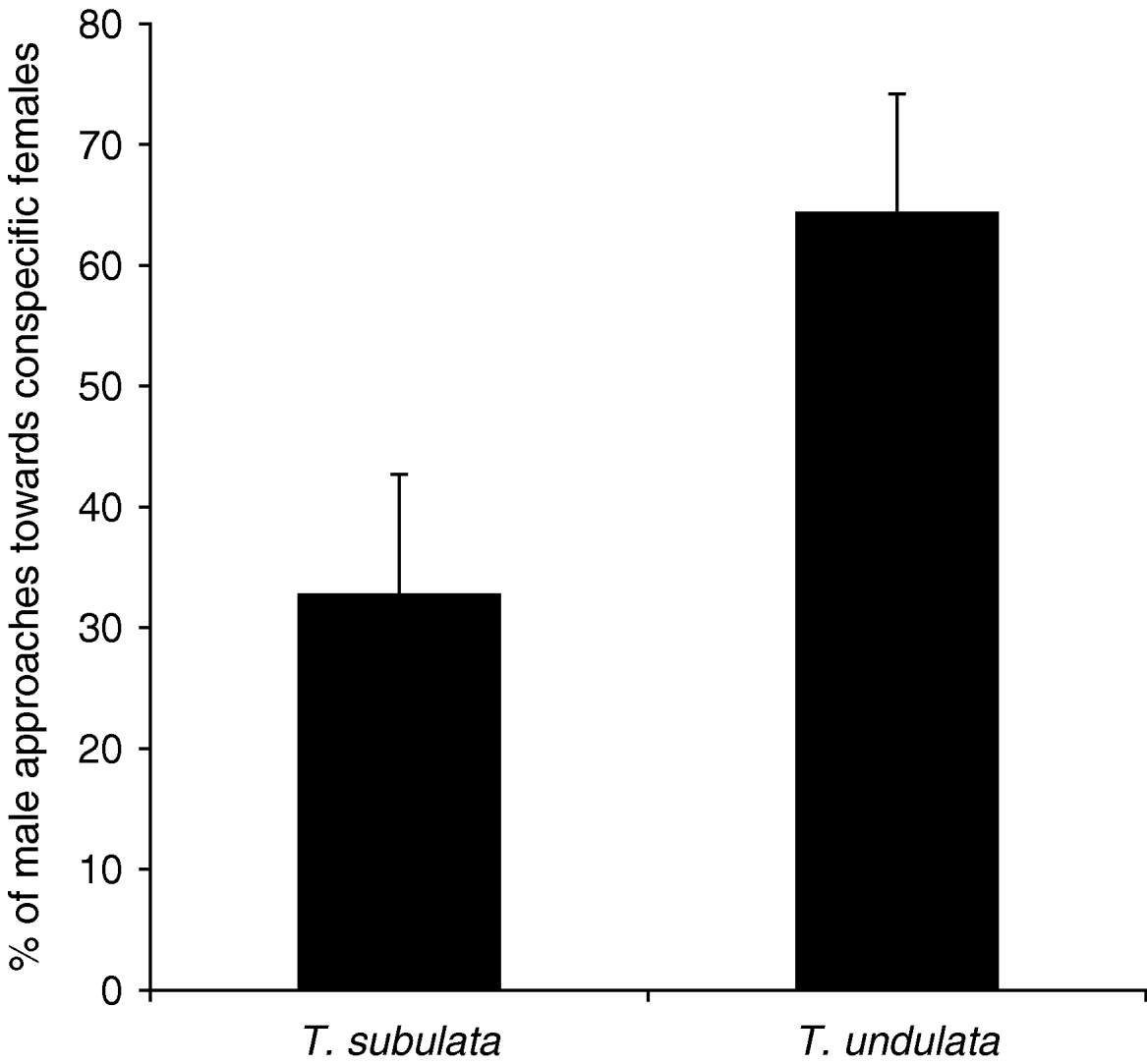
Fig. 3. Average proportion of male approaches towards conspecific females in the mixed treatment. The direction of male approaches differed significantly between the two species. T. undulata males were more attracted by females of their own species, T. subulata by heterospecific females (N=20 males for each species). Error bars are standard errors.
Defensive behaviour in Tetrigidae mainly includes leg movements and body shaking. This type of behaviour is usually related to the repulsion of male mating attempts, whereas the two species are not territorial and, therefore, show virtually no intrasexual aggression. Hence, females spent more time with defensive behaviour than males, but we also found a significant effect of the treatment on this type of behaviour (table 1). Generally, the ground-hoppers invested more time in defensive behaviour in the heterospecific and mixed treatments than in the conspecific treatment. In the mixed treatment, the relative frequency of defensive reactions of females towards male approaches did not differ between the species (Kruskal-Wallis test: N subulata=21, N undulata=31, χ12=0.05, P=0.827). Moreover, females of both species did not discriminate between con- and heterospecific males and rejected males of both species to a similar proportion (Kruskal-Wallis test: N=26, χ12=0.26, P=0.608, fig. 4).
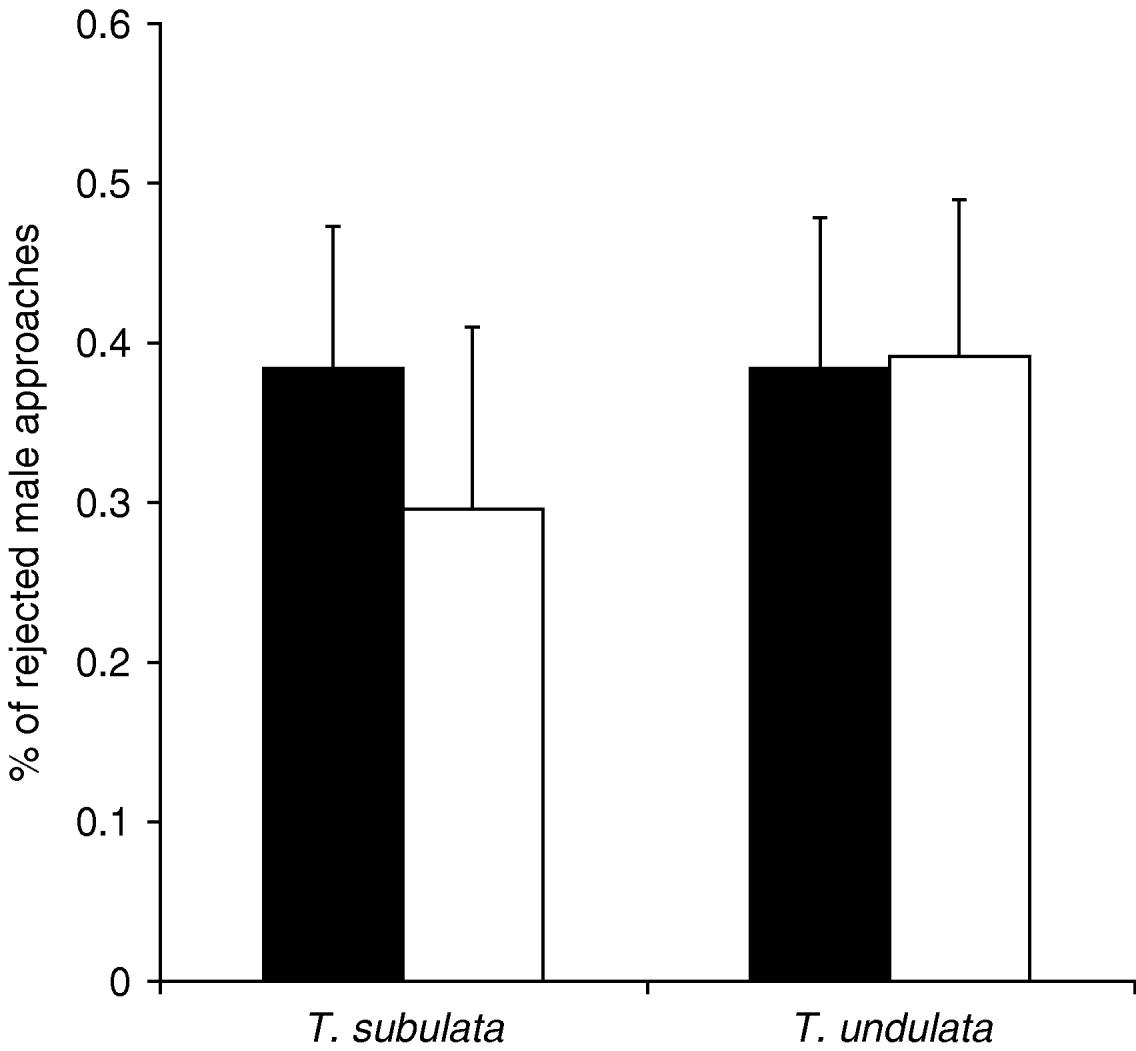
Fig. 4. Average percentage of female defensive reactions towards approaches of conspecific males (black columns) and heterospecific males (white columns) in the mixed treatment. Females of both species rejected approaches of con- and heterospecific males to a similar proportion (T. subulata: 11 replicates with conspecific and ten with heterospecific male approaches; T. undulata: 15 replicates with conspecific and 16 with heterospecific male approaches). Error bars are standard errors.
Discussion
Our results provide evidence for reproductive interference in terms of misdirected courtship, heterospecific mating attempts, erroneous female choice and heterospecific matings between T. undulata and T. subulata. These results support the hypothesis that the coexistence of the two ground-hopper species might be influenced by sexual interactions. In the choice-treatment, T. subulata males preferred heterospecific females over their own females (in terms of male approaches), whereas T. undulata preferred conspecific females (fig. 3). Only when conspecific females were missing, T. undulata males copulated with T. subulata females. Since the females of both species did not discriminate against heterospecific males in the mixed treatment, interspecific matings readily occurred. However, it remains unknown why no heterospecific matings between T. subulata males and T. undulata females were observed in the heterospecific treatment. This system, therefore, differs from the type of reproductive interference found between T. subulata and Tetrix ceperoi, in which females of T. subulata reject heterospecific males more often than conspecific males (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). Males of T. undulata and T. subulata performed more mating attempts when heterospecifics were present than in the conspecific treatment. Subsequently, the females increased the number of defensive reactions. Interestingly, T. subulata males reduced their temporal investment in courtship displays when conspecific females were missing and spent more time with mating attempts. One might argue that the reduced mating frequency of T. subulata in the mixed treatment could be explained by the lower conspecific density. However, in T. undulata, the number of matings per male remained more or less constant in the conspecific and the mixed treatment, suggesting that conspecific density alone cannot account for this pattern. Moreover, in a similar experiment with T. subulata and T. ceperoi, the mating frequency of T. subulata remained constant in the mixed treatment (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007).
Based upon the male preferences for heterospecific females, T. subulata seems to be more adversely affected by reproductive interference than T. undulata. In the presence of T. undulata, T. subulata was not able to achieve any intraspecific copulation. However, since females of both species are not choosy and males of both species preferably attempt to mate with T. undulata, the costs might also be high in the latter species. The costs for males are mainly a function of time and easier to estimate than for females, in which the fitness costs depend on the mating system and the degree of postmating isolation. Conspecific sperm precedence is a widespread type of cryptic isolation (Howard, Reference Howard1999), which might decrease the costs of heterospecific matings. The degree of postmating isolation between T. undulata and T. subulata remains to be tested. Although they represent sister taxa, the genetic distance is rather high (p-distance: 7.8% in the mitochondrial ND1 gene, Hochkirch & Gröning, unpublished results), suggesting that successful hybridization is unlikely. Nevertheless, the presence of heterospecific sperm might negatively affect the reproductive success of a female (Gröning & Hochkirch, in press). The reproduction of females of some grasshopper species is constrained by the number of eggs they can produce (Ingrisch & Köhler, Reference Ingrisch and Köhler1998). Their sexual receptivity decreases after a copulation; and, hence, the conspecific mating success could be substantially reduced after a heterospecific mating. This is particularly true if the heterospecific is more attractive than the conspecific female (Deering & Scriber, Reference Deering and Scriber2002).
Reproductive interference has been reported in a wide range of animal taxa, but is rarely considered in ecological research as a possible force structuring communities (Gröning & Hochkirch, in press). The most apparent and most frequently discussed type of reproductive interference is hybridization (Rhymer & Simberloff, Reference Rhymer and Simberloff1996; Mallet, Reference Mallet2005), which is more related to negative heterosis than to competition (Spencer et al., Reference Spencer, Mcardle and Lambert1986). Hybridization is often regarded as the most costly form of reproductive interference, as it involves gamete wastage and gene-pool swamping (Barton & Hewitt, Reference Barton and Hewitt1985; Bull & Burzacott, Reference Bull and Burzacott1994; Rhymer & Simberloff, Reference Rhymer and Simberloff1996). However, there is accumulating evidence that, in some cases, hybrid fitness could be higher than the fitness of non-hybrid offspring (Fitzpatrick & Shaffer, Reference Fitzpatrick and Shaffer2007; Pfennig, Reference Pfennig2007). Mating without hybridization and other types of reproductive interference represent high fitness costs as well, as time, energy and nutrients are wasted (Liou & Price, Reference Liou and Price1994; Ardeh et al., Reference Ardeh, De Jong, Loomans and Van Lenteren2004). This fitness loss often results in a decreased reproductive success of one species, as has been shown in field experiments with T. subulata and T. ceperoi (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). Species which mate only once in their lifetime may be effectively sterilised by heterospecific matings (Vick, Reference Vick1973; Nasci et al., Reference Nasci, Hare and Willis1989).
Based on laboratory experiments, it is difficult to assess the significance of reproductive interference in the field, as the costs of this interaction can be altered by several factors (Gröning & Hochkirch, in press). First, reproductive interference is likely to be density-dependent (Westman et al., Reference Westman, Savolainen and Julkunen2002). In the field, the frequency of heterospecific encounters can be substantially lower than in small arenas with unnaturally high abundances. Indeed, we already demonstrated for T. ceperoi and T. subulata that encounter rates were at least tenfold lower in the field than in the laboratory experiment. Males of these species were indiscriminate in the field, and the number of mating attempts was strongly correlated with the encounter frequencies (Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007), whereas they both preferred females of T. subulata in a laboratory experiment (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). It remains to be tested whether such a discrepancy between results from laboratory experiments and field observations is also found in the case of T. subulata and T. undulata. The relative frequencies of both species could be decisive for the fitness consequences of reproductive interference (Gröning & Hochkirch, in press). If one species is much more abundant, the risk to mate with heterospecifics will be substantially reduced in the more common species (Westman et al., Reference Westman, Savolainen and Julkunen2002). Therefore, it might be more difficult for a species to colonise a habitat, which is already occupied by heterospecifics. Second, different dispersal abilities might allow one species to colonise new habitats rapidly and persist as long as heterospecifics are missing or occur in low abundance. Indeed, T. subulata is a pioneer species, which is adapted to highly dynamic ecosystems (Detzel, Reference Detzel1998). It is usually fully winged and, therefore, it has a high potential to colonise new emerging ponds and shores. Third, several ecological and ethological mechanisms might allow both species to coexist on a site despite reproductive interference (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). Both Tetrix species typically have clumped dispersions (Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007). Such intraspecific aggregations might reduce the number of heterospecific encounters in the field (Ficetola & De Bernardi, Reference Ficetola and De Bernardi2005). However, the most obvious mechanism of coexistence in these two species seems to be habitat segregation, a pattern which also has been reported for two sexually interacting beetle species (Kawano, Reference Kawano2004). Although both ground-hoppers overlap in their habitat preferences, T. subulata mainly occurs in floodplain habitats, such as ephemeral ponds, shores of pools, lakes and rivers, whereas T. undulata seems to have a broader ecological niche and inhabits heathland, peat bogs, forest edges, moist grassland and pits. On sites, where both species are found, T. undulata occupies drier zones and T. subulata moist areas of the habitat (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005). The question remains whether habitat partitioning is a consequence of reproductive interference, or whether it is caused by differences in the fundamental niches of the species. In the latter case, reproductive interference might be of lower significance in nature, because the two species rarely encounter each other. It might, therefore, not represent a strong selective force driving the evolution of mate recognition systems. Similar to the ‘ghost of competition past’ (Connell, Reference Connell1980), niche partitioning could also have become genetically fixed in response to past selective pressures of reproductive interference. Thus, it would be valuable to test whether habitat choice is influenced by the presence of heterospecifics.
Most reported cases of reproductive interference are asymmetric, with one species suffering higher costs than the other (Wirtz, Reference Wirtz1999). This effect might be further complicated by an asymmetric response of both sexes. In our system, the males of both species seem to have stronger preferences for females of T. undulata (fig. 3). The reason for this pattern remains to be examined, but it might be related to differences in body size. T. undulata has a raised pronotum, giving it a larger appearance. A preference for larger females has been shown in a number of other insect species (Thornhill & Alcock, Reference Thornhill and Alcock2000; Bonduriansky, Reference Bonduriansky2001; Deering & Scriber, Reference Deering and Scriber2002; Suzuki et al., Reference Suzuki, Nagano and Trumbo2005) and might represent an ancestral sensory bias (Ryan, Reference Ryan1998). This might be caused by the greater potential fecundity of larger females (Thornhill & Alcock, Reference Thornhill and Alcock2000). Interestingly, the females of both species do not seem to be able to discriminate heterospecific males. The strong overlap in the visual courtship displays (Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006) might be one reason for this missing ability, but the significance of the courtship displays for female choice remains to be tested. Recent studies suggest that vibrational communication might also play a role in communication of Tetrigidae (Benediktov, Reference Benediktov2005). It remains to be tested if reproductive interference also occurs between T. ceperoi and T. undulata. The ranges of both species overlap substantially (Kleukers et al., Reference Kleukers, Van Nieukerken, Odé and Van Wingerden1997), but they are rarely found at the same site in northwestern Germany (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005). This might be influenced by differences in their habitat preferences. The niche overlap between T. ceperoi and T. subulata is rather strong, as both species prefer damp, open patches with a warm microclimate (Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007). T. undulata differs from both species, as it inhabits also drier zones and occurs in a variety of habitats (Gröning et al., Reference Gröning, Kochmann and Hochkirch2005). As males of Tetrix species seem to be rather indiscriminate in their mate choice and even attempt to mate with flies (Hochkirch et al., Reference Hochkirch, Deppermann and Gröning2006), it is likely that reproductive interference also occurs between T. ceperoi and T. undulata.
Since the outcome of reproductive interference (decreased reproduction) seems to be similar to competition (Ficetola & De Bernardi, Reference Ficetola and De Bernardi2005), the consequences of both types of interaction are comparable. ‘Sexual exclusion’ (Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007) is a reasonable effect of reproductive interference (Kuno, Reference Kuno1992; Söderbäck, Reference Söderbäck1994; Westman et al., Reference Westman, Savolainen and Julkunen2002) and might explain the missing coexistence of several closely related species. Reproductive interference can, thus, become a threat to endangered species. Although T. undulata is much more common than T. subulata in Central Europe, it remains speculation whether reproductive interference influences this pattern. Nevertheless, sexual interactions between species should receive more attention in ecological studies and in conservation management. Recent trends in nature conservation are strongly influenced by the idea that landscape connectivity enhances the viability of populations (Beier & Noss, Reference Beier and Noss1998). Negative interactions between species are rarely considered in this context. The creation of corridors could increase the number of encounters of two sexually interacting species and subsequently threaten the more sedentary species or the one which is more severely affected by reproductive interference.
Conclusions
There is accumulating evidence that reproductive interference may have strong ecological effects, which may be similar or even stronger than those of resource competition (Kuno, Reference Kuno1992; Kawano, Reference Kawano2004; Hochkirch et al., Reference Hochkirch, Gröning and Bücker2007). Studies on reproductive interference have mainly focused on narrow hybrid zones (e.g. Butlin & Ritchie, Reference Butlin and Ritchie1991; Kawano, Reference Kawano2004; Pfennig, Reference Pfennig2007) or invasive species (Söderbäck, Reference Söderbäck1994; Liu et al., Reference Liu, De Barro, Xu, Luan, Zang, Ruan and Wan2007). Our results show that sexual interactions may also affect species with broadly overlapping ranges and may provide a more powerful explanation for allotopic distribution patterns than resource competition. The consequences of reproductive interference may involve displacement of one species (Kuno, Reference Kuno1992; Liu et al., Reference Liu, De Barro, Xu, Luan, Zang, Ruan and Wan2007), habitat segregation (Kawano, Reference Kawano2004), changes of dispersion patterns (Gröning et al., Reference Gröning, Lücke, Finger and Hochkirch2007) and the evolution of mate recognition systems (Dobzhansky, Reference Dobzhansky1937; Servedio & Noor, Reference Servedio and Noor2003). There is a strong need for more detailed studies on reproductive interference, which should measure fitness costs in terms of lifetime reproductive success. Moreover, more field studies are needed to examine the magnitude of sexual interactions under natural conditions and the potential ecological mechanisms to decrease the costs associated with reproductive interference.
Acknowledgements
We are grateful to Anselm Kratochwil and Kathrin A. Witzenberger for critical comments that helped to improve the manuscript. Special thanks go to Axel Tschuschke for technical assistance. Till Eggers gave essential advice regarding statistics. We thank Anselm Kratochwil for his constant support throughout this project. Günter Grein provided information concerning the distribution of the investigated species.







