Acute rheumatic fever is an inflammatory disease occurring after acute tonsillopharyngitis caused by beta-haemolytic group A streptococci. Acute rheumatic fever is a common problem in developing countries, and the most common cause of acquired cardiac disease in childhood.Reference Gerber1 The disease can affect many systems, such as the heart, joints, skin, subcutaneous tissues, and the central nervous system. The prognosis in acute rheumatic fever is related to the cardiac involvement.Reference Tani2
Prolonged atrioventricular conduction in acute rheumatic fever is a common finding, and is one of the minor Jones criteria.3 It has been shown that atropine reverses the PR interval prolongation in patients with acute rheumatic fever.Reference Keith4 The main effect of atropine in the heart is to block vagal receptors in the sinoatrial and atrioventricular nodes.Reference Guyton and Hall5 Therefore, the PR prolongation in patients with acute rheumatic fever is thought to be due to increased vagal activity. Increased vagal activity is an indicator of autonomic imbalance. Heart rate variability is a useful tool that might provide indices of autonomic modulation of the heart.6 To our knowledge, there has been no previous study investigating heart rate variability in children with acute rheumatic fever. In this study, we aimed to show the balance of the autonomic nervous system by analysing heart rate variability in children with acute rheumatic fever.
Materials and methods
This study was performed between June, 2006 and April, 2010. Children with the diagnosis of acute rheumatic fever – during either first attack or recurrence – were included in the study group. Children with an innocent murmur were included in the control group. All patients and control cases were evaluated by history, physical examination, chest radiography, electrocardiography, and echocardiographic study.
PR intervals were measured from lead II. Prolonged PR interval was defined as a PR interval longer than the upper limit of the normal for age and heart rate.Reference Park and Guntheroth7
The first attack of acute rheumatic fever was diagnosed strictly on the basis of Jones criteria. In the diagnosis of recurrences, criteria offered by the World Health Organization were used.Reference Bisno, Butchart, Ganguly and Ghebrehiwet8 Patients were classified according to the findings of the major Jones criteria. Subclinical carditis, diagnosed by echocardiography, was not accepted as a major finding, and the patients with subclinical carditis were classified as patients without carditis.
Written informed consent was obtained from all parents.
Using DMS 300-7 Holter recorder (DMS Inc., New York, United States of America), 24-hour electrocardiography recordings were obtained from the study – soon after diagnosis – and control groups. The recordings included a complete day and night cycle. All recordings were analysed using DMS Cardioscan program (DMS Cardioscan 11 Holter analysis program, DMS Inc.), and QRS complexes were identified as artefacts and ectopic and normal beats. Further, all were re-evaluated by a paediatric cardiologist (Mehmet Karacan). Heart rate variability was measured by calculating time- and frequency-domain indices from 24-hour recordings.
The following time-domain indices were calculated: standard deviation of all normal sinus R–R intervals; mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording; standard deviation of the averages of R–R intervals in all 5-minute segments of the entire recording; root mean square of the successive normal sinus R–R interval difference; and percentage of successive normal sinus R–R intervals longer than 50 milliseconds. The calculated frequency-domain indices were: variance of all R–R intervals – total power; power in the very low frequency range – very low frequency; power in the low frequency range – low frequency; low frequency power in normalised units – normalised low frequency; power in the high frequency range – high frequency; and high frequency power in normalised units – normalised high frequency.
The exclusion criteria for this study included administration of any medication for other reasons; presence of congenital cardiac diseases, systemic disease, or metabolic abnormalities; and abnormal QRS complexes comprising more than 85% of the recorded QRS complexes.
Data were expressed as proportions, means plus or minus standard deviations. The data were compared with the Student t-test and chi-square test. A p-value less than 0.05 was accepted as statistically significant. All statistical analysis was done using the SPSS 11.0 package program (Statistical Package for the Social Sciences, Chicago, Illinois, United States of America).
Results
Recordings of 61 consecutive children with acute rheumatic fever were evaluated. Of the 61 patients, 11 patients were excluded, that is, eight patients due to lack of 24-hour electrocardiography and three patients due to the presence of excessive artefact. The remaining 50 patients were included in the study group.
The mean age of the study group was 11.6 years, with a range from 4 to 12 years and a median of 12 years), including 30 (60%) girls. The mean age of the control group was 10.7 years, with a range from 5 to 14 years and a median of 11 years, including 22 (59%) boys. There was no statistical difference in terms of mean, age, and sex distribution between the groups, with a p-value greater than 0.05.
Of the patients, 48 (96%) were evaluated during the first attack and two (4%) during recurrence. A total of 39 patients (78%), with or without other major findings, had carditis; in 11 (22%) of them, carditis was isolated. Eleven patients (22%) did not have carditis (isolated arthritis = 7, isolated chorea = 4). In 10 (20%) of the patients from the study group, of whom five had isolated carditis and four had carditis and arthritis, the PR interval was longer for age and heart rate, whereas only one patient in the control group had prolonged PR interval.
Total 24-hour electrocardiography recording times were 1251 plus or minus 155 minutes and 1198 plus or minus 282 minutes in the study and control groups, respectively. The mean total recording times were not statistically different, with a p-value greater than 0.05.
Comparison of study and control cases
The mean values of the minimum, maximum, and average heart rates obtained from 24-hour electrocardiography recordings are given in Table 1. The mean, minimum, and average heart rates in the study group were significantly higher than in the control group, with a p-value less than 0.001.
Table 1 Comparison of heart rate parameters.

Heart rate variability parameters in the study and control groups are shown in Table 2. All time-domain heart rate variability parameters in the study group were significantly lower than in the control group, with a p-value less than 0.001. The results of the frequency-domain heart rate variability parameters in the study group showed significant alterations in favour of sympathetic dominance when compared with the control group – increased normalised low frequency and the ratio of low frequency to high frequency, and decreased normalised high frequency (Table 2).
Table 2 Comparison of the time- and frequency-domain heart rate variability parameters.

pNN50 = the amount of adjacent R–R intervals that are greater than 50 milliseconds for the whole analysis; rMSSD = the square root of the mean of the sum of squares of differences between adjacent R–R intervals over the length of the analysis; SDANN = the standard deviation of the means of all R–R intervals for all 5-minute segments of the analysis; SDNN = the standard deviation of all R–R intervals over 24 hours; SDNN index = SDNNi, mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording
Comparison of patients with isolated carditis and without carditis
The mean values of heart rate – minimum, maximum, and average – and heart rate variability parameters in patients with isolated carditis and without carditis, and comparisons with the control group, are given in Tables 3 and 4, respectively. All heart rate variability parameters – time- and frequency-domain – were similar in patients with isolated carditis and without carditis, with a p-value greater than 0.05. The mean values of heart rate parameters in patients with isolated carditis were significantly higher than in the control group, with a p-value less than 0.05, whereas there was no difference between patients without carditis and the control group, with a p-value greater than 0.05. All heart rate variability parameters in patients with isolated carditis and without carditis were found to be influenced in favour of sympathetic overstimulation when compared with the control group, with a p-value less than 0.05. Owing to the low patient numbers, we did not compare the results of the patients with isolated arthritis and chorea separately.
Table 3 The comparison of heart rate and heart rate variability parameters in patients with isolated carditis and without carditis.
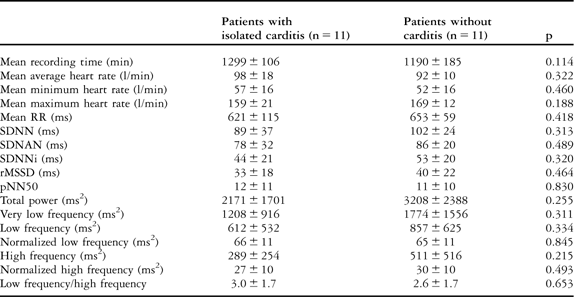
pNN50 = the amount of adjacent R–R intervals that are greater than 50 milliseconds for the whole analysis; rMSSD = the square root of the mean of the sum of squares of differences between adjacent R–R intervals over the length of the analysis; SDANN = the standard deviation of the means of all R–R intervals for all 5-minute segments of the analysis; SDNN = the standard deviation of all R–R intervals over 24 hours; SDNN index = SDNNi, mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording
Table 4 The heart rate and heart rate variability of patients with isolated carditis and without carditis and the control group.
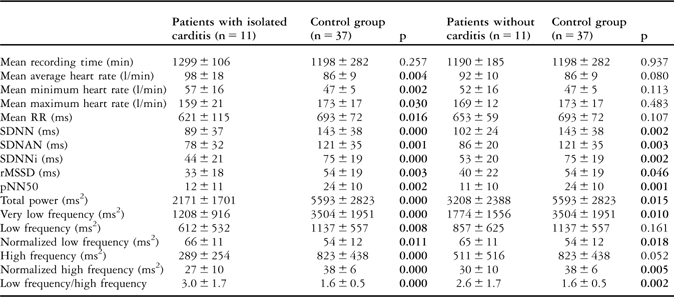
pNN50 = the amount of adjacent R–R intervals that are greater than 50 milliseconds for the whole analysis; rMSSD = the square root of the mean of the sum of squares of differences between adjacent R–R intervals over the length of the analysis; SDANN = the standard deviation of the means of all R–R intervals for all 5-minute segments of the analysis; SDNN = the standard deviation of all R–R intervals over 24 hours; SDNN index = SDNNi, mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording
Comparison of patients with prolonged and normal PR interval
The mean average heart rate in patients with prolonged and normal PR interval was similar, with a p-value greater than 0.05. There was no significant difference between mean time-domain heart rate variability parameters of patients with prolonged and normal PR interval, with a p-value greater than 0.05, whereas the mean normalised low frequency was significantly lower, with a p-value of 0.01 and the mean normalised high frequency was significantly higher, with a p-value of 0.045 in patients with prolonged PR interval (Table 5).
Table 5 The comparison of heart rate and heart rate variability parameters in patients with prolongedand normal PR interval.

pNN50 = the amount of adjacent R–R intervals that are greater than 50 milliseconds for the whole analysis; rMSSD = the square root of the mean of the sum of squares of differences between adjacent R–R intervals over the length of the analysis; SDANN = the standard deviation of the means of all R–R intervals for all 5-minute segments of the analysis; SDNN = the standard deviation of all R–R intervals over 24 hours; SDNN index = SDNNi, mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording
The mean average heart rate in patients with normal PR interval was significantly higher than in the control group, with a p-value less than 0.001; however, it was not different between patients with prolonged PR and the control group, with a p-value of 0.66 (Table 6). The time- and frequency-domain heart rate variability parameters – except for the normalised low frequency – in patients with prolonged PR and normal PR interval showed significant changes in favour of sympathetic dominance when compared with the control group, with a p-value less than 0.05. However, the significance was lower in patients with prolonged PR interval (Table 6).
Table 6 The comparison of heart rate and heart rate variability parameters in patients with prolonged PR interval, normal PR interval and the control group.

pNN50 = the amount of adjacent R–R intervals that are greater than 50 milliseconds for the whole analysis; rMSSD = the square root of the mean of the sum of squares of differences between adjacent R–R intervals over the length of the analysis; SDANN = the standard deviation of the means of all R–R intervals for all 5 minute segments of the analysis; SDNN = the standard deviation of all R–R intervals over 24 hours; SDNN index = SDNNi, mean of the standard deviations of all normal sinus R–R intervals for all 5-minute segments of the entire recording
Discussion
Various degrees of decreased atrioventricular conduction velocity – prolonged PR interval – in patients with acute rheumatic fever have been identified.Reference Keith4 This information has been known almost since the beginning of the last century, and the PR interval prolongation is one of the minor diagnostic criteria in patients with acute rheumatic fever.Reference Keith4 Although KeithReference Keith4 found longer PR intervals more frequently in patients with carditis, subsequent studies showed that there was no correlation between carditis and the length of the PR.3, Reference Clark and Keith9, Reference Karacan, Işıkay, Olgun and Ceviz10 KeithReference Keith4 also showed that atropine reverses the prolongation of PR interval in patients with acute rheumatic fever. The effect of atropine is to block vagal receptors located in the sinoatrial and atrioventricular nodes in the heart.Reference Guyton and Hall5 It is known that acetylcholine may produce cardiac block via action on the atrioventricular node.Reference Guyton and Hall11 KeithReference Keith4 thus stated that the prolongation of PR interval in patients with acute rheumatic fever can be explained by overstimulation of vagal activity.
The autonomic balance can be evaluated by the analysis of heart rate variability.6 The cardiac contractions in healthy subjects are irregular, and the change in the intervals between cardiac contractions is a physiological phenomenon. The heart rate varies continuously with exercise, physical and mental stress, and respiratory and metabolic problems. Heart rate variability can be measured with continuous monitoring of electrocardiography, which provides information about the autonomic balance.6
We could not find any study evaluating heart rate variability in children with acute rheumatic fever in the current English literature. This is the first study to provide information about the state of autonomic balance in children with acute rheumatic fever.
Time-domain heart rate variability parameters are closely associated with the recording time, and therefore time-based heart rate variability parameters will increase with longer recording times. If the time-based heart rate variability parameters are to be compared, total recording time should be similar between groups.6 Furthermore, age and gender can affect the heart rate variability parameters.Reference Silvetti, Drago and Ragonese12 In our study, the mean recording times and the mean age and sex distribution were similar between the study and control groups, with a p-value greater than 0.05.
In the study group, the mean values of average and minimum heart rates were significantly higher than those of the control group, with a p-value less than 0.001 (Table 1), which was thought to be due to carditis, fever, and pain caused by arthritis. In addition, the possible autonomic dysfunction may have contributed to this physiological response.
Although the standard deviation of all normal sinus R–R intervals gives an overview of all heart rate variability, standard deviation of the averages of R–R intervals in all 5-minute segments of the entire recording provides information about long-term components of heart rate variability; the root mean square of the successive normal sinus R–R interval difference and percentage of successive normal sinus R–R intervals longer than 50 milliseconds values are reliable indicators of parasympathetic efferent activity.6, Reference Kardelen, Tezcan, Akcurin, Ertug and Yesilipek13 The significantly decreased time-domain parameters, especially root mean square of the successive normal sinus R–R interval difference and percentage of successive normal sinus R–R intervals longer than 50 milliseconds, in the study group (Table 2), suggest an autonomic dysfunction in favour of sympathetic overstimulation.
Frequency-domain heart rate variability parameters contain components of sympathetic–parasympathetic interactions.6 An increased high frequency indicates a dominant parasympathetic activity, whereas increased low frequency and the ratio of low frequency to high frequency show an increased sympathetic activity. The physiological interactions of very low frequency are not fully known. Measurement of very low frequency, low frequency, and high frequency power components is usually made in absolute values of power; however, low frequency and high frequency may also be measured in normalised units.6 The normalised units of low frequency and high frequency represent the relative value of each power component in proportion to the total power minus the very low frequency component.6 Moreover, normalisation tends to minimise the effect of the changes in total power on the values of the low frequency and high frequency components.6 Spectral analysis of heart rate variability in our study group revealed a significant reduction in total and in the individual power spectral components. However, when the power of low frequency and high frequency was calculated in normalised units, a significantly increased normalised low frequency, with a p-value less than 0.001, and a significantly decreased normalised high frequency, with a p-value less than 0.001, were detected in the study group compared with the control group. In addition, the ratio of low frequency/high frequency was significantly higher in the study group, with a p-value less than 0.001. These changes indicate a shift of sympathovagal balance towards sympathetic predominance and reduced vagal tone. In contrast to Keith'sReference Keith4 proposal, our findings indicated an impaired autonomic balance in favour of sympathetic overstimulation in patients with acute rheumatic fever.
It is known that tachycardia is an important finding in patients with rheumatic carditis.Reference Tani2 We compared the results of heart rate variability parameters in patients with isolated carditis and without carditis to investigate the effect of the presence of carditis on heart rate variability. The mean average heart rate was similar between patients with isolated carditis and without carditis (Table 3). When compared with the control group, it was significantly higher in patients with isolated carditis, but it was not significantly different in patients without carditis (Table 4).
In patients with rheumatic mitral stenosisReference Ozdemir, Alyan and Soylu14 and different left ventricular load conditions due to different rheumatic cardiac diseases,Reference Dzimiri, Moorji, Kumar and Halees15 presence of sympathetic overactivity has been shown previously. In both studies, sympathetic overactivity was thought to be related to the result of the haemodynamic effects of these valvular cardiac diseases. In our study, the time- and frequency-domain heart rate variability parameters were similar between patients with isolated carditis and without carditis. However, all heart rate variability parameters were found to be influenced in patients with isolated carditis and without carditis in favour of sympathetic overstimulation when compared with the control group. This result indicates that in patients with acute rheumatic fever the presence of carditis does not cause an additional risk of autonomic imbalance, and that the sympathetic overactivity should be a result of a different pathophysiological mechanism other than the volume overload of valvular damage.
Demonstration of sympathetic overstimulation in patients with acute rheumatic fever makes the PR prolongation more interesting. We compared the heart rate variability parameters of patients with prolonged PR interval in surface electrocardiography – 10 patients – to that of the patients with normal PR interval – 40 patients – and control cases (Tables 5 and 6).
The mean average heart rate in patients with prolonged PR interval was not statistically different from that of the patients with normal PR interval, with a p-value greater than 0.05 (Table 5). Although the mean average heart rate in patients with prolonged PR interval was extremely close to that of the control group, with a p-value greater than 0.05, it was significantly higher in patients with normal PR interval, with a p-value less than 0.05 (Table 6).
When compared with the control group, the time- and frequency-domain heart rate variability parameters in patients with prolonged and normal PR interval showed significant changes in favour of sympathetic overstimulation (Table 6). Nevertheless, the significance was relatively lower in patients with prolonged PR interval.
Although there was no significant difference between mean time-domain heart rate variability parameters of patients with prolonged and normal PR interval, with a p-value greater than 0.05, the mean normalised low frequency was significantly lower, with a p-value of 0.01, and the normalised high frequency was significantly higher, with a p-value of 0.045, in patients with prolonged PR interval (Table 5). These results indicate that the sympathetic overstimulation in patients with prolonged PR interval is relatively lower than that of patients with normal PR interval.
KeithReference Keith4 found that the effect of atropine administration on PR interval is more prominent in patients with acute rheumatic fever with prolonged PR interval than in the control cases, and suggested that the prolonged PR interval in patients with acute rheumatic fever is associated with vagal overstimulation. The increase in heart rate after atropine administration may be effective on the shortening of the PR interval. However, the increase in heart rate after atropine administration was similar between patients with acute rheumatic fever and the control cases. On the basis of those results, KeithReference Keith4 suggested that the increased heart rate is not effective on the PR interval, and the effect of atropine on the prolonged PR interval in acute patients with rheumatic fever is a result of the direct effect of atropine on the conduction system. KeithReference Keith4 also administered adrenalin to six patients with acute rheumatic fever, with a PR interval of greater than 0.19 seconds. He observed a mean shortening of 0.027 seconds. Owing to the fact that this rate was significantly shorter than that of atropine, he proposed that the prolonged PR interval is the main evidence of vagal overstimulation in patients with acute rheumatic fever. In the same study, the heart rate was found to be low and stayed low despite the continuing rheumatic activity. This was also accepted as another indicator of increased vagal activity. Furthermore, the increased frequency of abdominal pain and vomiting in patients with acute rheumatic fever was determined to be associated with the increased vagal stimulation of the gastrointestinal system.
KeithReference Keith4 evaluated the previous attempts that aimed to explain the possible reasons for the changes in conduction time in patients with acute rheumatic fever, and concluded that the main feature of the mechanism that causes prolongation in conduction time is the overstimulation of the vagus. The reason for this increased activity is unclear. However, the evidences presented by KeithReference Keith4 indicate a peripheral impairment, because the changes in conduction time occurred without a generalised reaction, and frequently continued for weeks or months even after cessation of the acute infection and generalised inflammatory process. In these children, the pathological findings of the disease are mainly in the heart and are very limited in the other parts of the body. For these reasons, it seems more likely that the site of overstimulation of the vagus lies in its terminations in the heart rather than in the medulla. Furthermore, it may be significant that the sites of the most abundant vagal nerve supply in the heart are where one finds such a high incidence of pathological change in rheumatic fever.Reference Keith4
In contrast to the proposal of Keith,Reference Keith4 the results of our study clearly demonstrated sympathetic overstimulation in the acute period of acute rheumatic fever. Sympathetic overstimulation even in patients with prolonged PR interval makes the explanation of prolonged PR interval harder. Our results suggest that the prolongation in PR interval may not be a result of parasympathetic overstimulation, but rather a consequence of the pathological process on the atrioventricular node and sinus node. Nevertheless, why this functional problem affects only some of the patients with acute rheumatic fever remains to be explained. This may be a result of the difference in immunological mechanisms controlling susceptibility to acute rheumatic fever.








