Introduction
Alzheimer's disease (AD) is the most frequent cause of dementia. Due to population aging, the increasing global prevalence of AD will pose huge challenges to public health and elderly care systems in all countries across the world (GBD 2015 Neurological Disorders Collaborator Group, 2017). Given the current lack of any curative treatment, identifying new modifiable risk factors and their associated biological changes could provide new insights on AD physiopathology and pave the way for prevention strategies. Previous studies have identified dementia risk factors, such as health and lifestyle factors (for instance, diabetes mellitus, smoking, hypertension, and physical activity) and also psychological risk factors [for instance, depression (Vos et al., Reference Vos, van Boxtel, Schiepers, Deckers, de Vugt, Carrière and Köhler2017), chronic psychological distress (Sutin, Stephan, & Terracciano, Reference Sutin, Stephan and Terracciano2018)]. Many of these psychological risk factors are associated with personality traits themselves involved in AD. These traits include mainly neuroticism and negative thoughts (Marchant et al., Reference Marchant, Lovland, Jones, Pichet Binette, Gonneaud and Arenaza-Urquijo2020; Terracciano et al., Reference Terracciano, Sutin, An, O'Brien, Ferrucci, Zonderman and Resnick2014) whereas openness and agreeableness are associated with a slightly reduced risk of AD (Terracciano et al., Reference Terracciano, Sutin, An, O'Brien, Ferrucci, Zonderman and Resnick2014). Personality traits influence lifestyle patterns such as health behavior, dietary habits, cognitive activity, and social relationships (Roberts, Kuncel, Shiner, Caspi, & Goldberg, Reference Roberts, Kuncel, Shiner, Caspi and Goldberg2007; Stephan, Boiché, Canada, & Terracciano, Reference Stephan, Boiché, Canada and Terracciano2014; Weston, Edmonds, & Hill, Reference Weston, Edmonds and Hill2020), and these factors in turn are related to the risk of developing dementia in later life (Scarmeas & Stern, Reference Scarmeas and Stern2003; Stern, Reference Stern2012). Risk traits are relatively stable (Just & Alloy, Reference Just and Alloy1997) but like other traits, they can also be modified through intervention (Gu, Strauss, Bond, & Cavanagh, Reference Gu, Strauss, Bond and Cavanagh2015; Watkins et al., Reference Watkins, Mullan, Wingrove, Rimes, Steiner, Bathurst and Scott2011). Therefore, personality characteristics may represent important determinants of dementia risk.
To date, few studies have focused on personality traits and looked at premorbid personality traits as possible predictors of cognitive decline and dementia (Cipriani, Borin, Del Debbio, & Di Fiorino, Reference Cipriani, Borin, Del Debbio and Di Fiorino2015; Tautvydaitė et al., Reference Tautvydaitė, Kukreja, Antonietti, Henry, von Gunten and Popp2017), some personality traits have yet to be explored. Cynical hostility (CH), as defined by Cook & Medley (Reference Cook and Medley1954), refers to a specific dimension of hostility that consists in the mistrust of others, a psychological attitude towards others characterized by the belief that they are mainly motivated by selfish concerns (Gurol et al., Reference Gurol, Irizarry, Smith, Raju, Diaz-Arrastia, Bottiglieri and Greenberg2006). CH has been prospectively associated with higher cardiovascular morbidity and related mortality, as well as all-cause mortality (Tindle et al., Reference Tindle, Chang, Kuller, Manson, Robinson, Rosal and Matthews2009). Although many cardiovascular risk factors have been associated with dementia, only one study investigated whether people with higher levels of CH in late-life were more susceptible to dementia (Neuvonen et al., Reference Neuvonen, Rusanen, Solomon, Ngandu, Laatikainen, Soininen and Tolppanen2014). Despite the scarce literature, it has been hypothesized that psychological factors, such as CH, could influence the risk of a disease like dementia through different biological mechanisms, for instance dysregulated β-adrenergic receptor function (Hughes, Sherwood, Blumenthal, Suarez, & Hinderliter, Reference Hughes, Sherwood, Blumenthal, Suarez and Hinderliter2003), elevated pro-inflammatory activity mediated by cytokine production (Janicki-Deverts, Cohen, & Doyle, Reference Janicki-Deverts, Cohen and Doyle2010), and telomere shortening (Brydon et al., Reference Brydon, Lin, Butcher, Hamer, Erusalimsky, Blackburn and Steptoe2012). However, no study has assessed the link between CH and AD-related early brain alterations, or brain alterations in general. While previous neuroimaging studies have provided insight into neural correlates of personality traits, the current knowledge of relationships between brain structures, especially white matter, and personality traits remains limited. Knowledge of white matter changes underlying a personality trait such as CH holds significant implications for understanding the neurobiological factors related to behavioral tendencies and cognitive skills likely to promote the emergence of AD (Privado, Román, Saénz-Urturi, Burgaleta, & Colom, Reference Privado, Román, Saénz-Urturi, Burgaleta and Colom2017; Xu & Potenza, Reference Xu and Potenza2012).
Only one study demonstrated significant correlations between CH and regional gray matter density in the right anterior mid-cingulate cortex, bilateral dorsolateral prefrontal cortex, left dorsomedial prefrontal cortex and premotor cortex (Nakagawa et al., Reference Nakagawa, Takeuchi, Taki, Nouchi, Sekiguchi, Kotozaki and Kawashima2017). To our knowledge, no study has investigated white matter integrity in CH, or whether brain alterations in people with CH could be related to early neuroimaging biomarkers of dementia.
Among the core neuroimaging markers of AD, hippocampal atrophy is the best described and validated (Jack et al., Reference Jack, Barkhof, Bernstein, Cantillon, Cole, Decarli and Foster2011). Classically, the medial temporal lobe, which contains hippocampus, entorhinal cortex, and amygdala, is affected very early during AD development (Braskie & Thompson, Reference Braskie and Thompson2014). Cortical atrophy then extends to the remainder of the cortex along a temporal-parietal-frontal trajectory that correlates with the disease severity and the appearance of clinical symptoms (Apostolova et al., Reference Apostolova, Lu, Rogers, Dutton, Hayashi, Toga and Thompson2008; Lerch et al., Reference Lerch, Pruessner, Zijdenbos, Hampel, Teipel and Evans2005). This progression is consistent with the spatial distribution of the neurodegenerative changes known to occur in AD from post-mortem studies (Braak & Braak, Reference Braak and Braak1991; Vemuri et al., Reference Vemuri, Whitwell, Kantarci, Josephs, Parisi, Shiung and Jack2008). Evidence also supports white matter degradation in the early disease stages in the cingulum, fornix, and prominently in the corpus callosum (CC) (Radanovic et al., Reference Radanovic, Pereira, Stella, Aprahamian, Ferreira, Forlenza and Busatto2013; Teipel et al., Reference Teipel, Meindl, Wagner, Stieltjes, Reuter, Hauenstein and Hampel2010).
The first objective of this study was to investigate the association between late-life CH and incident AD in a community sample of older adults from Southern France during an 8-year follow-up. The second objective was to examine the association between early structural MRI brain markers of AD and CH level.
Methods
Study population
Subjects were selected among the population recruited for the ESPRIT project (Montpellier, France) between 1999 and 2001 (Ritchie et al., Reference Ritchie, Artero, Beluche, Ancelin, Mann, Dupuy and Boulenger2004). The ESPRIT study (N = 1863) is part of a wider, multi-site cohort study (three-City Study; 3C) of community-dwelling people aged 65 and older selected from the electoral rolls of Bordeaux, Dijon and Montpellier, France. The main aim of the ESPRIT study was to construct a comprehensive database that incorporates clinical, biological, genetic, and environmental risk factors of neurological and psychiatric diseases, including neuroimaging data. Random sampling was carried out separately for each of the 15 electoral registers of the Montpellier District between the dates of March 1999 and February 2001, in order to obtain 1/15 of the total population for the purposes of the ESPRIT study. Non-participants were replaced by other participants drawn from the same electoral register through an additional random sampling procedure so that each electoral division was equally represented. Non-participants were slightly older than participants and more likely to live alone (Ritchie et al., Reference Ritchie, Artero, Beluche, Ancelin, Mann, Dupuy and Boulenger2004). Standardized interviews, neuropsychological tests, neurological and psychiatric examinations were carried out at baseline (phase 1) and at year 2 (phase 2), 4 (phase 3), 7 (phase 4), and 10 (phase 5) of the follow-up. At baseline (phase 1), 760 randomly selected participants younger than 80 years of age underwent an MRI brain scan. The study protocol was approved by the Bicêtre University Hospital Ethical Committee (France), and written informed consent was obtained from each participant after receiving a complete description of the study. The present study included only subjects from the ESPRIT cohort who completed the Buss and Durkee Hostility Inventory (BDHI) for CH assessment (Buss & Durkee, Reference Buss and Durkee1957) at phase 2 (N = 1388). Brain MRI data were available for N = 508 of the selected individuals (Fig. 1). All patients with dementia at phase 1 and 2 were excluded.

Fig. 1. Flow-chart with the different study phases. Neuropsychological evaluations and diagnosis of dementia are conducted at each phase.
Assessment of cynical hostility
The BDHI (Buss & Durkee, Reference Buss and Durkee1957) is a measure of general aggression and hostility, composed of 75 items with true-false answers. It has eight subscales, seven of which are designed to measure different components of hostility: assault, verbal aggression, indirect hostility, irritability, negativism, resentment, and suspicion. Using the suspicion component, factor analyses allowed extracting three questions related to CH (questions 14, 46, and 54) that showed good internal reliability (α = 0.7): ‘I tend to be on my guard with people who are somewhat more friendly than I expected’; ‘My motto is “never trust strangers”’; and ‘I commonly wonder what hidden reason another person may have for doing something nice for me’. The sum of these three questions' scores gives a total score between 0 and 3. Based on this score, participants were divided into three groups: none (score = 0), moderate (score = 1), and high levels (score = 2–3) of CH.
Diagnosis of dementia
At each phase, the diagnosis of dementia was based on a three-step procedure. First, trained psychologists administered a battery of neuropsychological tests that assessed different cognitive domains including verbal fluency (Isaacs Set Test) (Isaacs & Kennie, Reference Isaacs and Kennie1973), immediate visual memory (Benton Visual Retention Test) (Benton, Reference Benton1965), attention and visuomotor processing speed and executive function (Trail Making Test) (Reitan, Reference Reitan1958), verbal episodic memory (Free and Cued Selective Reminding Test) (Grober, Buschke, Crystal, Bang, & Dresner, Reference Grober, Buschke, Crystal, Bang and Dresner1988). The Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975) was used as an index of global cognitive performance. Second, all participants were examined by a neurologist. Third, an independent committee of neurologists reviewed all potential prevalent and incident cases of dementia to obtain a consensus on the diagnosis and etiology, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR). Cases of dementia, including AD, were classified according to the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association Criteria (McKhann et al., Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984), and cases of mixed/vascular dementia according to the National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria (Román et al., Reference Román, Tatemichi, Erkinjuntti, Cummings, Masdeu, Garcia and Hofman1993). Mixed dementia was defined as a diagnosis of AD with either cerebrovascular lesions on brain imaging, or a documented history of stroke and presence of prominent executive function deficits in addition to an AD-type cognitive profile. During the 8-year follow-up (Fig. 1), the following cases of dementia were identified: AD (N = 52), mixed (N = 12), Lewy body (N = 8), vascular (N = 7), Parkinson (N = 2), frontotemporal (N = 1), and others (N = 2). In the context of this work, ‘AD’ refers to dementia due to Alzheimer's disease.
Socio-demographic and clinical parameters
The baseline interview contained items on socio-demographic characteristics, such as age, sex, marital status, education level (no formal education or primary school, lower secondary education, higher secondary education, and university degree), height, weight, smoking status (non-smoker, ex-smoker, and current smoker), and current alcohol consumption (>12 g/day). The body mass index (BMI) was calculated as follows: weight (kg)/height (m)2. Each participant completed self-reported questionnaires on medical disorders, lifestyle, and subjectively evaluated health. History of cardiovascular disease included stroke, myocardial infarction, angina pectoris, arteritis, and coronary surgery.
The current level of depressive symptoms was evaluated with the Center for Epidemiologic Studies-Depression Scale (CESD) (Radloff, Reference Radloff1977); a >16 cut-off indicated a high level of symptoms. A standardized psychiatric examination was administered using the Mini International Neuropsychiatric Interview (MINI; French version 5.00) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998), based on the DSM IV and whose procedures have been well described elsewhere (Ritchie et al., Reference Ritchie, Artero, Beluche, Ancelin, Mann, Dupuy and Boulenger2004). Mood and anxiety disorders, psychotic syndrome, and suicidal risk were assessed.
Blood pressure was measured twice during the interview using an OMRON M4 digital electronic tensiometer. Subjects were considered hypertensive when the mean of the two measurements was ⩾160/95 mm Hg, or if they were taking antihypertensive drugs. Blood was collected only at baseline after overnight fasting, for standard measurements of serum C-reactive protein, lipids, and glucose levels. Hypercholesterolemia was defined as total cholesterol ⩾6.2 mmol/L and/or cholesterol-lowering therapy, and diabetes as treated diabetes or fasting blood glucose ⩾7 mmol/L. All drugs used in the previous month were recorded from medical prescriptions and drug packages. Apolipoprotein E (APOE) genotyping (presence or absence of the allele ε4) was performed by Lille Génopôle, as previously described (Dufouil et al., Reference Dufouil, Richard, Fiévet, Dartigues, Ritchie, Tzourio and Alpérovitch2005). An ultrasound examination of the carotid arteries was performed, as previously described (Plichart et al., Reference Plichart, Celermajer, Zureik, Helmer, Jouven, Ritchie and Empana2011).
MRI data analysis
A Sigma 1.5T GE Imaging System (General Electric Medical Systems, Milwaukee, Wisconsin) was used to acquire T1-weighted sequences for volumetric estimations (repetition time = 12 ms, echo time = 2.8 ms, inversion time = 600, matrix size = 256 × 256, pixel spacing = 0.9375 × 0.9375 mm2, number of excitations = 1, slice thickness = 1.0 mm). Slices were then converted to isotropic (0.9375 mm3) and re-sliced to 1.00 mm3. T2-weighted 2D axial MR images were also obtained by using the spoiled gradient-echo sequence (RT = 97 ms, ET = 4 ms) that consisted of a set of 124 adjacent transverse sections parallel to the anterior commissure–posterior commissure line with a section thickness of 1.5 mm (no gap).
Automated measurements for grey matter region of interests (ROIs) and CC volumes
Regional reconstruction and segmentation were performed with the FreeSurfer version 6.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu/). The first stage included the reconstruction of the cortical surface (Dale, Fischl, & Sereno, Reference Dale, Fischl and Sereno1999). Normalized intensity images were created, corrected for variations in intensity due to magnetic field inhomogeneity. Voxels beyond the cerebral cortex, namely the skull, were then removed before beginning the segmentation. Segmentation was based on the geometric structure where grey and white matter interface, and subsequently the left and right hemispheres were separated as well as the cortical from subcortical structures. The resulting cortical volume was covered by a triangular tessellation and deformed to more accurately represent the grey and white matter interface and the pial surface. Once the cortex was reconstructed, this volume was registered to a spherical atlas (Fischl, Sereno, & Dale, Reference Fischl, Sereno and Dale1999a, Reference Fischl, Sereno, Tootell and Dale1999b) and parcellated into regions based on the sulcal and gyral structures (Desikan et al., Reference Desikan, Ségonne, Fischl, Quinn, Dickerson, Blacker and Killiany2006; Fischl et al., Reference Fischl, van der Kouwe, Destrieux, Halgren, Ségonne, Salat and Dale2004). Each T1-weighted scan was segmented into cortical and subcortical ROIs in each hemisphere. The CC was identified and divided into five subsections (anterior, midanterior, central, midposterior, posterior) by the mri_cc command of FreeSurfer. The five subsections were derived from evenly spaced partitions along the primary eigenaxis, closely corresponding to the longitudinal axis. Data from the FreeSurfer output, expressed in volumes (mm3) for each ROI, were then converted to be exported into SPSS for analysis as described below.
Estimation of white matter lesions (WML) volume
WML volumes (in cm3) were estimated using a semi-automatic method (Brickman et al., Reference Brickman, Sneed, Provenzano, Garcon, Johnert, Muraskin and Roose2011; Gurol et al., Reference Gurol, Irizarry, Smith, Raju, Diaz-Arrastia, Bottiglieri and Greenberg2006). Supratentorial WMLs appearing as hyperintensity areas were segmented on T2-weighted sequences using the MRIcro software (Rorden & Brett, Reference Rorden and Brett2000). This method was described in detail elsewhere (Mortamais et al., Reference Mortamais, Reynes, Brickman, Provenzano, Muraskin, Portet and Artero2013). WML values were transformed by a log10 (x + 0.01) function given their highly asymmetric distribution and possible null values.
Intracranial volume (ICV) estimation
ICV was computed from T1-weighted anatomical images using the segment m-file of the SPM5 software (Wellcome Department of Cognitive Neurology, London, UK). The ICV (gray plus white matter plus cerebrospinal fluid) was used as a covariate in the models to minimize the effect due to global brain-size differences. All outputs were manually inspected to ensure accurate and valid data.
Statistical analyses
To assess the association between CH levels and sociodemographic, lifestyle, clinical and psychiatric variables, descriptive analyses were carried out using the χ2, analysis of variance (ANOVA), and Kruskal–Wallis tests, according to the variable characteristics. Multivariate Cox proportional hazards models with delayed entry and age as time-scale were used for the longitudinal analysis of dementia and AD onset, during the 8-year follow-up (Commenges, Letenneur, Joly, Alioum, & Dartigues, Reference Commenges, Letenneur, Joly, Alioum and Dartigues1998). The assumption of hazard log-linearity with Schoenfeld partial residuals and residual normality (dfbeta) was checked for each CC area measure. The checks did not indicate a violation of the proportional hazard assumption and non-normality of residuals. The date of dementia onset was set half way between the date of the last follow-up visit, when the subject was classified as normal, and the date of diagnosis. The models were adjusted for sex, education level, and for potential confounders reported in the literature: MMSE, APOE ε4, history of cardiovascular disease, diabetes, hypertension, BMI, hypercholesterolemia, smoking status, alcohol use, depressive symptoms, and lifetime anxiety. The results of the Cox proportional hazards regression analyses were expressed as hazard ratios (HR) with 95% confidence intervals (CI). Additional sensitivity analyses were performed to assess the reliability of our findings. Specifically, the association between CH and dementia risk was re-examined after exclusion of all incident cases of dementia at phase 3 (4 years of follow-up). MRI brain ROIs were compared according to the CH level using ANOVA after adjustment for age, sex, educational level, and ICV. False Discovery Rate (FDR) correction (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) was used for multiple comparisons in case of significance. Except for WML data that were transformed, MRI measures were normally distributed based on the Shapiro–Wilks test. Analyses were two-tailed and significance was set at p ⩽ 0.05.
We also performed a mediation analysis to examine the potential mediating role of white matter integrity on the relationship between CH and dementia. Age, sex, education, and APOE status were included as covariates in the models. The hypothesized mediation model was analyzed using the PROCESS macro for SPSS (Hayes, Reference Hayes2017), based on ordinary least squares (OLS) regression. The bias-corrected 95% CI was calculated with 5000 bootstrapping re-samples to estimate the conditional indirect effect (the indirect effect refers to the effect that is through the mediator under study). If the interval does not include zero, the effect is statistically significant at p < 0.05.
All statistical analyses were done with IBM SPSS Statistics (SPSS Inc., Chicago, Illinois, USA, version 20).
Results
Baseline characteristics of the study population
The mean age of the 1388 participants was 73.6 years (standard deviation = 4.7), and 782 (56.3%) were women. Among all participants, 291 (21%) did not have CH (score = 0), whereas 432 (31.1%) had moderate CH (score = 1), and 665 (47.9%) high CH (score = 2 or 3, score 2 = 411 subjects, score 3 = 254 subjects) (Table 1). Participants with moderate or high CH were more likely to have fewer years of formal education, current high depressive symptoms, and more lifetime anxiety disorders, especially lifetime generalized anxiety. Participants without MRI (N = 880 63% of the individuals with CH evaluation) were older (72.5 v. 70.8 years, p < 0.01) and more often women (61.3% v. 47.8%, p < 0.01) than the other participants (N = 508).
Table 1. Comparison of the baseline demographic and clinical characteristics in participants categorized according to their cynical hostility status
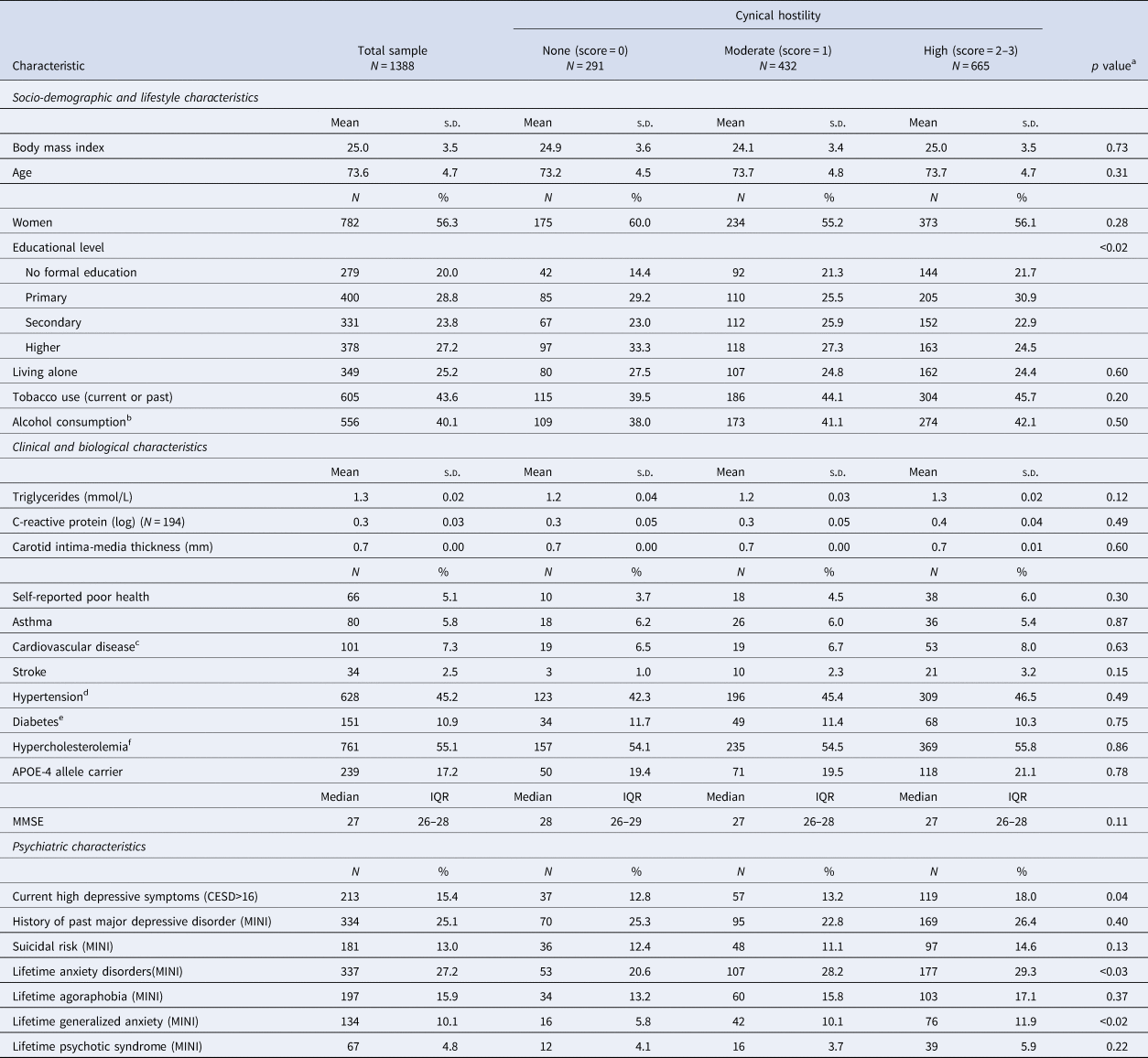
CESD, Center for Epidemiologic Studies Depression; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MINI, Mini International Neuropsychiatric Interview; MMSE, Mini-Mental State Examination.
a χ2, analysis of variance or Kruskal–Wallis tests, according to the variable characteristics.
b Alcohol consumption ⩾12 g/day.
c History of stroke, myocardial infarction, angina pectoris, arteritis or cardiovascular surgery (coronary surgery/angioplasty, endarterectomy).
d Systolic blood pressure ⩾160 or diastolic blood pressure ⩾95 mm Hg, or use of antihypertensive medication.
e Treatment for diabetes, or glucose ⩾6.2 mmol/L.
f Total cholesterol ⩾6.2 mmol/L.
Cynical hostility and Alzheimer's disease or all dementia
Analysis of the association between baseline CH status and the incident dementia and AD rates at the 8-year follow-up visit (adjusted Cox proportional HR values and 95% CI in Table 2) showed that compared with participants without CH, a high CH score was associated with higher rates of dementia (p = 0.027) and AD (p = 0.030). The results remained significant after the exclusion of participants with incident dementia (N = 10) at phase 3 (Table 2). Figure 2 shows AD incidence in the function of the CH status over time.

Fig. 2. Survival curve obtained from a Cox regression analysis to assess the association between the levels of cynical hostility (CH) and incident Alzheimer's disease (AD). The cumulative risk of AD is shown along the y-axis with follow-up time on the x-axis. The risk of incident dementia over 10-year follow-up is significantly higher in individuals with high CH (![]() ) than moderate (
) than moderate (![]() ) or no CH (
) or no CH (![]() ).
).
Table 2. Association between baseline cynical hostility level and incidence of dementia and Alzheimer's disease during the 8-year follow-up

a Hazard ratios were calculated using the Cox proportional hazard models with delayed entry, age as a time-scale, and after adjustment for sex, educational level, baseline MMSE, APOE ε4, history of cardiovascular disease, diabetes, hypertension, BMI, hypercholesterolemia, smoking status, alcohol use, depressive symptoms, and lifetime anxiety.
Cynical hostility and brain MRI markers
Analysis of the association (ANOVA) between CH levels and volumes of brain ROIs after adjustment for age, sex, educational level, and ICV (Fig. 3 and online Supplementary Table S1 for complete results) showed that CH was not associated with significant differences in grey matter ROIs. Conversely, for white matter ROIs, anterior CC volumes were smaller, and WML volumes were larger in individuals with high CH level than in those without CH. This last association did not remain significant after FDR correction for multiple comparisons in white matter volumes. The analyses performed after adjusting for depressive symptoms (model 2), lifetime anxiety disorders (model 3), and suicidal risk (model 4) gave similar results.

Fig. 3. Association between cynical hostility levels and MRI volumes of brain regions of interest (ANOVA). Analyses were performed after adjustment for age, sex, educational level, and intracranial volume. (a) Grey matter volumes. (b) Corpus callosum volumes. (c) White matter lesions (log) volumes. *p < 0.05. †Non-significant level after False Discovery Rate (FDR) correction q* = 0.0083 (Benjamini & Hochberg, Reference Benjamini and Hochberg1995). CC, corpus callosum; L, left; R, right.
Role of white matter on the relationship between CH and dementia (mediation analysis)
The β total effect of CH on white matter integrity was 0.0522 with s.e. = 0.2971. The β indirect effect was 0.00428 with the CI (−0.112 to –0.1518). The mediating effect of white matter integrity (anterior CC) on the relationship between CH and dementia was not significant.
Discussion
This study shows that CH is significantly related to dementia and AD. Specifically, a high CH score was associated with a greater incidence of dementia and AD over an 8-year follow-up, and with MRI white matter alterations, particularly smaller anterior CC volume.
Only one previous study on 622 elderly subjects found an association between higher cynical distrust level and incident dementia (Neuvonen et al., Reference Neuvonen, Rusanen, Solomon, Ngandu, Laatikainen, Soininen and Tolppanen2014). The risk of dementia was higher in people with the highest level of cynical distrust compared with people with low cynical distrust, after accounting for age, sex, cardiovascular risk factors, socioeconomic factors, lifestyle, health status, and APOE-4 carrier status. However, AD was not differentiated from other types of dementia due to the overall smaller number of dementia cases. Consistent with this study, in our sample, higher CH levels were not associated with higher mortality (online Supplementary Table S2), which was entirely explained by behavioral and socioeconomic confounding factors. More recently, a study on a cohort of 99 013 community-dwelling older adults also reported that hostility, and also anxiety, pessimism, hopelessness, and perceived constraints, were associated with a 20–30% increased risk of dementia during a 6–8-year follow-up, independently of depressive symptoms, history of mental disorders, and common behavioral and vascular risk factors, such as obesity, diabetes, hypertension, smoking, and physical activity (Sutin et al., Reference Sutin, Stephan and Terracciano2018). However, this study did not use clinical criteria for the diagnosis of dementia. In our study, a high level of CH was significantly associated with more lifetime anxiety disorders, and this could have biased our findings. Nevertheless, after adjustment for potential confounding factors, including anxiety disorders, our results remained similar. Our study brings new data to the small, but growing literature on the links between personality and dementia, specifically highlighting the potential role of early structural brain alterations. The present study suggests that CH-associated white matter alterations, particularly in the CC, the main white matter interhemispheric commissure, might be early pathological markers of cognitive decline and subsequent dementia. These findings are in line with a set of neuroimaging studies. A growing body of evidence suggests that white matter degeneration is an early neuropathological process of AD pathogenesis (Sachdev, Zhuang, Braidy, & Wen, Reference Sachdev, Zhuang, Braidy and Wen2013) preceding grey matter degeneration and thereby contributing to more differentiated pre-AD diagnoses (Amlien & Fjell, Reference Amlien and Fjell2014). In the present study, we did not show a significant mediating effect of white matter integrity (anterior CC) on the relationship between CH and dementia. However, this negative result might be explained by the small number of subjects in the analysis (n = 508 with only 14 dementia cases) when combining the personality and brain imaging samples.
The biological mechanisms by which CH may predispose to AD and more generally to dementia are still unknown. However, elevated pro-inflammatory activity may constitute a pathway linking CH to AD and dementia via white matter alterations. Indeed, it has been demonstrated that CH may influence inflammation by promoting cytokine production by helper T lymphocytes (Th1) (Janicki-Deverts et al., Reference Janicki-Deverts, Cohen and Doyle2010). In the whole ESPRIT cohort, we found a significant association between CRP and CC volume, suggesting that low-grade inflammation is associated with CC structural integrity alterations during aging (Cyprien et al., Reference Cyprien, Courtet, Maller, Meslin, Ritchie, Ancelin and Artero2019). Moreover, CC atrophy extent has been associated with the degree of cognitive decline: atrophy in the anterior portion in patients in the prodromal stage of dementia, and anterior and posterior atrophy in patients with AD (Wang et al., Reference Wang, Ren, Zhu, Gao, Zhang, Shen and Gao2015). These results are consistent with our findings of a smaller anterior CC volume among participants with high CH. Hence, inflammatory mechanisms could provide a plausible link between CH with AD. The association of CH and dementia with cardiovascular risk factors could provide an alternative explanation for our findings. However, in our analysis, CH emerged as an independent risk factor of dementia after controlling for these factors. Consequently, it is unlikely that cardiovascular risk factors mediate the association between high CH level and incident AD.
The results of this study are strengthened by the large sample size, the population-based design, and the relatively long follow-up, supporting the robustness of the association between baseline CH and incident dementia that was evaluated with a strict procedure by neurologists. The fact that the population was well characterized allowed us to take into account a large number of potential confounding factors.
Some potential limitations need to be acknowledged. Participants who were prospectively diagnosed with dementia could have been already in the prodromal phase when completing the assessments (protopathic bias). This might have overestimated the association between CH and AD risk. To overcome this bias, sensitivity analyses were also conducted to eliminate participants with incident dementia at phase 3 (i.e. 4 years after the baseline assessment), and the association still remained significant. Although the 4-year lag time should be sufficient to correct for the reverse causation bias, this latter cannot be formally ruled out because AD can have a very long prodromal phase, even more than a decade (Amieva et al., Reference Amieva, Le Goff, Millet, Orgogozo, Pérès, Barberger-Gateau and Dartigues2008). However, only a lifetime cohort study would provide a definitive answer to the question whether hostility is a stable personality trait contributing to dementia or an early neuropsychiatric symptom of a developing neurodegenerative disease.
MRI imaging was performed at phase 1 (baseline), whereas the BDHI for the assessment of CH was completed at phase 2 (2 years of follow-up). This could have biased CH data collection. However, personality traits are considered to be stable patterns across adult life (Damian, Spengler, Sutu, & Roberts, Reference Damian, Spengler, Sutu and Roberts2019; Roberts, Wood, & Caspi, Reference Roberts, Wood, Caspi, John, Robins and Pervin2008). Therefore, it is highly unlikely that a change in CH occurred between baseline and phase 1. Moreover, the BDHI is not a conventional tool for CH assessment, like the Cook-Medley Hostility Scale or its derived eight-item Cynical Distrust Scale. However, the CH score used for this study was based on three related questions extracted from factor analyses that showed good internal reliability. Thus, the construct of the CH score is statistically valid, and our results are consistent with the findings by Neuvonen et al. (Reference Neuvonen, Rusanen, Solomon, Ngandu, Laatikainen, Soininen and Tolppanen2014) with the Cynical Distrust Scale. Finally, longitudinal MRI data were not available to monitor brain changes in relation to CH.
In summary, the present study provides evidence to support an association between higher CH and incident AD rate in agreement with previous research. Furthermore, our findings suggest that in subjects with vulnerable personality (high CH score), white matter changes, particularly in the anterior CC, occur before grey matter loss during the neurodegenerative process that leads to AD. Additional long-term epidemiological studies with repeated administration of the CH scale, or implementation of a history of CH reported from a family member or from medical records, are needed to extend these findings and explore the relationship between CH and longitudinal white matter changes at AD onset. We also need to identify lifestyle and biological factors that mediate the detrimental effect of CH on AD that might give insights into AD physiopathology and might be used to develop prevention strategies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721000416.
Acknowledgements
The authors wish to thank Dr Elisabetta Andermarcher for English proof-reading.
Financial support
The ESPRIT project was supported by the regional government of Languedoc-Roussillon; the Agence Nationale de la Recherche; and an unconditional grant from Novartis. This study is also supported by France Alzheimer. The 3C Study was conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Medicale (INSERM), the Victor Segalen – Bordeaux II University, and Sanofi-Synthelabo. The Fondation pour la Recherche Medicale supported the preparation and initiation of the study. None of the funding organizations or sponsors played a role in the design of the study; in the collection, analysis, and interpretation of the data; in the writing of the report or in the decision to submit the article for publication.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








