Gastrointestinal complications are a significant problem in patients with critical congenital heart disease (CHD), including those with single ventricle physiology. These gastrointestinal complications lead to increased morbidity and mortality.Reference Golbus, Wojcik, Charpie and Hirsch1, Reference Jeffries, Wells, Starnes, Wetzel and Moromisato2 Infants with critical CHD are at a much higher risk of developing necrotising enterocolitis in particular because of a variety of aetiologies including impaired systemic blood flow; the stress of cardiac surgery and cardiopulmonary bypass; underlying baseline elevation of circulating cytokines; and diminished mesenteric flow during feedings.Reference Giannone, Luce, Nankervis, Hoffman and Wold3, Reference McElhinney, Hedrick and Bush4 Additional contributing factors include umbilical artery catheterisation as well as hypoxemia and hypotension.Reference Giannone, Luce, Nankervis, Hoffman and Wold3 While necrotising enterocolitis occurs more frequently in premature infants, approximately 10–15% of cases occur in full-term infants. It has been shown that critical CHD is a risk factor for full-term infants, with up to 25% of full-term infants with necrotising enterocolitis also having critical CHD.Reference Ostlie, Spilde and St Peter5
The most common presenting symptoms for necrotising enterocolitis among critical CHD patients are haematochezia and feeding intolerance.Reference Giannone, Luce, Nankervis, Hoffman and Wold3, Reference Hunter, Podd, Ford and Camerini6 Haematochezia in infants can be due to a diverse set of aetiologies. These aetiologies range from being relatively benign, including swallowed maternal blood or anal fissures, to severe causes such as necrotising enterocolitis. Other possibilities include milk protein allergy, the use of medications such as aspirin or ibuprofen, vitamin K deficiency, volvulus, or intussusception.Reference Pai and Fox7 In the otherwise healthy infant, aetiologies are often benign. In infants with critical CHD, more serious causes such as necrotising enterocolitis need to be considered.
In infants with critical CHD, enteral feeding is introduced and titrated to goal caloric needs slowly to ensure feeding tolerance and minimise serious gastrointestinal complications. These patients are monitored for signs and symptoms of intolerance including emesis, abdominal distension, worsening somatic near-infrared spectroscopy values, occult blood, and haematochezia.Reference Sharma and Hudak8 Guaiac testing of stools is often used to evaluate occult blood in stools.
The utility of guaiac testing of stools in this patient population is undetermined, as the test has the potential for false positives. In paediatric patients, minute blood loss can be associated with perianal dermatitis or passage of stool, which would yield a faecal blood-positive test and subsequent unnecessary diagnostic procedures.Reference Boyle9 In addition, studies have shown that the sensitivity, specificity, and positive predictive values of a positive faecal occult blood test among very low birth weight infants in the neonatal intensive-care unit were 0, 34.4, and 0%, respectively.Reference Pickering, White and Davis10 Due to the limited proven association between occult haematochezia and necrotising enterocolitis, some studies have found that routine faecal occult blood testing is not a useful diagnostic tool for neonatal patients.Reference Pinheiro, Clark and Benjamin11, Reference Abramo, Evans, Kokomoor and Kantak12 As a result, some centres have discontinued routine testing. Furthermore, Pinheiro et al,Reference Pinheiro, Clark and Benjamin11 hypothesised that false-positive faecal occult blood testing can have several adverse effects, including increased usage of radiographs and withholding feedings in patients who are already nutritionally compromised. Withholding feeds can negatively impact patient survival, growth, and development due to increased metabolic and infectious risks.Reference Pinheiro, Clark and Benjamin11, Reference Krishnamurthy, Gupta, Debnath and Gomber13
Although the utility of guaiac testing is questioned by some, other literature indicates that occult blood can be indicative of serious gastrointestinal complications.Reference Boyle9 As such, some centres still conduct routine guaiac testing, particularly for patients at greater risk for life-threatening complications. In our tertiary care centre’s paediatric cardiac surgery programme, our current feeding protocol involves routine guaiac testing of stools for infants with critical CHD who are receiving enteral feedings (Fig 1). We use guaiac testing to evaluate for potential feeding intolerance and serious gastrointestinal complications. When an infant has a guaiac-positive stool, enteral feedings are paused, and the patient receives an abdominal examination as well as an abdominal radiograph. Similar guaiac-positive stool protocols are considered common practice with enteral feedings, as shown in several study protocolsReference Krishnamurthy, Gupta, Debnath and Gomber13, Reference Caple, Armentrout and Huseby14 If the clinical exam and abdominal radiograph are benign, enteral feedings are resumed and the patient continues to be monitored for any further signs or symptoms of feeding intolerance and gastrointestinal complications.
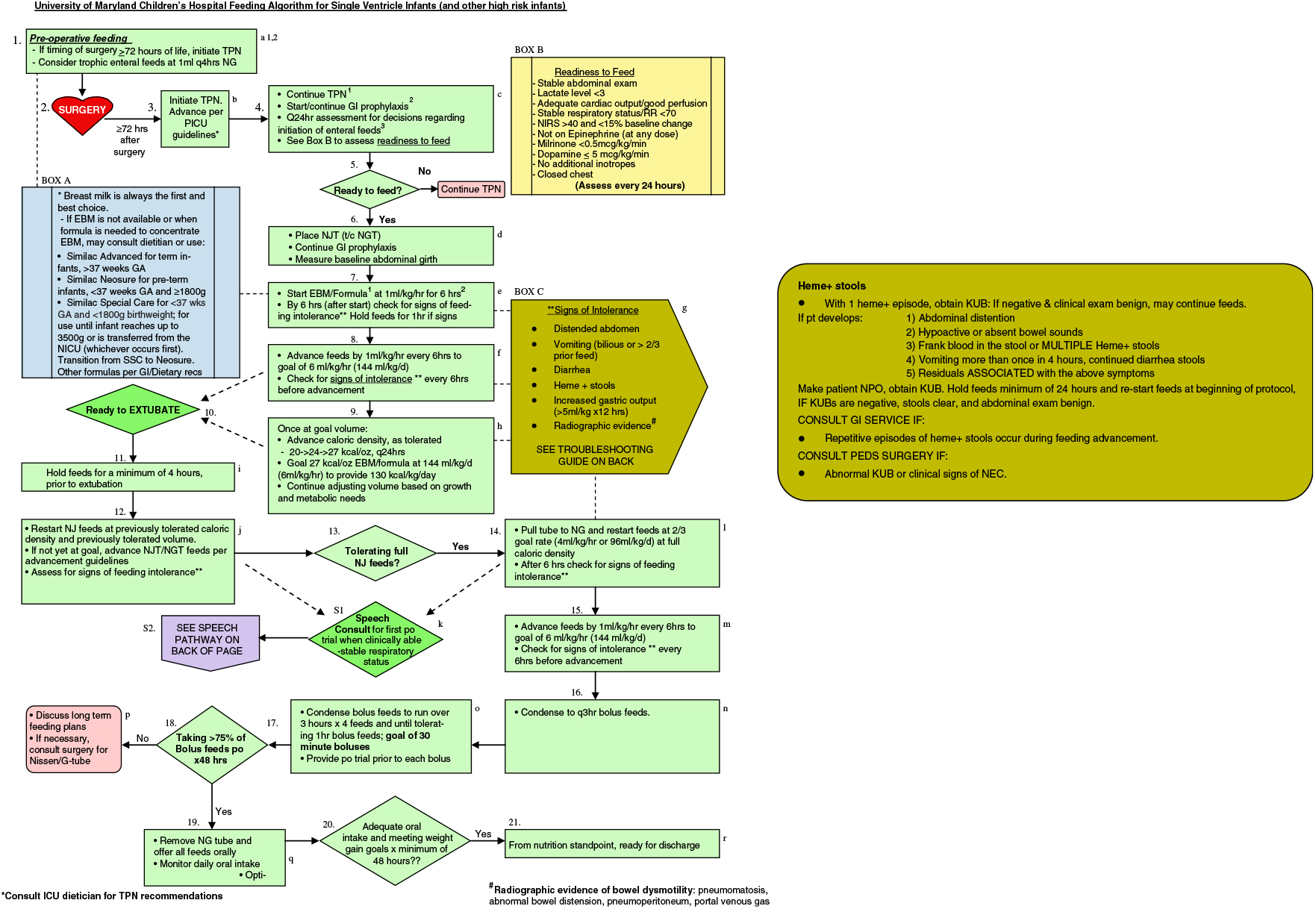
Figure 1. Feeding algorithm for single ventricle infants.
While there is research on the utility of guaiac testing in neonates in general, there is a paucity of information in the literature discussing this practice of routine guaiac testing of stool in infants with critical CHD. Due to concerns regarding adverse effects of false-positive guaiac testing, we aimed to evaluate our use of guaiac testing of stools among patients with single ventricle physiology who underwent the stage one palliation for CHD. Specifically, we investigated the role of guaiac testing in detecting gastrointestinal complications, defined as a gastrointestinal surgical emergency or necrotising enterocolitis, as well as the association with feeding interruptions, time to achieving goal enteral feedings, placement of a gastrostomy tube, and frequency of radiographic exposure.
Material and methods
A retrospective chart review was performed on patients with single ventricle physiology who underwent stage one palliation (Norwood, Damus–Kaye–Stansel, or aortopulmonary shunt) at the University of Maryland Children’s Hospital between 7 October, 2012, and 30 September, 2016 Patients were excluded from the analysis if their admission occurred prior to the initiation of the programme’s single ventricle feeding protocol or if they were never able to achieve post-operative enteral nutrition. Patient characteristics are listed in Table 1.
Table 1. Patient characteristics (n = 19)

ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; LAZ = length for age z score; LV = left ventricle; PA/IVS = pulmonary atresia with intact ventricular septum; RV = right ventricle; UGI = upper gastrointestinal; WAZ = weight for age z score.
Continuous data are presented as medians with standard deviation.
Data were abstracted from electronic and paper medical records. Baseline demographic data included gender, birth length and weight, gestational age, cardiac diagnosis (classified as hypoplastic left heart syndrome, other single right ventricle, tricuspid atresia, pulmonary atresia with intact ventricular septum, double inlet left ventricle, or other single left ventricle), genetic syndromes, gastrointestinal abnormalities, other comorbidities, and type of feeds received preoperatively. Surgical data included type of stage one palliation surgery (Norwood/Damus–Kaye–Stansel or aortopulmonary shunt), age at surgery, cardiopulmonary bypass time, cross-clamp time, and surgical complications. Post-surgical information included history of cardiac arrest, need for extracorporeal mechanical oxygenation, post-operative duration of intubation, need for reintubation, need for repeat surgeries, or cardiac catheterisation (excluding delayed sternal closure), delayed sternal closure, number of days on inotrope or vasopressor support, multiple guaiac-positive stools, vocal cord dysfunction, or chylothorax). The presence or absence of heme-positive stools was evaluated, as well as time (in days) to goal caloric intake, presence or absence of serious gastrointestinal complication (defined as a gastrointestinal surgical emergency or necrotising enterocolitis), placement of a gastrostomy tube, and number of abdominal radiographs performed on each patient.
Results
There were 19 patients included in this analysis. During hospitalisation, 12 out of the 19 (63%) patients experienced heme-positive stools. The median days to goal caloric intake (n = 19) was 6 days (IQR = 7). Days to goal caloric intake for the heme-positive group (mdn = 5, IQR = 8) was not significantly different (U = 43.5, p = 0.967) compared to the heme-negative group (mdn = 7, IQR = 9). The data for days to goal caloric intake were not normally distributed and a Mann–Whitney U-test was used to compare days to goal caloric intake for patients with and without heme-positive stools.
During the study period, 3 out of the 19 patients had a serious gastrointestinal complication (Fig 2). There was no statistically significant difference (p = 0.117) in the proportion of heme-positive and heme-negative stools between patients with and without a serious gastrointestinal complication. Those patients with serious gastrointestinal complication also experienced grossly bloody stools, had a diagnosis of medical necrotising enterocolitis, or had an abdominal radiograph showing dilated bowel loops. In the study, 12 out of 19 patients required placement of a gastrostomy tube. There was no statistically significant difference (p = 0.356) in the heme-positive and heme-negative stool proportion between patients who did and did not require a gastrostomy tube. Given the sample size, and because expected frequencies were small, a Fisher’s exact test was used to compare both serious gastrointestinal complications and gastrostomy tube placements between patients with and without heme-positive stools.

Figure 2. Display of results of haemoccult stool tests and patients with gastrointestinal complications.
The number of abdominal radiographs performed for each patient was collected for patients with and without guaiac-positive stools. These numbers were evaluated using a Mann–Whitney U-test. A p value less than 0.05 was used for statistical significance. Analysis revealed significant difference in the number of abdominal radiographs for patients with heme-positive stools (Md = 4, n = 11) and patients without heme-positive stools (Md = 0, n = 8), U = 6, z = −3.187, p = 0.001, r = 0.73 (Fig 3).

Figure 3. Comparison of abdominal radiographs in patients with and without heme-positive stools.
Discussion
In our study, 11 out of 19 (58%) patients experienced haemoccult-positive stools during their post-operative course. Details for these patients are included in Table 2. Heme-positive stools were not found to increase time to achieve full enteral nutrition. Thus, although some literature asserts that heme-positive stools do not justify withholding feedings, doing so did not delay time to achieving full enteral nutrition.Reference Stiles, Simpson, Vaughn and Thullen15 In addition, heme-positive stools were not found to correlate with increased frequency of gastrointestinal emergencies, including necrotising enterocolitis. This is supported by other studies that found that there is limited association between occult haematochezia and necrotising enterocolitis, and thus limited utility of routine faecal occult blood testing in infants.Reference Pickering, White and Davis10– Reference Abramo, Evans, Kokomoor and Kantak12 Although there is no correlation between heme-positive stools and increased incidence of gastrointestinal emergencies, there were no gastrointestinal emergencies in the patients with heme-negative stools in our study.
Table 2. Detailed diagnosis and medical information for patients that experienced heme-positive stools.

ASA = aspirin; AXR = abdominal radiograph; BT = Blalock–Taussig; DSC = delayed sternal closure; EDP = end diastolic pressure; GI = gastrointestinal; GJT = gastro-jejunal tube; GT = gastrostomy tube; HLHS = hypoplastic left heart syndrome; LPA = left pulmonary artery; LV = left ventricle; MBS = modified barium swallow study; NEC = nectrotising enterocolitis; NG = nasogastric; PA/IVS = pulmonary atresia with intact ventricular septum; PO = by mouth; RV = right ventricle; SSI = surgical site infection; SVC = superior caval vein; TA = triscuspid atresia; TR = tricuspid regurgitation.
Our study did show that there was an association between heme-positive stools and an increased number of abdominal radiographs. Increased abdominal radiograph in children has been associated with an increased cumulative cancer lifetime mortality risk.Reference Baird, Tessier, Guilbault, Puligandla and Saint-Martin16 Given that the presence of heme-positive stools did result in an increased number of abdominal radiographs but did not result in an increased detection of gastrointestinal emergencies, haemoccult testing does not seem to be a valuable diagnostic tool and may place the patient at increased risk of radiation exposure. This is supported by the results of additional studies that assert that there are no advantages to routine guaiac testing of stool in the absence of other indicators for feeding intolerance or gastrointestinal complications, such as emesis, abdominal distension, and gastric residuals.Reference Carter17
There are several limitations to this study. Due to our specific population, the sample size is small and thus limits the generalisability of our findings. Also, since this was a retrospective chart review over several years, there could have been some variability in documentation of procedures over time, leading to misclassification bias. Moreover, our study population is heterogeneous with various diagnoses and post-operative courses, and thus a few outliers could have disproportionally affected the measures we investigated. Despite these limitations, this study serves as an initial evaluation of the utility of guaiac testing in critical CHD patients, which is a little-researched field. More research needs to be done in this field with larger sample sizes to further investigate our findings.
Conclusion
Infants with critical CHD are at a heightened risk of having gastrointestinal complications while advancing feeds. Current practice utilises routine guaiac stool testing as a marker for gastrointestinal complications. Our results show that heme-positive stools are not associated with gastrointestinal complications in this population, but they are associated with an increased number of abdominal radiographs. This suggests that routine guaiac testing is of low yield in detecting problems and may lead to evaluations that place our patients at future risk.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.







