Significant outcomes
-
∙ The GAT-1−/− mice displayed increased hyperactivity, impaired sustained attention.
-
∙ Morris water maze results indicated that GAT-1−/− mice exhibited impairment of spatial learning and memory.
-
∙ It could be a new animal model for attention-deficit hyperactivity disorder.
Limitations
-
∙ Small sample size of the study.
-
∙ Study only from the perspective of animal behaviours and no further study on the mechanism.
-
∙ Similar but methodology is different.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurobehavioural disorder defined by symptoms of inappropriate attention, impulsivity and hyperactivity, with a worldwide prevalence estimate of 5.29% and a heritability estimate of about 76% (Reference Faraone, Sergeant, Gillberg and Biederman1,Reference Faraone2). However, the pathophysiology and aetiology of ADHD remain inconclusive so far. There are undoubtedly complex gene–gene and gene–environment interactions to the aetiology of ADHD. To date, polymorphisms in several candidate genes have been associated with the disorder, including the D4 and D5 dopamine receptor (Reference Faraone, Perlis and Doyle3,Reference Wu, Xiao, Sun, Zou and Zhu4), the dopamine transporter (DAT) and synaptosomal-associated protein 25 (Reference Lesch and Waider5,Reference Thapar, O’Donovan and Owen6), etc. Dopaminergic and noradrenergic systems are thought to have primary roles in the aetiology of ADHD, based on the efficacy of the DAT and the norepinephrine transporter (NET), substrate amphetamine and the DAT and NET inhibitor, and benzyl piperidine derivative methylphenidate for ADHD (Reference Bidwell, McClernon and Kollins7,Reference Stein, Waldman and Sarampote8).
Gamma aminobutyric acid (GABA) is the principal inhibitory neurotransmitter widely distributed in mammalian central nervous system, and about 50% of the central synapses with GABA neurotransmitter. It has postsynaptic inhibition effect and can make postsynaptic neuron in protective inhibition condition, through inducing postsynaptic membrane hyperpolarisation, reducing ion flow, and cell metabolism and oxygen consumption (Reference Reichling and Basbaum9,Reference Krajnc, Neff and Hadjiconstantinou10).
As ADHD is a disorder of ‘disinhibition’, close interaction of dopaminergic, noradrenergic and GABAergic systems, and the role of dopamine and norepinephrine in the aetiology of ADHD (Reference Garbutt and van Kammen11–Reference van der Kooij and Glennon13), it is conceivable that GABA neurotransmission is implicated in the pathophysiology of ADHD. The GABA transmission is decreased or terminated by the re-uptake of the released GABA in synaptic cleft through GAT, a family of membrane proteins located on vesicle, presynaptic and glial cell membrane. GAT also plays an important role in the pathophysiology of ADHD. So far, four GABA transporters (GAT-1–GAT-4) have been identified. The affinity for GABA is GAT-1>GAT-3>GAT-2>GAT-4 (Reference Ueda and Willmore14). Among these transporters, GAT-1 has the largest ability to uptake GABA in brain. GAT-1 is mainly localised in neurons and astrocytes in the cerebral cortex (Reference Guastella, Nelson and Nelson15,Reference Chiu, Jensen and Sokolova16). More than 75% of GABA re-uptake can be attributed to GAT-1 in the central nervous system (Reference Jensen, Chiu, Sokolova, Lester and Mody17), indicating that GAT-1 plays an important role in the metabolism of GABA. Yang et al. (Reference Yang, Cai, Cai, Fei and Liu18) have also proposed that gat-1 −/− mice display low levels of attentively focusing and increased impulsivity, and can be used as a new animal model for ADHD.
In the current study, three kinds of behavioural tests [the open field test, elevated O-maze (EZM) and Morris water maze] were used to evaluate behavioural traits relevant to ADHD (e.g. hyperactivity, impaired sustained attention, impulsivity and learning ability) in the gat-1 −/− mice and wild-type (WT) mice.
Materials and methods
Animals
The GAT-1−/− and WT mice were obtained from Shanghai South Biomodel Organism Company, and bred in animal centre of Jinshan Hospital. The animal rooms were kept neat and uncluttered. All of the animals were maintained under a 12/12 h light/dark cycle (lights on at 7:00 h) at 22°C and 50% humidity, and had ad libitum access to food and water. GAT-1−/− and WT mice at the age of 6–8 weeks were examined in behavioural tests. All animals were weighed at 9:00–10:00 h everyday. Age and body weight-matched male mice were used for behavioural experiments. Investigators observed animal behaviours through a video monitor in another room without any information about the genotype of the mice. All the experimental protocols were performed in compliance with the Institutional Animals Care and Use Committee of the Institute of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Behavioural tests
Open field test
The open field test is used to test the differences in locomotor behaviour of WT and gat-1 −/− mice (Reference Fuss, Ben and Vogt19), which is widely used in laboratories to quantify anxiety-like and locomotor behaviours in mice. Mice have the innate tendency to escape bright, open new surroundings. The open field is a square arena (50×50×50 cm) with white acrylonitrile butadiene styrene (ABS) engineering plastic walls and floor. Mice were placed in the centre of the box and allowed to freely explore for a 10-min period. Motor activity was videotaped using a camera fixed above the floor and analysed with a video tracking system, which divided the arena into ‘margin’ and ‘centre’ fields. The ‘centre’ field is defined as the central 25% area of the open field. A video camera automatically recorded mice motion curve, and identified the movement and stationary state of mice.
EZM
The EZM is modified on the basis of elevated plus maze. The advantage of the O-maze is that it solves the ambiguous central square of the traditional plus maze. The EZM consisted of a circular platform (46 cm in outer diameter, 5.5 cm in width) that was elevated 40 cm above the floor. Above the platform were two open and two enclosed segments. The closed segments were constituted by walls extending 20 cm above the surface of the platform. Each test started by placing the mouse in any closed sectors. The test session lasted for 5 min. Mice were recorded using a video camera placed above the EZM. The video tracking software recorded the path moved and per cent of time spent in the open and closed segments, and number of open and closed segment entries. The EZM were cleaned with water between trials.
Morris water maze
The Morris water maze task was based on the method described previously (Reference Tang, Shimizu and Dube20,Reference Morris, Garrud, Rawlins and O’Keefe21). The water maze was a circular swimming pool (100 cm in diameter, 50 cm high) with black ABS engineering plastic walls and floor. The swimming pool filled with water maintained at 24–26°C to a depth of 30 cm, the experimental conditions (room extra cues, water temperature, room temperature, light) were constant throughout the experiment. Water was added with milk powder so as to hide the underwater platform and show the black mice clearly. The pool was divided into four equal quadrants by four entry points marked on the pool wall and a white escape platform was set in the centre of the target quadrant (1.5 cm below the water level). Each quadrant was marked with a different shape so as to provide visual clues to help the mice find the escape platform. The position of the platform was fixed throughout the place navigation test. The platform was the only escape of mice in the water, so they had to search for the hidden underwater platform. This task consisted of place navigation tests four times per day for 5 consecutive days, followed by probe trials on the 6th day. In each trial, a mouse was released into the water facing the pool wall, from one of the starting positions randomly. Mice were allowed to swim for a maximum of 60 s until they found the platform. If the mouse failed to find the platform in 60 s, it was gently placed on the platform and allowed to stay on it for 30 s before the next trial. On the probe trial day, the platform was removed and each mouse was released into the pool from the same position. The swimming paths of the mice were recorded for 60 s and monitored by a camera mounted above the centre of the pool.
Statistical analysis
Data are represented as mean±SE of the mean. Two groups in navigation test of Morris water maze test use paired samples t-test, other data adopt independent sample t-test. A value of p<0.05 denotes a statistically significant difference. All statistical analyses were performed using Stata11.0 statistical software.
Result
Motor activity
In the open field test, the total distances moved, velocities, the rest times during observation period (motor activity of the first 10 min was compared) were recorded. The GAT-1−/− mice travelled longer and displayed enhanced kinematic velocity with significantly reduction of rest time during the initial open field exposure in the 1st week (p<0.01) and the re-exposure to the open field in the second (p<0.01) or 3rd weeks (p<0.01) (Figs 1a and b). This phenotype manifested hyperactivity and enhanced locomotor activity compared with WT mice.
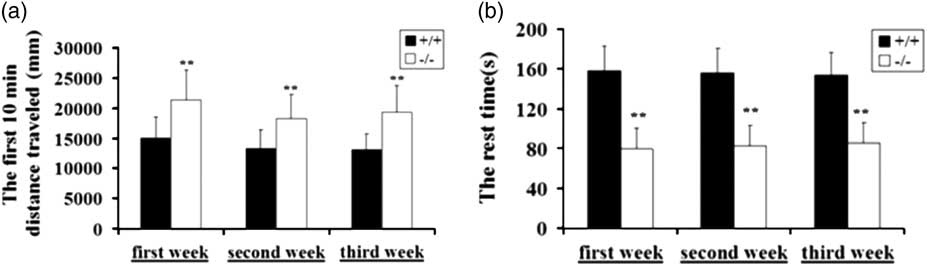
Fig. 1 GAT-1 −/− mice showed hyperactivity and enhanced locomotion in open field test. Performance of GAT-1−/− mice in open field compared with the wild-type (WT) mice in novel environments and the re-exposure to the open field in the 2nd and the 3rd weeks. (a) An increase in movement distance. (b) A reduction in rest time; WT mice group, n +/+=10; GAT-1 −/− group, n −/−=10. *p<0.05, **p<0.01. GAT-1, GABA transporter 1.
EZM test
The results showed that GAT-1−/− mice displayed a significant increase in total entries into the open sectors and the closed sectors compared with the WT mice (p<0.01) (Fig. 2a). GAT-1−/− mice manifested hyperactivity and enhanced motoricity in the EZM test compared with WT mice. No significant differences were found in the percentage of entries into open sectors compared with total entries (p>0.05) (Fig. 2b).
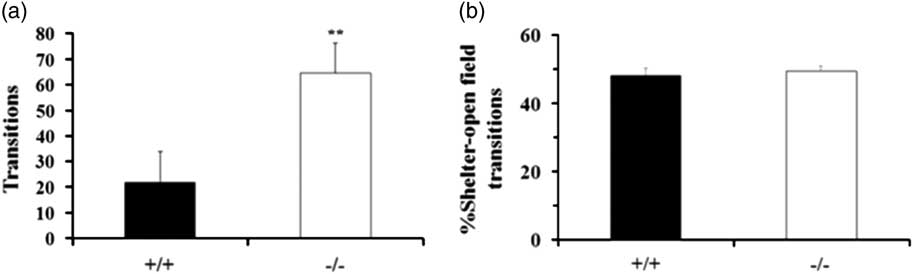
Fig. 2 GAT-1−/− mice manifested hyperactivity and enhanced motoricity in the Elevated O-Maze (EZM) test. Effect of GAT-1 deficiency on EZM test. (a) An increase in total entries into the open sectors and the closed sectors. (b) No significant differences were found in the percentage of entries into open sectors compared with total entries; wild-type mice group, n +/+=10; GAT-1 −/− group, n −/−=10. **p<0.01. GAT-1, GABA transporter 1.
Morris water maze test
Morris water maze task was performed to assess a complex process of spatial learning and memory, including collecting, dealing with, sorting, memorising and strengthening visual information. Escape latency in the navigation test and time spent in each quadrant in the probe trial were recorded. Escape latencies of GAT-1−/− mice were significantly longer than those of WT mice during the place navigation test. The WT mice showed shorter latencies after the training session (p<0.01), whereas the GAT-1−/− mice made no difference (Fig. 3a). The GAT-1−/− mice showed no significant difference in preference for the target quadrant during the probe test, whereas the WT mice spent 44.24% of the total time on the target quadrant and only 12.95% on the opposite quadrant. Compared with the WT mice, the GAT-1−/− mice spent less time in the target quadrant (p<0.01) (Fig. 3b). These results indicated that GAT-1−/− mice exhibited impairment of spatial learning and memory.

Fig. 3 GAT-1 −/− mice exhibited impairment of spatial learning and memory in Morris water maze. Effect of GAT-1 deficiency on Morris water maze test. (a) During learning session, the latency to find the platform was significantly longer for GAT-1−/− mice compared with wild-type (WT) mice. (b) During probe test, GAT-1−/− mice spent less time in the target quadrant but more time in the opposite quadrant than WT mice; WT mice group, n +/+=10; GAT-1 −/− mice group, n −/−=10. *p<0.05, **p<0.01. GAT-1, GABA transporter 1.
Body weight
The initial body weights of the WT and GAT-1−/− mice at the 4th weeks were similar (p>0.05). However, after 8th weeks, the GAT-1−/− mice had a significant weight loss compared with the WT mice (p<0.05) (Fig. 4).
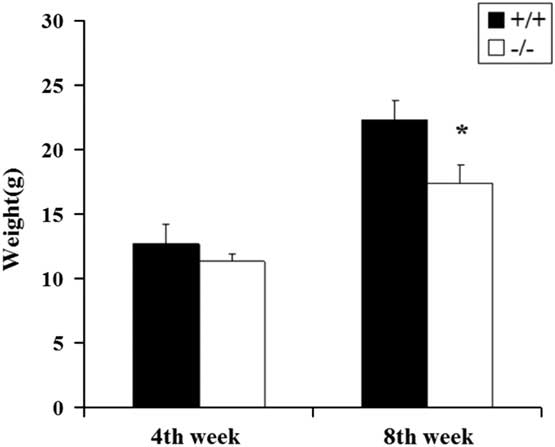
Fig. 4 Body weights of the two group mice at 4th and 8th weeks. Wild-type mice group, n +/+=10; GAT-1−/− mice group, n −/−=10. *p<0.05. GAT-1, GABA transporter 1.
Discussion
In this study, the GAT-1−/− mice manifested hyperactivity and enhanced locomotion compared with WT mice in open field test and EZM test. In the EZM test, even two GAT-1−/− mice fell to the ground because of hyperactivity in the open area. The open field test is applicable to the assessment of motor behaviour and anxiety caused by a deficit in habituation. A deficit in habituation often leads to hyperactivity and anxiety. In our study, the GAT-1−/− mice showed higher locomotor activity than the WT mice, when they were re-exposed to the same environment three times, suggesting that hyperactivity in the GAT-1−/− mice was not owing to a deficit in habituation to novel environment. At the same time, the GAT-1−/− mice had a significant weight loss compared with the WT mice. There were few researchers who took the body weight change into consideration in the ADHD model. We found that the change of body weight in GAT-1 knockout mice had a statistical significance compared with the even-aged wild mice. We speculated that this phenomenon might be related to the hyperactivity of the mice. Mass movement of the GAT-1−/− mice caused the energy consumption that finally lead to the weight loss. Therefore, the change of body weight indirectly proved the hyperactivity of the GAT-1−/− mice.
The water maze was used to measure spatial learning and memory ability (Reference Gong, Li and Cai22,Reference Huang, Hu, Liu, Zhou and Zhang23). In this study, the GAT-1−/− mice exhibited impairment of spatial learning and memory. Children with ADHD often have comorbid learning problems. In addition, when the GAT-1−/− mice were placed on the platform after failing to find the platform in 60 s in a navigation test, most of them stayed only 3–5 s on the platform and jumped into the water again. The GAT-1−/− mice exhibited distinct hyperactivity and low levels of attentively focusing compared with WT mice, which might also be one of the reasons that led to the deficiency of platform positioning ability. Actually, Yang et al. (Reference Yang, Cai, Cai, Fei and Liu18) had reported the idea that GAT-1 knockout mice could mimic ADHD model mainly from the angle of movement and balance skills. They demonstrated the low levels of attentively focusing and increased impulsivity of the mice. However, we largely focused on the cognitive function and change of body weight in GAT-1 knockout mice compared with the WT mice. We used the Morris water maze to detect the spatial learning and memory ability of GAT-1 knockout mice and indicated that the mice showed a similar cognitive dysfunction in line with ADHD patients. Some researchers gave the water maze task to the spontaneously hypertensive rat that was the most widely used animal model of ADHD for evaluation of its spatial learning and memory (Reference Yin, Cao, Yu, Guo, Sun and Lei24–Reference Liu, Yang, Lei, Wang, Wang and Sun26). We drew lessons from this method to test the cognitive function of GAT-1 knockout mice as a model of ADHD.
Close interaction between dopaminergic, noradrenergic and serotonergic systems has been known in ADHD. Changes in any one system can alter the function of the other monoaminergic systems or nonmonoaminergic systems, which contain glutamate and GABA systems, and then alter the underlying neural circuits of behaviour. Study found that stimulation of the GABA (B) receptor decreased dopamine release in the pedunculopontine tegmental nucleus and this phenomenon seemed to be behaviourally relevant (Reference Steiniger and Kretschmer27). More than 75% of GABA re-uptake could be attributed to GAT-1 in the central nervous system, so GAT-1 deficiency leads to enhanced extracellular GABA levels resulting in an overactivation of GABAA receptors, which is responsible for a postsynaptic tonic conductance, and overactivation of postsynaptic GABAA receptors inhibits dopaminergic neurons in the GAT-1−/− mice, and finally leads to enhanced locomotion in the GAT-1−/− mice compared with WT mice. In addition, several studies have suggested that serotonergic and adrenergic systems might be modified in GAT-1−/− mice and the modified dopaminergic, serotonergic and adrenergic systems following alteration in the GABAergic system might together be involved in the aetiology of ADHD in the GAT-1−/− mice (Reference Jensen, Chiu, Sokolova, Lester and Mody17,Reference Liu, Cai and Cai28).
ADHD is a complex psychiatric and polygenic disorder, and the GAT-1−/− mice may only mimic a subset of its symptoms (Reference Willcutt29). In further study, we will use clinical ADHD drugs to test effects on GAT-1 knockout mice and detect the contents of related neurotransmitters in the brain neurons such as dopamine and γ-GABA in GAT-1 knockout mice in order to explore the pathogenesis of ADHD.
Taken together, we showed that the GAT-1−/− mice had phenotypes of hyperactivity, impaired sustained attention, learning deficiency and the performances of GAT-1−/− mice were similar to ADHD symptoms. However, the underlying molecular mechanism of ADHD is yet to be ascertained and the GABAergic system may be the key factor (Reference Shi, Cai and Liu30). Thus, the study of the GAT-1−/− mice may provide new insights into the mechanisms and the discovery of novel therapeutics for the treatment of ADHD.
Acknowledgements
Authors’ contributions: Yinghui Chen and Biqin Chen carried out the conception and design of the entire study. Long Chen, Xiaoyong Zhou and Xue Gong carried out the behaviours tests. Cuicui Wang contributed to the development of project and the design of experiments. Xiaobo Yang and Long Chen contributed to the data analysis and wrote the manuscript.
Financial Support
This work was supported by grants from the Science and Technology Commission of Jinshan District, Shanghai (2012-3-3) to Cuicui Wang.
Conflicts of Interest
The authors report no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals, and approved by the Ethic Committee of Animal Care of the Jinshan Hospital, Fudan University (2013-034-01).







