INTRODUCTION
Leishmaniasis is a widespread parasitic disease caused by protozoa of the genus Leishmania, with approximately 12 million of cases in tropical and temperate areas (WHO, 2009). Cutaneous, mucocutaneous and visceral are the major forms of leishmaniasis in humans. Leishmania (Leishmania) amazonensis is the aetiological agent of the diffuse and disseminated cutaneous leishmaniasis, which is characterized by a decreased immune response in infected patients (Convit et al. Reference Convit, Pinardi and Rondón1972; Barral et al. Reference Barral, Pedral-Sampaio, Grimaldi Junior, Momen, McMahon-Pratt, Ribeiro de Jesus, Almeida, Badaro, Barral-Neto, Carvalho and Johnson1991). Despite the world prevalence and the elevated number of cases, there were few advances in chemotherapy in the last years, and there is still no vaccine for this disease.
Parasites from the genus Leishmania have 2 major life forms: the promastigote forms that reside in the sandfly insect vector and the amastigote forms that reside in macrophages from the mammalian host. The life cycle involves transmission of amastigote-infected host macrophages to the female sandfly upon taking a bloodmeal. The amastigotes then rapidly undergo differentiation to promastigotes within the sandfly gut and ultimately migrate to the foregut where, upon a subsequent bloodmeal, they are transmitted to a mammalian host.
Ecto-enzymes are glycoproteins located at the external surface of the plasma membrane. The activities of these enzymes can be measured using intact cells (De Pierre and Karnowsky, Reference De Pierre and Karnovsky1973, Reference De Pierre and Karnovsky1974; Furuya et al. Reference Furuya, Zhong, Meyer-Fernandes, Lu, Moreno and Docampo1998; Meyer-Fernandes, Reference Meyer-Fernandes2002). Ecto-nucleoside triphosphate diphoshydrolases are ecto-enzymes that hydrolyse tri- and/or di-phosphate nucleotides (Plesner, L. Reference Plesner1995; Zimmermann, H. Reference Zimmermann1999). There is evidence that these enzymes are related to virulence and infectivity in protozoan parasites. Virulent strains of Toxoplasma gondii express and secrete 2 types of ecto-NTPases (ecto-NTPase I and II), while avirulent strains express only one type (ecto-NTPase II) (Nakaar et al. Reference Nakaar, Beckers, Polotsky and Joiner1998). Similarly, the invasive form of Entamoeba histolytica presents an ecto-ATPDase activity significantly higher than the free-living Entamoeba moshkovskii (Barros et al. Reference Barros, De Menezes, Pinheiro, Silva, Lopes, De Souza and Meyer-Fernandes2000). Virulent promastigote forms of Leishmania amazonensis presented a Mg2+-dependent ecto-ATPase activity 2-fold higher than avirulent promastigote forms (Berrêdo-Pinho et al. Reference Berrêdo-Pinho, Peres-Sampaio, Chrispim, Belmont-Firpo, Lemos, Martiny, Vannier-Santos and Meyer-Fernandes2001). Furthermore, short-term cultivated promastigotes are able to infect higher levels of peritoneal macrophages than long-term cultivated parasites, which present reduced ecto-ATPase activity. The elevated ecto-ATPase activity is also related to a reduced immune response by the host cells (de Souza et al. Reference De Souza, De Assis, Gomes, Marques da Silva, Melo, Fietto and Afonso2010). Santos et al. (Reference Santos, Pôssa, Bastos, Guedes, Almeida, Demarco, Verjovsky-Almeida, Bahia and Fietto2009) showed that an ecto-ATPase activity is important for infectivity of Trypanosoma cruzi, since infective trypomastigote forms presented 2·5-fold higher levels of ecto-ATPase activity when compared with amastigote-like forms. They also observed that ATPase/ADPase activity ratios of cell-derived trypomastigotes decreased 3- to 6-fold during sequential subcultivation, and infectivity was substantially reduced. The activities of ecto-enzymes are also related to infectivity in other different pathogens, such as Candida parapsilosis (Kiffer-Moreira et al. Reference Kiffer-Moreira, Fernandes Sampaio, Alviano, Axelband, Cesar, Cosentino-Gomes, Rodrigues, Nimrichter, Vieyra, Alviano and Meyer-Fernandes2010), Mycoplasma hominis (Hopfe et al. Reference Hopfe and Henrich2008), Legionella pneumophila (Sansom et al. Reference Sansom, Riedmaier, Newton, Dunstone, Müller, Stephan, Byres, Beddoe, Rossjohn, Cowan, d'Apice, Robson and Hartland2008), Cryptococcus neoformans (Collopy-Junior et al. Reference Collopy-Junior, Esteves, Nimrichter, Rodrigues, Alviano and Meyer-Fernandes2006), Tritrichomonas foetus (Jesus et al. Reference De Jesus, de Sá Pinheiro, Lopes and Meyer-Fernandes2002) and Trichomonas vaginalis (de Jesus et al. Reference De Jesus, de Sá Pinheiro, Lopes and Meyer-Fernandes2002).
CrATP (bidentate chromium (III) ATP complex) is a Mg-ATP analogue, in which the ion Cr3+ substitutes the Mg2+ ion (Depamphilis and Cleland, Reference DePamphilis and Cleland1973). It has been described as an inhibitor that forms a stable complex with P-ATPases, leading to their inhibition. This mechanism has been described for sarcoplasmic reticulum Ca2+-ATPase (Vilsen and Andersen, Reference Vilsen and Andersen1992a,b; Einholm et al. Reference Einholm, Vilsen and Andersen2004), plasma membrane Ca2+-ATPase (Moreira et al. Reference Moreira, Rios and Barrabin2005) and Na+, K+-ATPase (Pauls et al. Reference Pauls, BredenBröcker and Schoner1980; Linnertz et al. Reference Linnertz, Thonges and Schoner1995). In a previous study, we showed that CrATP acts as a reversible inhibitor of ecto-ATPase activity in monoxenic and heteroxenic trypanosomatids, such as Herpetomonas sp., Trypanosoma cruzi and Leishmania amazonensis. The ecto-ADPase and ecto-phosphatase (assessed by a p-nitrophenylphosphate as substrate) activities were not sensitive to CrATP (Moreira et al. Reference Moreira, Rios, Esteves, Meyer-Fernandes and Barrabin2009).
In this work, we investigated the contribution of ecto-ATPase activity in the infectivity of Leishmania amazonensis, evaluating the interaction of promastigote forms of the parasite with resident peritoneal macrophages from BALB/c mice in the presence of CrATP. We observed that both adhesion and internalization indices were strongly reduced in the presence of CrATP. This effect was reversible, such as the inhibition of ecto-ATPase activity by CrATP. Furthermore, this compound was able to inhibit the multiplication of cells in axenic cultivation, with virtually no toxicity to murine macrophages.
The net negative charge of CrATP at pH 7·2 makes this compound most probably membrane impermeable in intact cells. Thus, it can be considered a specific inhibitor of the ecto-ATPase activity, as DIDS and suramine (Bernardes et al. Reference Bernardes, Meyer-Fernandes, Saad-Nehme, Vannier-Santos, Peres-Sampaio and Vercesi2000; Bisaggio et al. Reference Bisaggio, Peres-Sampaio, Meyer-Fernandes and Souto-Padron2003). Therefore, this is the first time that a specific inhibitor of ecto-ATPase activity has been used to investigate the role of this enzyme in L. amazonensis-macrophage interaction.
MATERIALS AND METHODS
Reagents
All reagents were purchased from E. Merck (Darmstadt, Germany) or Sigma Chemical Co. (St Louis, MO). [γ 32P]ATP was synthesized as described by Walseth, and Johnson (Reference Walseth and Johnson1979).
CrATP synthesis
CrATP was synthesized as described by Depamphilis and Cleland (Reference DePamphilis and Cleland1973), from an equimolar mixture of 5 mm Na2-ATP and 5 mm CrCl3 and the bidentate isomer was isolated through a cation-exchange column (Dowex 50-H+ resin). The product was confirmed by spectrophotometry, according to the absorption spectrum from 400 to 800 nm and quantified by its absorption at 610 nm (ε 610 nm=20 M−1cm−1). The final product was free of contaminating Cr3+ (Moreira et al. Reference Moreira, Rios, Esteves, Meyer-Fernandes and Barrabin2009).
Cultivation methods
Promastigote forms of Leishmania (Leishmania) amazonensis (MHOM/BR/77/LTB0016) were obtained from lesions of the hind footpads of BALB/c mice. The recently isolated promastigotes were maintained in axenic cultivation for up to 1 month, by weekly transfers in brain-heart-infusion (BHI) medium, supplemented with 10 mg/ml haemin and 10% (v/v) heat-inactivated fetal calf serum, at 28°C. After 4 days of growth, cells were harvested by centrifugation, washed 3 times in a medium containing 50 mm Hepes-KOH buffer, pH 7·2, 116 mm NaCl, 5·4 mm KCl and 5·4 mm D-glucose, and re-suspended in the same medium.
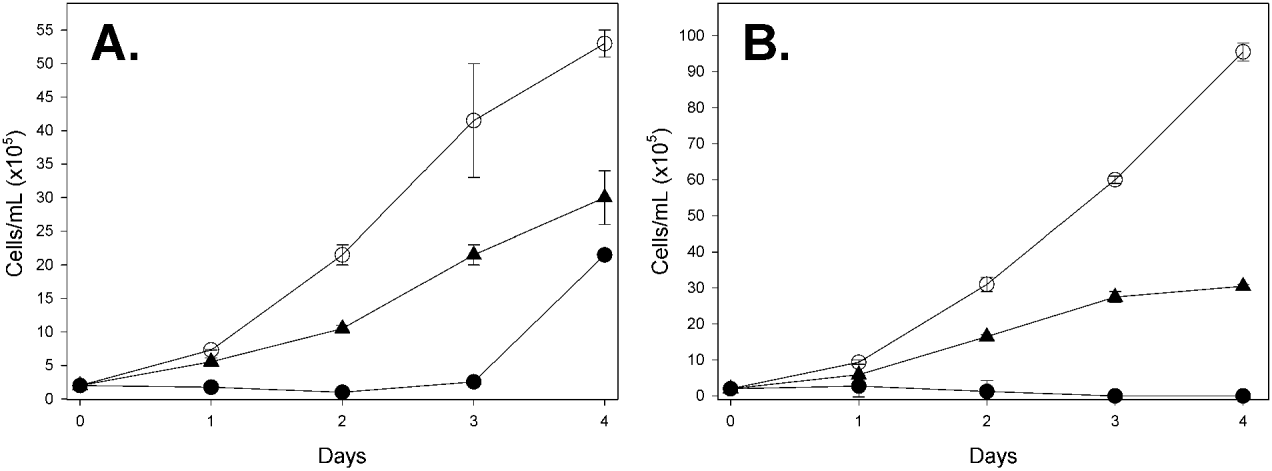
The effect of CrATP on the multiplication of promastigote forms of L. amazonensis in axenic cultivation was assayed in culture cell plates by incubating 2·0×105 cells/ml in 200 μl of cultivation medium, as described previously, in the presence or absence of 50 μ m or 500 μ m CrATP. After 24, 48, 72 and 96 h of growth, 10 μl aliquots were withdrawn from the cultivation medium, under aseptic conditions, and the number of cells was measured by counting in a Neubauer chamber (Fig. 3A). Alternatively, to reduce the possible effect of CrATP liability in the growth medium, the cells were harvested daily by centrifugation and re-suspended in a fresh medium, supplemented with new aliquots of 50 μ m or 500 μ m CrATP (Fig. 3B).
Ecto-ATPase activity measurements
The ecto-ATPase activity was measured as described by Berredo-Pinho et al. (Reference Berrêdo-Pinho, Peres-Sampaio, Chrispim, Belmont-Firpo, Lemos, Martiny, Vannier-Santos and Meyer-Fernandes2001) with slight modifications. Briefly, all intact parasites were incubated at 28°C for 1 h in 500 μl of a medium containing, unless otherwise specified, 50 mm Hepes-KOH buffer, pH 7·2, 116 mm NaCl, 5·4 mm KCl, 5·4 mm D-glucose, 2 mm ATP and 1·0×108 cells/ml, in the presence or absence of 5 mm MgCl2. In the case of absence of MgCl2, 300 μ m EDTA was added. The Mg2+-dependent ecto-ATPase activity was calculated from the difference between the total activity, measured in the presence of 5 mm MgCl2, and the basal activity, measured in the absence of 5 mm MgCl2 (Figs 1 and 2). ATPase activity was determined by measuring the hydrolysis of [γ 32P]ATP (104 Bq/nmol ATP). The experiments were started by the addition of intact promastigotes and stopped by the addition of 1 ml of a cold mixture containing 200 mg charcoal in 0·1 m HCl. The tubes were centrifuged at 1500 g for 10 min at 4°C. Then, 750 μl aliquots of the supernatants containing the released inorganic phosphate (32Pi) were deposited on filter paper and, after drying, transferred to scintillation vials containing 9 ml of scintillation fluid (2 g/L 2,5-diphenyloxazole (PPO) in toluene). The ATPase activity was calculated by subtracting the non-specific ATP hydrolysis measured in the absence of cells.
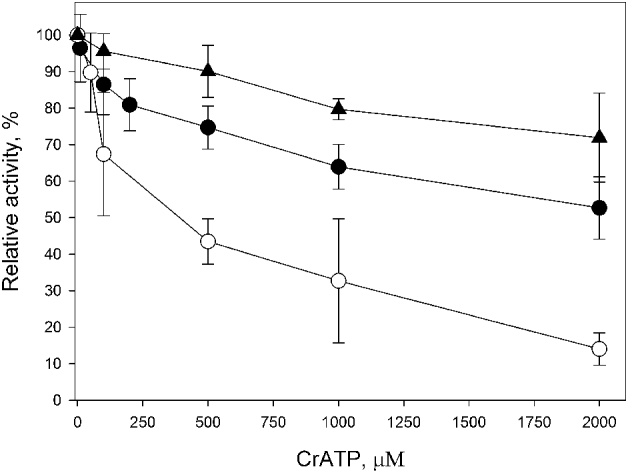
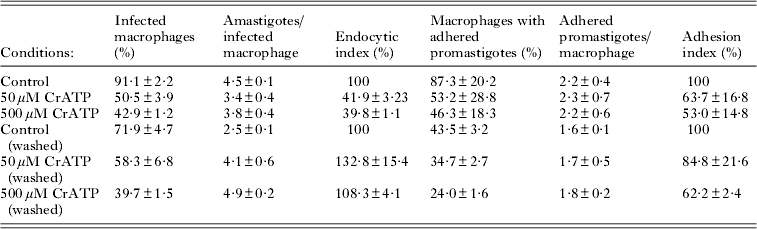
Fig. 1. Effect of increasing concentrations of CrATP on the ecto-ATPase activity (n=3). Intact cells were incubated for 1 h at 28°C, in a medium containing 50 mm Hepes-KOH buffer, pH 7·2, 116 mm NaCl, 5·4 mm KCl, 5·4 mm D-glucose, 2 mm [γ-32P]ATP (4000 cpm/nmol), in the presence (●) or absence (○) of 5 mm MgCl2. In the latter, 300 μ m EDTA was added. The Mg2+-dependent ecto-ATPase activity (▲) was estimated by the difference between the activities measured in the presence and absence of 5 mm MgCl2. Values representing 100% activities and Ki were, respectively, 137·5±2·3 nmol Pi/108 cel×h and 575·7±199·1 μ m (○), 45·4±3·4 nmol Pi/108 cel×h and 383·5±79·0 μ m (●) and 92·1±3·0 nmol Pi/108 cel×h and 1961±926 μ m (▲).

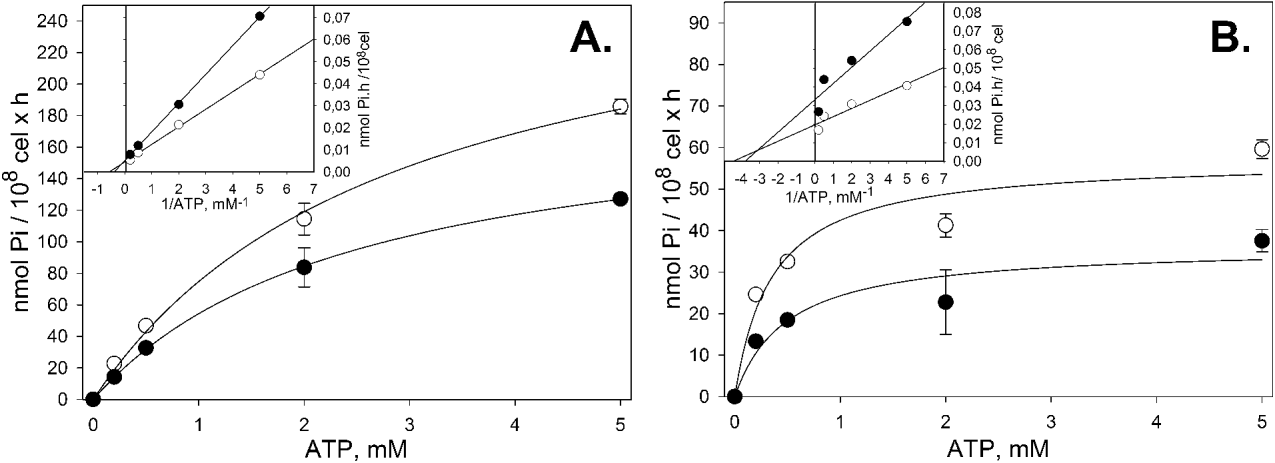
Fig. 2. ATP dependence in the CrATP inhibition of Mg2+-dependent (A) and Mg2+-independent (B) ecto-ATPase activities (n=3). (A) The Mg2+-dependent ecto-ATPase activity: intact cells were incubated for 1 h at 28°C, in the presence (●) or absence (○) of 200 μ m CrATP, in a medium containing 50 mm Hepes-KOH buffer, pH 7·2, 116 mm NaCl, 5·4 mm KCl, 5·4 mm D-glucose and increasing concentrations of [γ-32P]ATP. The Mg2+-dependent ecto-ATPase activity corresponds to the difference of activities measured in the presence and absence of 5 mm MgCl2. In the latter, 300 μ m EDTA was added. Control activity: Vmax=290·2±23·4 nmol Pi/108 cel×h; KM=2·9±0·5 mm. CrATP-inhibited activity: Vmax=190·2±3·5 nmol Pi/108 cel×h; KM=2·5±0·1 mm. (B) The Mg2+-independent ecto-ATPase activity: intact cells were incubated as described in (A). The Mg2+-independent ecto-ATPase activity corresponds to the activity measured only in the absence of MgCl2 (300 μ m EDTA added). Control activity: Vmax=57·2±7·7 nmol Pi/108 cel×h; KM=0·4±0·2 mm. CrATP-inhibited activity: Vmax=36·2±7·0 nmol Pi/108 cel×h; KM=0·5±0·3 mm. In both graphics, the insets show the Lineweaver-Burk plots of the experiments: (○) Control curve, (●) 200 μ m CrATP curve.
Cellular viability was assessed, before and after incubation, by motility and trypan blue exclusion. For trypan blue staining, the cells were incubated in the presence of 0·01% trypan blue for 10 min in the buffer used in each experiment (Dutra et al. Reference Dutra, Dias, Santos, Rodrigues, Romeiro, Attias, De Souza, Lopes and Meyer-Fernandes2001a). The viability was not affected under the conditions employed here.
Toxicity of CrATP on mammalian and Leishmania cells
In order to investigate the toxic effects of CrATP on mammalian or Leishmania amazonensis cells, uninfected resident peritoneal macrophages, collected from BALB/c mice (Menna-Barreto et al. Reference Menna Barreto, Corrêa, Pinto, Soares and de Castro2007), or promastigote forms of L. amazonensis were incubated, respectively, at 37°C or 28°C with 50 or 500 μ m of this compound for 48 h, and the cell death rate was measured by a dye-reduction assay (Mosmann et al. Reference Mosmann1983). Briefly, the cells were incubated with 0·5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide dye (MTT) for 3 h in the dark at 37°C. Subsequently, dimethylsulfoxide (DMSO) was added to dissolve the formazan crystals formed and the absorbance was read at 490 nm. Concentrations of CrATP capable of maintaining more than 85% macrophage viability were used in the interaction assays.
Leishmania-macrophage interaction assay
Parasite-macrophage interaction was performed as follows. BALB/c mice peritoneal resident macrophages were re-suspended in 300 μl of Dulbecco's modified Eagle's Medium (DMEM), supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (0·1 mg/ml), added to 24-well plates, and maintained for 24 h at 37°C in 5% CO2. Stationary-phase promastigotes were harvested by centrifugation and re-ssuspended with Hanks’ balanced salt solution, and parasites (3·0×107 cells/ml), previously treated with 50 or 500 μ m CrATP for 1 h in a sterile medium containing 50 mm Hepes-KOH buffer, pH 7·2, 116 mm NaCl, 5·4 mm KCl, 5·4 mm D-glucose and 5 mm MgCl2, were washed or not in a similar fresh medium and then added to the macrophage cultivation (ratio 10:1 parasites/host cell). After 2 h of interaction, free parasites were removed by successive washes with PBS and the cells were fixed in methanol, Giemsa-stained and dehydrated in acetone solutions progressively replaced by xylol. The assembly of the slides was done with Permount. The percentage of infected macrophages was determined by randomly counting at least 100 cells in each of duplicated cover-slips using a light microscope. The adhesion index was obtained by multiplying the percentage of macrophages with adhered parasites by the average of the ratio of adhered parasites per macrophage. The endocytic index was obtained by multiplying the percentage of infected macrophages by the average of the ratio promastigotes per infected macrophages. Experiments were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA L-0006/07, Protocol 019-07).
Statistical analysis
All experiments were repeated at least 3 times in triplicate, and the medium and standard deviation of the results of these experiments are shown. The data were analysed statistically using Student's t-test from SigmaPlot for Windows Version 11.0 Build 11.0.0.77 computer software.
RESULTS
As described previously, L. amazonensis presents Mg2+-dependent ecto-ATPase activity on its external surface (Berredo-Pinho et al. Reference Pinheiro, Martins-Duarte, Ferraro, Fonseca De Souza, Gomes, Lopes, Vannier-Santos, Santos and Meyer-Fernandes2001; Pinheiro et al. Reference Pinheiro, Martins-Duarte, Ferraro, Fonseca De Souza, Gomes, Lopes, Vannier-Santos, Santos and Meyer-Fernandes2006; De Souza et al. Reference De Souza, De Assis, Gomes, Marques da Silva, Melo, Fietto and Afonso2010). Here, we showed that, in the absence of Mg2+ (obtained by EDTA treatment), intact cells showed a basal ecto-ATPase activity of 45·4±3·4 nmol Pi/108 cel×h, that increased to 137·5±2·3 nmol Pi/108 cel×h by the addition of 5 mm MgCl2, showing a Mg2+-dependent ecto-ATPase activity of 92·1±3·0 nmol Pi/108 cel×h (Fig. 1). CrATP inhibited the ecto-ATPase activities either in the presence or absence of MgCl2, in a dose-dependent manner: in the absence of MgCl2, CrATP inhibited the ecto-ATPase activity with a Ki of 383·5±79·0 μ m. In the presence of 5 mm MgCl2, the Ki was 575·7±199·1 μ m (Fig. 1). In the range tested, CrATP partially inhibited the Mg2+-dependent ecto-ATPase activity, with a Ki of 1961±926 μ m. However, in the absence of MgCl2, the basal ecto-ATPase activity seemed to be fully sensitive to CrATP inhibition. Furthermore, inhibition of both activities was reversible, since washing the cells after 1 h of incubation with 1 mm CrATP fully restored the ecto-ATPase activity (data not shown).
In order to elucidate the mechanism of Mg2+-dependent and Mg2+-independent ecto-ATPase inhibition, the ATP dependence on the CrATP inhibition was investigated (Fig. 2). An increasing Mg2+-dependent ecto-ATPase activity was observed, with a Vmax=290·2±23·4 nmol Pi/108 cel×h and a KM=2·9±0·5 mm. In the presence of 200 μ m CrATP, a lower Vmax=190·2±3·5 nmol Pi/108 cel×h was detected, but with a similar KM=2·5±0·1 mm (Fig. 2A). A similar profile was observed to the Mg2+-independent ecto-ATPase activity, with a Vmax=57·2±7·7 nmol Pi/108 cel×h and a KM=0·4±0·2 mm. In the presence of 200 μ m CrATP, also, a lower Vmax=36·2±7·0 nmol Pi/108 cel×h was observed, but with a similar KM=0·5±0·3 mm (Fig. 2B). The insets in Fig. 2 show the Lineweaver-Burk plots for this experiment. To investigate the effect of CrATP on the L. amazonensis multiplication in axenic cultivation, we incubated promastigote forms with 50 μ m or 500 μ m CrATP and followed the time-course of cellular growth. We observed that 50 μ m CrATP partially inhibited the cellular growth of L. amazonensis. On the other hand, 500 μ m CrATP fully inhibited the cellular growth up to the third day (Fig. 3A). After this time, the cells resumed growth, probably due to the self-degradation of CrATP at pH 7·2 after a long period (Dephamphilis and Cleland, Reference DePamphilis and Cleland1973). In order to confirm this hypothesis, we performed a similar time-course cellular growth experiment, in which the cultivation medium was replaced daily for a fresh sample, supplemented with 50 μ m or 500 μ m CrATP (Fig. 3B). Thus, fresh CrATP remained in contact with L. amazonensis throughout the experiment. In this condition, we observed that 500 μ m CrATP completely inhibited the cellular growth until the end of the growth curve. In the case of 50 μ m, as expected, only a partial inhibition was observed.

Fig. 3. Effect of CrATP on the growth profile of promastigote forms of Leishmania amazonensis (n=3). 2×105 L. amazonensis cells/ml were cultivated at 28°C in 200 μl of BHI medium, supplemented with 10% fetal bovine serum. (A) Cells were cultivated in the (○) absence or in the presence of (▲) 50 μ m CrATP or (●) 500 μ m CrATP for 4 days. Daily, 10 μl aliquots were withdrawn from the cultivation medium, under aseptic conditions, and the number of cells was measured by counting in a Neubauer chamber. (B) Cells were cultivated as described in (A) with modification as follows. After the daily estimation of cell number, cells were harvested by centrifugation and re-suspended in a fresh medium, supplemented with fresh aliquots of (▲) 50 μ m or (●) 500 μ m CrATP. (○) Control curve, with no CrATP addition.
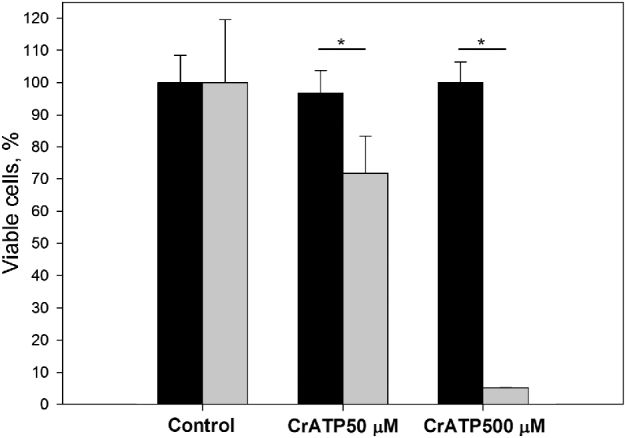
In order to evaluate the possible toxic effects of CrATP in L. amazonensis and murine macrophages, 50 μ m or 500 μ m CrATP was incubated with murine macrophages or promastigote forms of L. amazonensis for 48 h, followed by evaluation of cell viability through the MTT assay, as described in the Materials and Methods (Fig. 4). We observed no deleterious effect for either 50 μ m or 500 μ m CrATP on BALB/c macrophages. In contrast, less than 5% of promastigote forms of L. amazonensis remained viable when incubated with 500 μ m CrATP under this condition.

Fig. 4. Evaluation of CrATP cytotoxicity to BALB/c mouse macrophages and promastigote forms of Leishmania amazonensis (n=6). The viability of macrophages (black bars) and L. amazonensis (grey bars) was determined spectrophotometrically at 490 nm by means of MTT assay, after incubation with CrATP for 48 h, at 37°C (macrophages) or 28°C (L. amazonensis), as described in the Materials and Methods section. *P<0·05.
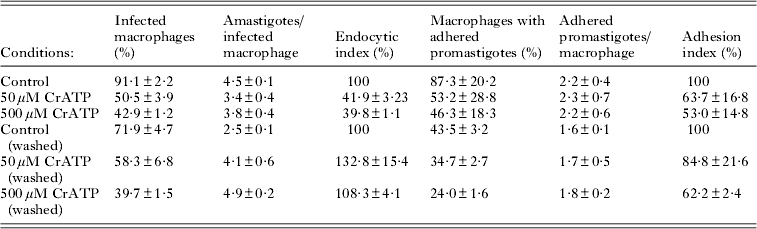
The effects of CrATP on the L. amazonensis-macrophage interaction are shown in Table 1. At the drug concentrations tested (50 and 500 μ m), the parasites maintained their viability after the treatment for 1 h, as judged by their morphology, motility, trypan blue exclusion and MTT assay, in which more than 95% of the promastigotes were viable (data not shown). Therefore, after this time, cells were washed or not with PBS, and treated or untreated parasites were allowed to interact with macrophages for 2 h (Table 1). Under this experimental condition, CrATP treatment significantly decreased both endocytic and adhesion indices. Surprisingly, when CrATP-treated promastigotes were washed before the interaction assay, the endocytic index recovered to about 132·8% and 108·3% in relation to the control, respectively for 50 and 500 μ m CrATP samples. This effect was less pronounced for the adhesion index, where the washing raised the index from 63·7 to 84·8%, and from 53·0 to 62·2%, respectively for 50 and 500 μ m CrATP treatment. These results are in agreement with the observed reversible inhibition of ecto-ATPase activity by CrATP.
Table 1. The effect of CrATP on the adhesion and internalization of promastigote forms of Leishmania amazonensis by resident peritoneal macrophages of BALB/c mice (n=5)
Representative images of the L. amazonensis-macrophage interaction are shown in Fig. 5. In the control (A), it is possible to observe promastigote forms adhered to the macrophage surface, in addition to amastigotes and promastigotes turning into amastigotes inside macrophages. In (B) and (C), the promastigotes were pre-incubated respectively with 50 μ m or 500 μ m CrATP and directly transferred to the macrophage's interaction assay. It is possible to observe a similar image in both, with promastigotes of normal size and shape, although the number of macrophages with adhered or internalized parasites seems to be reduced.

Fig. 5. Representative images of the effect of CrATP in Leishmania amazonensis–macrophage interactions. (A) Control. Promastigotes incubated with (B) 50 μ m CrATP or (C) 500 μ m CrATP. The images represent the results of Leishmania amazonensis–macrophage interactions, without washing the promastigotes after incubation with CrATP, as described in Table 1. The Scale bars represent 10 μm.
DISCUSSION
Several hypotheses have been proposed for the function of ecto-ATPases in trypanosomatids, which include acquisition of adenosine from the media, necessary for normal growth, modulation of parasite infection and virulence, and involvement in cellular adhesion (Berrêdo-Pinho et al. Reference Berrêdo-Pinho, Peres-Sampaio, Chrispim, Belmont-Firpo, Lemos, Martiny, Vannier-Santos and Meyer-Fernandes2001; Meyer-Fernandes, Reference Meyer-Fernandes2002; Bisaggio et al. Reference Bisaggio, Peres-Sampaio, Meyer-Fernandes and Souto-Padron2003; Pinheiro et al. Reference Pinheiro, Martins-Duarte, Ferraro, Fonseca De Souza, Gomes, Lopes, Vannier-Santos, Santos and Meyer-Fernandes2006; Peres-Sampaio et al. Reference Peres-Sampaio, de Almeida-Amaral, Giarola and Meyer-Fernandes2008; Santos et al. Reference Santos, Pôssa, Bastos, Guedes, Almeida, Demarco, Verjovsky-Almeida, Bahia and Fietto2009; De Souza et al. Reference De Souza, De Assis, Gomes, Marques da Silva, Melo, Fietto and Afonso2010). However, the precise function of these enzymes is still an open question. CrATP has been used as a kinetic and structural tool because of its capacity to inhibit a series of enzymes that use the MgATP complex as substrate. In a previous study (Moreira et al. Reference Moreira, Rios, Esteves, Meyer-Fernandes and Barrabin2009), we characterized CrATP as an inhibitor of ecto-ATPase activity of monoxenic and heteroxenic trypanosomatids. We observed that CrATP could be used as a valuable selective tool for a better understanding of the properties and role of ecto-ATPases in the biology of parasites. In this work, this compound was used to investigate the involvement of ecto-ATPases in the infection of murine macrophages by Leishmania amazonensis. The effect of this compound on the cellular growth of L. amazonensis and its toxicity to BALB/c peritoneal macrophages and promastigote forms of L. amazonensis was also evaluated.
Ecto-enzymes that can hydrolyse phosphorylated compounds have been described in trypanosomatids as nucleoside triphosphate diphosphohydrolases (NTPDase1), ecto-ATPases (NTPDase2) and ecto-phosphatases. In L. amazonensis promastigotes, an ecto-ATPase activity, stimulated by Mg2+, was described on its external surface (Berrêdo-Pinho et al. Reference Berrêdo-Pinho, Peres-Sampaio, Chrispim, Belmont-Firpo, Lemos, Martiny, Vannier-Santos and Meyer-Fernandes2001; Pinheiro et al. Reference Pinheiro, Martins-Duarte, Ferraro, Fonseca De Souza, Gomes, Lopes, Vannier-Santos, Santos and Meyer-Fernandes2006). This activity was sensitive to the membrane-impermeable inhibitors DIDS (4,4′-diisothiocyanostylbene 2′,2′-disulfonic acid) and suramine, but was not affected by the presence of vanadate, which discarded the possibility of a plasma membrane P-type ATPase. On the other hand, this Mg2+-dependent-ecto-ATPase was not able to hydrolyse ADP whereas, in the absence of Mg2+, ADP hydrolysis was observed. It suggests that L. amazonensis presents both a Mg2+-dependent ecto-NTPDase 1 and a Mg2+-independent ecto-NTPDase 2. In this study, we have shown that CrATP is able to inhibit both enzymes in a dose-dependent manner, with Ki at the micromolar range. In the presence of Mg2+, the ecto-ATPase activity was less sensitive to CrATP, showing a nearly 2-fold Ki value when compared to that observed in the absence of Mg2+. Also, the inhibition of the Mg2+- dependent and Mg2+-independent ecto-ATPase activities was not dependent on ATP concentration. The KM values for these activities were very similar in the presence or absence of CrATP, but the maximal velocity was significantly lower in the presence of the inhibitor. Considering that CrATP is a reversible inhibitor of ecto-ATPase activities (Moreira et al. Reference Moreira, Rios, Esteves, Meyer-Fernandes and Barrabin2009), we suggest that it could be acting as a non-competitive inhibitor of Mg2+-dependent ecto-ATPase activity in L. amazonensis.
In order to test the cytotoxic effect caused by an inhibitor of ecto-ATPase, the ability of CrATP to inhibit L. amazonensis growth was also investigated. It was observed that 500 μ m CrATP drastically inhibited cellular growth in axenic cultivation until the third day. From there on, a moderate recovery of cell multiplication was observed. One possible explanation is that CrATP could be degraded in the cultivation medium, allowing the recovery of cellular growth after 3 days. Accordingly, DePamphilis and Cleland (1973) described that the active bidentate isomer of CrATP is unstable at neutral or alkaline pH. To test the CrATP degradation hypothesis, a similar growth curve was performed, in which the cells were harvested every day and incubated in a fresh medium supplemented with 50 μ m or 500 μ m fresh CrATP aliquots. In this scenario, CrATP degradation was virtually avoided. We observed an absolute growth inhibition of the promastigote forms of L. amazonensis with 500 μ m CrATP, which continued throughout all the experiment. When 50 μ m CrATP was used, a moderate effect was observed. It seems that a direct effect of CrATP in promastigote forms of L. amazonensis exists. Previous tests of our group showed that, in the absence of other nucleotides, CrATP was not hydrolysed by ecto-enzymes of some trypanosomatids (Moreira et al. Reference Moreira, Rios, Esteves, Meyer-Fernandes and Barrabin2009 and unpublished data). Moreover, due to the negative charge of ATP phosphates, it is unlikely that CrATP can directly cross the plasma membrane of L. amazonensis. Nevertheless, the possibility of a small fraction of CrATP reaching the cytoplasm by pinocytosis, mainly after several hours of incubation, cannot be discarded. Thus, regarding the ecto-enzymes, this compound can be considered a non-hydrolysable, membrane-impermeable inhibitor. In this sense, we can raise the possibility that the ecto-nucleotidase of L. amazonensis plays an important role on cell growth, since CrATP has caused a significant decrease in the cellular growth curve. Similar effects were observed in Trypanosoma cruzi, when the ecto-ATPase activity of the inhibitors DIDS and suramine arrested the growth of epimastigote forms (Bernardes et al. 2000; Bisaggio et al. Reference Bisaggio, Peres-Sampaio, Meyer-Fernandes and Souto-Padron2003), suggesting that these enzymes are involved in basic cellular processes that are related to parasite growth. More experiments are necessary to show how this compound affects the trypanosomatid's metabolism, probably through ecto-ATPases inhibition.
The effect of CrATP in murine macrophages and L. amazonensis was also investigated through the evaluation of cell viability by the MTT assay. We chose the same concentrations intended for the interaction assays, 50 and 500 μ m. No significant deleterious effect was observed for macrophages and L. amazonensis within 1 h of incubation (data not shown), allowing the use of CrATP in the interaction experiments. In contrast, a significant cellular viability reduction was observed for L. amazonensis, in 48 h of incubation with CrATP.
The investigation of the ecto-ATPase contribution on the L. amazonensis-macrophage interaction, in the presence of CrATP, was described in Table 1. When promastigotes were incubated with CrATP for 1 h before the interaction with macrophages, it clearly decreased both adhesion and endocytic indices. As CrATP is a reversible inhibitor of ecto-ATPase activity, this effect might be related to the inhibition of the enzyme, once the washing of promastigotes after pre-incubation recovered the index levels, mainly the endocytic index. Taken together, these results suggest that the ecto-ATPase plays an important role in the macrophage invasion process by L. amazonensis., which corroborates with previous data obtained by Pinheiro et al. (Reference Pinheiro, Martins-Duarte, Ferraro, Fonseca De Souza, Gomes, Lopes, Vannier-Santos, Santos and Meyer-Fernandes2006) and De souza et al. (2010).
Recently, Souza et al. (Reference Souza, Veras, Welby-Borges, Silva, Leite, Ferraro, Meyer-Fernandes, Barral, Costa and Freitas2011) used different strains of L. amazonensis (Ba125, Ba276 and Ba109), isolated from patients with different clinical forms of leishmaniasis to infect CBA mice. They observed differences in the evolution of the lesions, the inflammatory response, the number of parasites at the site of infection and the time of dissemination of parasites to internal organs, suggesting that, beyond the host immune response, intrinsic characteristics of different isolates of the parasite may influence the expression of different chemokines and attract different cells to the site of infection. In fact, they observed that those different isolates had presented, among other characteristics, distinct ecto-enzyme activities, including ecto-ATPase, ecto-ADPase and 5′-nucleotidase. Ba109 promastigotes displayed higher ecto-ATPase activity than those from Ba276 and Ba125. Since ATP is known to activate ligand-gated ion channel P2X7 purinergic receptors, contributing to the inflammatory response and inducing the secretion of pro-inflammatory cytokines and the migration of inflammatory cells (Mizumoto et al. Reference Mizumoto, Kumamoto, Robson, Sévigny, Matsue, Enjyoji and Takashima2002; la Sala et al. Reference la Sala, Ferrari, Di Virgilio, Idzko, Norgauer and Girolomoni2003), they suggest that an increased ability to hydrolyse ATP can contribute to the differences observed in the composition and intensity of the inflammatory infiltrate induced in CBA mice by the distinct isolates of L. amazonensis. In conjunction with other recent papers related to ecto-enzymes in trypanosomatids (Santos et al. Reference Santos, Pôssa, Bastos, Guedes, Almeida, Demarco, Verjovsky-Almeida, Bahia and Fietto2009; de Souza et al. Reference De Souza, De Assis, Gomes, Marques da Silva, Melo, Fietto and Afonso2010), our results suggest that ecto-ATPase is related to infectivity in L. amazonensis with a potential to be used as a new prototype for drug development. In this sense, the inhibitory effects observed for CrATP suggest that further investigations in animal models could help to determine whether CrATP is a promising leading compound to be used on leishmaniasis chemotherapy.
ACKNOWLEDGMENTS
The authors thank Dr Luciana P. Rangel for critical reading of the manuscript and English revision.
FINANCIAL SUPPORT
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Fundação Oswaldo Cruz (FIOCRUZ).