Severe right ventricular obstruction in the context of complex congenitally malformed hearts is often associated with duct-dependent pulmonary circulation and hypoplastic pulmonary arteries. In patients with complex left ventricular obstructions, as in hypoplastic left heart syndrome, the arterial duct provides the systemic blood flow.
Surgical creation of systemic-to-pulmonary shunts is still the first step of palliation in the newborn period in such patients, in order to guarantee pulmonary perfusion and provoke growth of the hypoplastic pulmonary arteries in the setting of the obstructed right heart, or to restrict the pulmonary circulation in hypoplastic left heart syndrome.Reference de Leval, McKay, Jones, Stark and Macartney1–Reference Sano, Ishino and Kawada7 Various types of shunt are currently created, such as a modified Blalock-Taussig shuntReference de Leval, McKay, Jones, Stark and Macartney1–Reference Norwood, Lang and Hansen4 from the right or left subclavian artery to the right or left pulmonary artery, a central aortopulmonary shuntReference Gates, Laks and Johnson5, Reference Alkhulaifi, Lacour-Gayet, Serraf, Belli and Planché6 from the ascending aorta to the central pulmonary artery, or a shunt from the right ventricular outflow tract to central pulmonary arteries.Reference Sano, Ishino and Kawada7 All shunts are usually made of polytetrafluorethylene (W.L. Gore & Associates, Inc., Medical Products, Flagstaff, USA).Reference de Leval, McKay, Jones, Stark and Macartney1–Reference Norwood, Lang, Casteneda and Campbell3, Reference Sano, Ishino and Kawada7, Reference Ilbawi, Grieco and DeLeon8 Kinking of these shunts, and/or thrombosis, causing severe stenosis or even occlusion, are acute and lifethreatening complications.Reference Norwood, Lang and Hansen4, Reference Ilbawi, Grieco and DeLeon8–Reference Fenton, Siewers, Rebovich and Pigula10 In some cases, the arterial duct can be reopened by the administration of prostaglandin, which may temporarily improve the state of the patient,Reference Peuster, Fink, Windhagen-Mahnert, Paul and Hausdorf11 albeit that immediate repeated surgery with revision or replacement of the shunt is usally necessary. In this report, we discuss interventional reopening and stenting of the shunt as an alternative to surgery.
Patients and methods
We encountered 5 patients (Table) with acute failure of their shunts. None of the patients had any type of clotting disorder. In all patients, heparin was administered, and the prothrombin time was prolonged to at least 60 seconds in the early postoperative period. For discharge, anticoagulation was continued with acetylsalicylic acid at a dose of 2-3 milligrammes per kilogramme per day in 4 of 5 patients.
Complications occurred from between 8 hours to 53 days after creation of the shunt, with a mean of 26 days. On auscultation, the murmur generated by the shunt was noted to have decreased, with echocardiography demonstrating stenosis or occlusion. The arterial saturation of oxygen dropped from a mean of 80% to a mean of 59% (Fig. 1).

Figure 1 Saturations of oxygen after construction of the systemic-to-pulmonary arterial shunts, and before and after implantation of the stents.
Our first patient was an 18-day old girl with a double outlet right ventricle and pulmonary stenosis. She was transferred to our hospital 7 days after surgery at another institution with suspected obstruction of a 4-millimetre central aortopulmonary shunt. Prostaglandin infusion had been started 6 days previously, leading to reopening of the arterial duct. Angiography showed severe obstruction of the shunt, which was thrombosed and sharply bent, with kinking at the aortic origin of the shunt. There was an additional antegrade flow to the pulmonary artery via the pulmonary valve, and the arterial duct appeared to be open (Fig. 2). We implanted 2 stents (Cordis BX Velocity 4.5 × 8 millimetres, Cordis Europe, N.V. 93016 LJ Roden, NL and Biotronik Lekton Motion 4 × 8 millimetres, Biotronik GmbH+Co.KG, Woermannkehre 1, D-12359 Berlin). The patient then developed severe instent thrombosis three days after implantation (Fig. 3), and required redilation of the stent (Fig. 4). Saturation increased again after the second procedure by up to 83%. The patient was put on warfarin until the elective next operation, which was performed three months after implantation without complications.
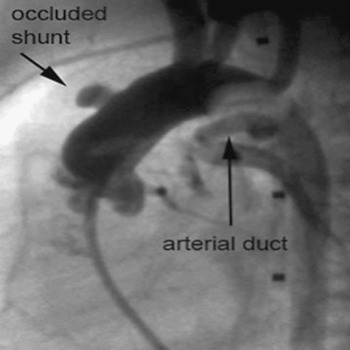
Figure 2 Occlusion of a central shunt with additional kinking before dilation. As seen in the lateral view, the arterial duct was re-opened by an infusion of Prostaglandin.
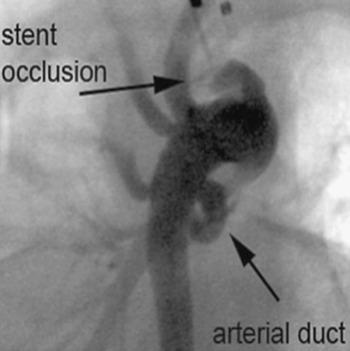
Figure 3 Secondary occlusion of a central shunt due to instent thrombosis, shown in an antero-posterior view angulated at 45° caudally to produce the “laid-back view”.
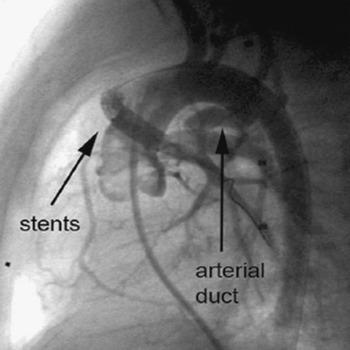
Figure 4 The result of re-dilation of the instent thrombosis is shown in the lateral view.
The second patient was a 48-day-old girl with tetralogy of Fallot and pulmonary atresia who was transferred to our hospital 23 days after surgical insertion of a 4-millimetre central aortopulmonary shunt at another institution. At an interval of 16 days after surgery, the saturation of oxygen dropped significantly, and treatment with infusion of prostaglandin was started, resulting in a reopening of the arterial duct. The patient reached our hospital in a poor clinical state, with cardiopulmonary arrest, and was resuscitated successfully before admission to the catheterization laboratory. Angiography showed thrombosis of a sharply bent shunt. We implanted 2 stents (Cordis BxSonic 3 × 8 millimetres and Cordis Genesis PG 1240 PSS 4 × 12 millimetres, Cordis Europe, N.V. 93016 LJ Roden, NL). She was transferred to the referring hospital on the day after the procedure, but sadly died one month later due to a septicaemia, unrelated to the procedure.
The third patient was a 12-day-old boy with tetralogy of Fallot, who had undergone surgery 8 hours previously with implantation of a 4-millimetre right-sided modified Blalock-Taussig shunt. There was additional antegrade flow via the pulmonary valve. Angiography showed subtotal obstruction of the shunt due to thrombosis despite infusion of heparin. The patient was treated by implantation of one stent (Cordis Genesis PG 1240 PSS 4 × 12 millimetres, Cordis Europe, N.V. 93016 LJ Roden, NL).
The fourth and fifth patients, aged 62 days and 30 days, both had hypoplastic left heart syndrome, and were seen 54 and 25 days, respectively after the first stage of the Norwood sequence, which had involved placement of 5 millimetre shunts from the right ventricle to the pulmonary arteries. Both shunts showed severe narrowing due to a short proximal kinking (Fig. 5). In this situation, we attempted to cover only the kinked part of the shunt, approximately onethird, by inserting relatively short stents, hoping to overcome and straighten the kinking. We inserted a Cordis Genesis PG 1260 PSS, 6 × 12 millimetres (Cordis Europe, N.V. 93016 LJ Roden, NL) shunt in the fourth patient, and an Evolution 2, 4 × 15 millimetres, (Orbus Medical Technologies Inc. 5363 NW, 35th Ave Fort Lauderdale, FL 33309, USA) in the final patient. In this latter patient, secondary kinking occurred after successful implantation, probably caused by the stent itself. We therefore placed a second stent, Evolution 2, 4 × 13 millimetres (Orbus Medical Technologies Inc. 5363 NW, 35th Ave Fort Lauderdale, FL 33309, USA), to correct the newly created kinking. It proved possible easily to advance and implant this second stent, using the guide wire which was still in place. The 2 stents covered over twothirds of the length of the shunt at the end of the interventional procedure.
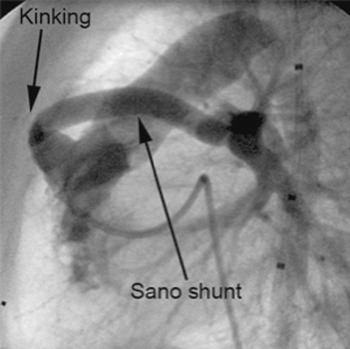
Figure 5 Kinking in the proximal part of a shunt placed from the right ventricle to the pulmonary arteries for palliation of hypoplasia of the left heart, shown in the lateral view.
Cardiac catheterization
All patients were admitted to the catheterization laboratory within less than one hour after diagnosis of shunt failure or after admission to our hospital. All procedures were performed under general anaesthesia (Table 1). Selective angiographies directly into the shunt or at the origin of the shunt were performed to visualize the anatomy using 4-French end-open catheters, such as Cobra catheters or right Judkins catheters (Cordis Europe N.V., LJ Roden, Netherlands).
Table 1 Characteristics of the patients.

Abbreviations used: DORV: double outlet right ventricle; PS: pulmonary stenosis; TOF: tetralogy of Fallot; TOF/PA: tetralogy of Fallot with pulmonary atresia; HLHS: hypoplastic left heart syndrome; TCPC: total cavopulmonary connection.
A 0.014-inch coronary guide wire could be pushed through the shunt with the tip far down into the peripheral pulmonary artery. In one patient with an open pulmonary artery, we were able to push the wire via the shunt across the right ventricular outflow tract and the tricuspid valve into the superior caval vein.
Rapid exchange coronary balloon systems (RX Driver, Medtronic, Inc. Minneapolis, MN 55432 USA) and premounted stent systems were used for predilation and stenting (Fig. 6). The diameter of the balloon for predilation was chosen exactly according to the diameter of the shunt. The balloons were inflated by the maximum pressure in order to provide optimal fixation.
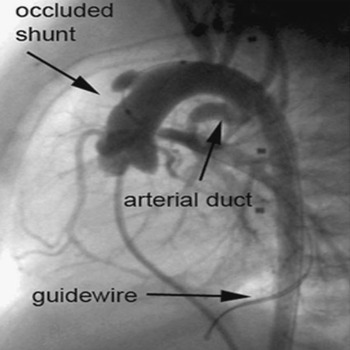
Figure 6 Immediate re-occlusion of a central shunt with additional kinking after dilation of the shunt, as seen in the lateral view.
Results
We implanted a total of 8 stents in our 5 patients during 6 procedures, choosing balloons with a ratio of their diameter to that of the shunt from 0.9 to 1. All procedures were straightforward and successful, but 3 patients needed 2 stents to reopen and stabilise the shunt. The period of fluoroscopy ranged between 8 and 69 minutes, with a mean of 25 minutes. Saturations of oxygen increased in all patients (Fig. 1). A complication occurred subsequent to the intervention in one patient when the shunt thrombosed 3 days after implantation of two stents, requiring interventional recanalization and redilation. Another patient experienced a problem at the site of cannulation of the femoral artery even though we used 4-French sheaths on the arterial side only. The pulses in this patient remained poor until discharge. None of the patients died in relation to the cardiac catheterization.
Follow-up
Angiography was performed in 3 patients to demonstrate the anatomy of the shunt and the pulmonary arteries before further surgery. Further surgical procedures were performed in the first and 4th patients, bidirectional cavopulmonary connections being created 3 and 6 months after implantation of the stents, while biventricular repair was achieved in the 3rd patient 10 months after the intervention.
During the operations, it proved easy to remove the shunts with the stents inside them. As judged macroscopically, there were multiple impressions showing how the shunt had been distorted by implantation of the stent and subsequent surgical manipulation. Histopathologic examination showed no signs of corrosion at the struts of the stent, while the shunt material remained intact. Intimal hyperplasia was visible in all parts of the examined tissue, but was not judged to have caused significant stenosis within the lumen. Thrombotic material was seen, but with no signs of cellular organisation, this finding being interpreted as intraoperative formation of thrombus (Figs 7 and 8). The next stage of surgical repair is awaited by one patient.

Figure 7 Macroscopic views of resected central shunt, (a) demonstrating intact struts. (b) The material of the shunt showed impressions of clamps used intraoperatively. Internal wall could not be assessed.
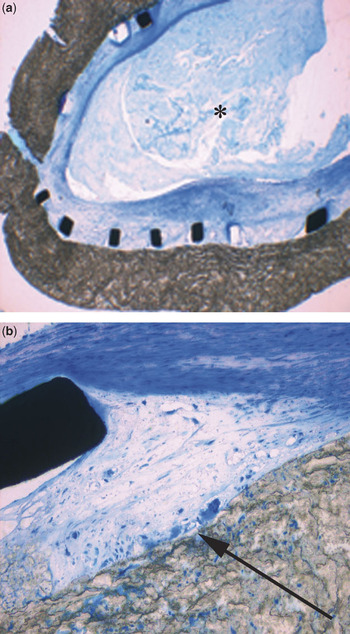
Figure 8 Microscopic views of resected central shunts. (a) The struts adjacent to the wall of the shunt show no signs of corrosion, although intimal hyperplasia is seen within the struts. Thrombus material inside the lumen (*) did not show signs of cellular organisation. (b) Apart from a minor foreign-body-reaction at the interface of the shunt and the stent (arrow), there are no signs of inflammatory reaction.
We transferred the patient with tetralogy of Fallot and pulmonary atresia, with the central aorto-pulmonary shunt, back to the referring hospital, where the patient died one month after the implantation due to a septicaemia unrelated to the procedure.
Discussion
Neonates with duct-dependent pulmonary circulation, or hypoplastic left heart syndrome, are usually treated with systemic-to-pulmonary shunts. Acute and chronic complications after such surgery are well recognised. Stenosis or scar formation at the systemic arterial or ventricular connection, or at the pulmonary arterial connection, are well-known long-term problems, as well as distortion of the pulmonary arteries and, subsequently, variability in supply of blood to, and varying growth of, the left and right pulmonary arteries.Reference Godart, Qureshi and Simha12–Reference Kirklin, Bargeron and Pacifico16 All of these problems can cause gradual deterioration of the general state of the patient, and reduction in saturations of oxygen. When occurring acutely, such complications can be lifethreatening events, producing a rapid decrease in arterial saturations of oxygen.
In our small group of patients, the acute complications encountered included thrombosis, kinking, or both. Kinking, along with a sharply bent course of the two central shunts, may have contributed to the thrombosis. Our only patient in whom a Blalock-Taussig-shunt remained without kinking had an additional antegrade source supplying blood to the lungs. All of these findings were associated with formation of thrombus within the shunt. None of the patients had an additional clotting disease, and all had been prescribed oral acetylsalicylic acid at a dose of 2 to 3 miligrams per kilogram per day after construction of the shunts.Reference Mullen, Lemermeyer and Bentley17
When acute complications occur, immediate treatment is essential. Before transferring such patients to a specialized centre, it is possible to administer prostaglandin E, providing the arterial duct has not been surgically closed.Reference Peuster, Fink, Windhagen-Mahnert, Paul and Hausdorf11 Administration of heparin, or an alternative form of lysis, should be considered at the same time.Reference Moszura, Ostrowska, Dryzek, Moll and Sysa18, Reference Malm, Holmqvist and Olsson19 Immediate transfer to a paediatric heart centre is crucial.
On arrival at the specialised centre, cardiac catheterization should be performed as soon as possible. Angiography can visualize precisely the cause of dysfunction, and offers interventional treatment at the same time.Reference Peuster, Fink, Windhagen-Mahnert, Paul and Hausdorf11, Reference Peuster, Fink, Bertram, Paul and Hausdorf20–Reference Sivakumar, Anil, Ravichandra, Natarajan, Kamath and Kumar24
Balloon dilation is able to increase the inner lumen of the shunts, but in presence of kinking or formation of thrombus can be followed by residual obstruction or re-occlusion. In contrast to dilation, implantation of stents leads to a definitive restoration of the shunts to their original diameter. In one patient with a sharply angled and stretched central shunt, however, thrombosis was observed 3 days after implantation of the stent, which was redilated successfully. Warfarin was given to this patient over a period of 3 months until it proved possible to perform the next elective surgical procedure. We continued to administer acetylsalicylic acid in the other 4 patients.
Dilation and stenting of the shunt in the setting of thrombotic obstruction theoretically produces a high risk of embolisation of the thrombotic material to the pulmonary arteries. This did not occur in our limited experience, so we presume that the thrombotic material was pressed against the inner side of the shunt. We excluded perfusion deficits in the central or peripheral pulmonary arteries by repeated angiographies. Histopathologic workup demonstrated regular endothelization without any instent restenosis due to severe intimal hyperplasia.Reference Sigler, Bartmus and Paul25
In conclusion, our experience has demonstrated the safety and efficacy of implantation of stents in stenosed or even occluded systemic-to-pulmonary arterial shunts, permitting patients to be returned to their planned surgical route. Larger series are needed to compare use of different patterns of stents in different types of shunt.











