INTRODUCTION
Representatives of the genus Haemogregarina Danilewsky, 1885 are heteroxenous intracellular parasites that infect various species of turtles worldwide (Acholonu, Reference Acholonu1974; Siddall and Desser, Reference Siddall and Desser1992; Mihalca et al. Reference Mihalca, Achelaritei and Popescu2002; Telford et al. Reference Telford, Norton, Moler and Jensen2009; Davis and Sterrett, Reference Davis and Sterrett2011; Hossen et al. Reference Hossen, Bandyopadhyay and Gürelli2013). Their life cycle includes gamogony and sporogony in leeches, and erythrocytic and extra-erythrocytic merogony in chelonian hosts (Desser, Reference Desser and Kreier1993; Siddall, Reference Siddall1995). The complete life cycle gives rise to numerous morphologically different life stages, often with temporary occurrence (Desser, Reference Desser and Kreier1993; Mihalca et al. Reference Mihalca, Achelaritei and Popescu2002; Telford, Reference Telford2009). In the leech host, gamonts are released from the digested blood and monosporoblastic oocysts mature within the intestinal epithelial cells. Sporozoites migrate to anastomosing lacunae of the leech's circulatory system, giving rise to primary merogony. Infectious merozoites enter into the bloodstream of the turtle as the leech feeds. Pre-erythrocytic meronts are formed in the internal organs of the turtle, such as the liver, lungs and spleen; released merozoites enter erythrocytes and become premeronts. After the host cells rupture, the next generation of merozoites infects other erythrocytes where they transform into erythrocytic meronts or gamonts (Reichenow, Reference Reichenow1910; Desser, Reference Desser and Kreier1993).
Haemogregarines were previously considered to be highly host-specific, and thus each parasite found often led to the description of a new species, yielding 29 named Haemogregarina species infecting turtles (Levine, Reference Levine1988). Further, the majority of them have been described on the basis of the morphological characteristics of a few detected life stages, mostly the gamonts and meronts found in the erythrocytes of intermediate turtle hosts, offering insufficient information for differential diagnoses. Data on sporogony in the vectors are usually missing; the complete life cycle is known for only a few species (Reichenow, Reference Reichenow1910; Paterson and Desser, Reference Paterson and Desser1976). Additionally, the host specificity of haemogregarines has also been questioned (Dvořáková et al. Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014).
Twenty three out of 39 Haemogregarina species infecting aquatic turtles were identified in Southeast Asia, but most of them are based on century-old-descriptions (e.g. Simond, Reference Simond1901; Laveran and Mesnil, Reference Laveran and Mesnil1902; Castellani and Willey, Reference Castellani and Willey1905; Patton, Reference Patton1908; Robertson, Reference Robertson1908, Reference Robertson1910; Laveran and Pettit, Reference Laveran and Pettit1910; Prowazek, Reference Prowazek1910; Laveran and Nattan-Larrier, Reference Laveran and Nattan-Larrier1912; Mello, Reference Mello de1932a , Reference Mello de b ; Misra et al. Reference Misra, Nandi, Raut and Choudhury1974; Saratchandra, Reference Saratchandra1981; Ray and Bhattacharjee, Reference Ray and Bhattacharjee1984; Chai and Chen, Reference Chai and Chen1990; Sinha, Reference Sinha1993). In contrast to all these recorded species and the time which has elapsed since their description, only 2 of them provide more comprehensive data (Robertson, Reference Robertson1910; Ray and Bhattacharjee, Reference Ray and Bhattacharjee1984). Morphological descriptions of a single life stage accompanied by simple drawings without measurements are attached to some of them, but, more often than not, incomplete development cycles and inadequate information on the morphological characteristics of life stages are presented. The incompleteness of morphological data together with relatively low host specificity provides a space for large synonymy in nomenclature of the genus Haemogregarina. A combination of microscopy with widely expanding molecular-genetic methods offers a new powerful tool for the identification of haemogregarines in recent studies, and also for a clarification of the alpha-taxonomy and phylogeny of the genus Haemogregarina (Perkins and Keller, Reference Perkins and Keller2001; Barta et al. Reference Barta, Ogedengbe, Martin and Smith2012; Dvořáková et al. Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014). Nevertheless, no molecular data have so far been made available for the haemogregarines of Southeast Asia.
In the present study, we examined samples from 5 turtle species originating from Southeast Asia for the presence of Haemogregarina species, their morphological comparison, phylogenetic analysis, evaluation of their host specificity, and using the literature data to carry out review of Haemogregarina species from the Oriental zoogeographic realm.
MATERIALS AND METHODS
Sampling, microscopy
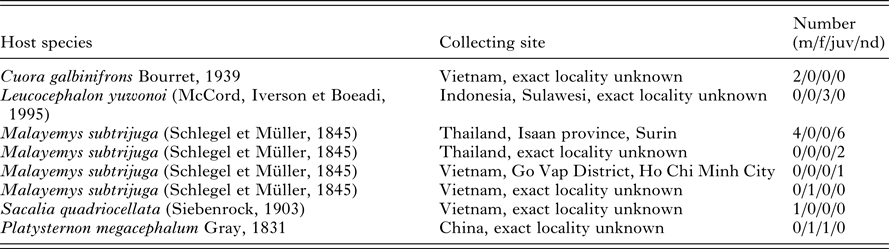
Twenty-two pet-traded turtles (2 Cuora galbinifrons, 3 Leucocephalon yuwonoi, 14 Malayemys subtrijuga, 2 Platysternon megacephalum and one specimen of Sacalia quadriocellata) originated from the wildlife in Sulawesi (Indonesia), China, Thailand and Vietnam (Table 1). Unfortunately, the exact capture localities are not known. All the turtles were inspected and sampled during veterinary screening in the Czech Republic. Among 22 sampled turtles, blood smears and blood samples were obtained from 12 turtles, whereas, only blood smears were provided for 10 of the M. subtrijuga specimens. Blood was collected from each turtle by puncture of the dorsal coccygeal vein, blood smears were prepared, and the remaining blood was stored in absolute ethanol for the forthcoming molecular-genetic analyses. Smears were fixed in absolute methanol for 5 min, and then stained with Giemsa (diluted 1:10 in distilled water, pH 7) for 15 min. The presence of parasites was observed microscopically using an Olympus BX53 microscope with 1000 × magnification and immersion oil. The intensity of parasitaemia was estimated for each infected turtle by examination of approximately 104 erythrocytes (Široký et al. Reference Široký, Kamler and Modrý2005). The maximum length and width were measured for all distinguished developmental stages of the parasites. Further, the LW value (length × width) and the L/W (length/width ratio), maximum length and width (with calculated LW) of nuclei were counted for the gamonts. All measurements are given in μm as a mean followed by SD, with ranges in parentheses. The images were acquired by Quick Photo Camera 3.0 software at 1000 × magnification.
Table 1. Examined turtles
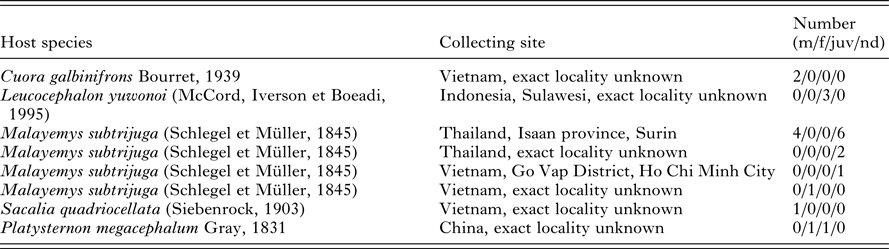
m – males, f – females, juv – juveniles, nd – not determined.
DNA extraction, PCR amplification and sequencing
Blood samples were incubated overnight in lysis buffer with proteinase K before the DNA extraction. Total genomic DNA was isolated using the NucleoSpin Tissue kit (Macherey-Nagel, Germany) according to the manufacturer's instructions, eluted in 100 μL of PCR water and stored at –20 °C. The specific primers ER and EF (Kvičerová et al. Reference Kvičerová, Pakandl and Hypša2008) were used for PCR amplification of approximately 1500 bp long fragment of 18S rDNA of apicomplexans. PCRs were carried out in the total volume of 25 μL using the reaction mixtures and PCR conditions according to Dvořáková et al. (Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014). Amplicons were visualized by electrophoresis on 1·2% agarose gel using the Midori Green (Elisabeth Pharmacon, Czech Republic). Positive PCR products were purified using the Gel/PCR DNA Fragments Extraction Kit (Geneaid Biotech Ltd., Taiwan) and DNA concentration was then measured with the spectrophotometer Nanodrop ASP-3700 (ACTGene, USA). Sequencing was carried out using an automatic ABI 3730XL DNA analyser (Macrogen Inc., The Netherlands).
Phylogenetic analyses
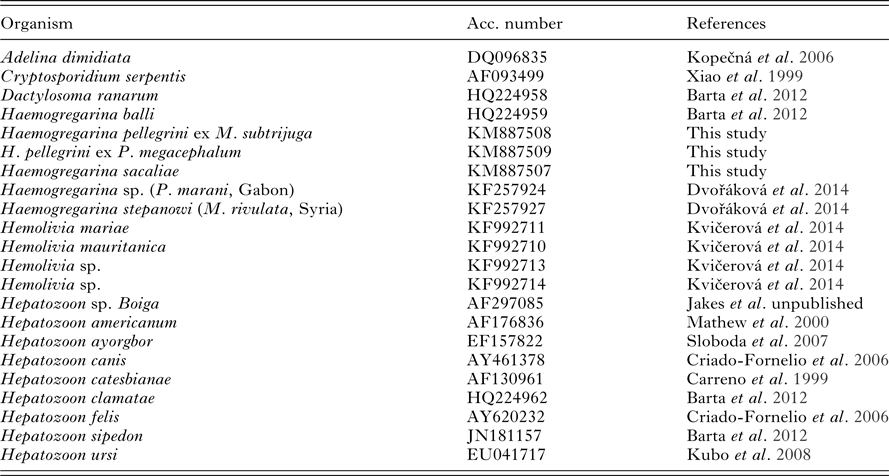
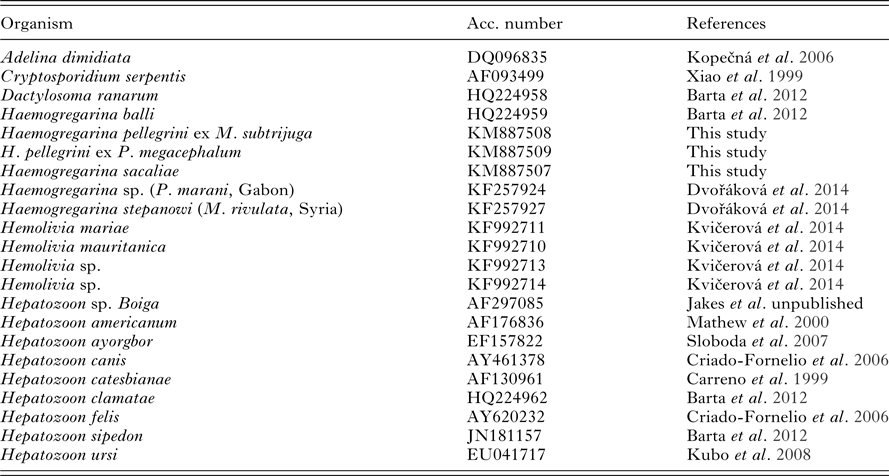
Obtained sequences were identified by BLAST analysis, edited using the DNASTAR program package (DNASTAR Inc.), and deposited in the NCBI GenBank database (accession numbers KM887507, KM887508 and KM887509). Additional sequences of available Haemogregarina species and closely related organisms of the genera Adelina, Cryptosporidium, Dactylosoma, Hemolivia and Hepatozoon were selected from the GenBank database (NCBI) to reconstruct the phylogeny (Table 2). Alignment was created using BioEdit (Hall, Reference Hall1999) with the Clustal W algorithm (Thompson et al. Reference Thompson, Higgins and Gibson1994). Genetic distances based on the 18S rDNA sequences of different species were generated by the Mega 5.0 (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Methods of Bayesian inference (BI), Maximum likelihood (ML) and Maximum parsimony (MP) were selected to infer the phylogenetic relationships within the related organisms. BI was performed in MrBayes 3.1.2. (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003) with a GTR+Γ+I model for 10 million generations. Analysis was completed after removing the burn-in of 630 trees. PHYML 2.4.4. (Guindon and Gascuel, Reference Guindon and Gascuel2003) was employed to carry out the ML analysis under the GTR+Г+I model; bootstrap support was calculated for 1000 replicates. PAUP 4.0b10 (Swofford, Reference Swofford2001) was used to compute the MP, with bootstrap support calculated for 1000 replicates. TreeView 1.6.6 (Page, Reference Page1996) was used to visualize the resulting trees, with Cryptosporidium serpentis as outgroup.
Table 2. List of taxa including GenBank accession numbers of sequences used in this study
RESULTS
Morphology of endogenous stages
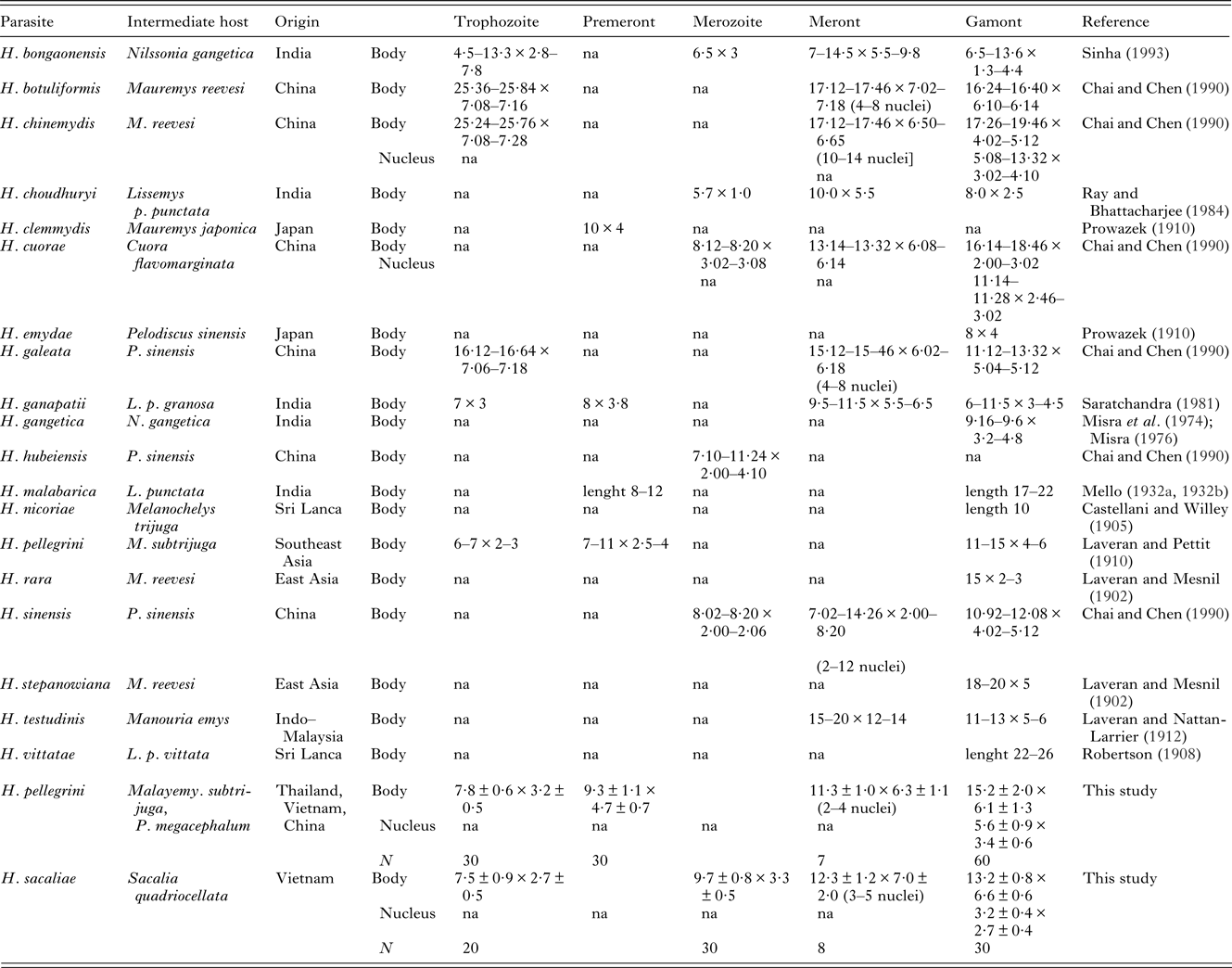
Blood parasites of the typical morphology of Haemogregarina sp. were found by microscopic examination in 6/22 (27·3%) examined turtles belonging to 3 species – M. subtrijuga 4/14 (28·6%), P. megacephalum 1/2 (50%) and Sacalia quadriocellata 1/1 (100%). The highest detected parasitaemia among species was recorded in S. quadriocellata (4·58%), followed by M. subtrijuga (0·88%) and P. megacephalum (0·01%). Morphological analysis of the developmental stages of Haemogregarina parasites found were carried out according to Telford (Reference Telford2009) and compared with already published morphological data on haemogregarines of turtles from the Oriental zoogeographic region (Table 3). In our study, one new species and one species identical to Haemogregarina pellegrini Laveran and Pettit (Reference Laveran and Pettit1910), were recorded. Both are described/redescribed as follows:
Table 3. Review of basic morphology of Haemogregarina species from turtles of East and South Asia
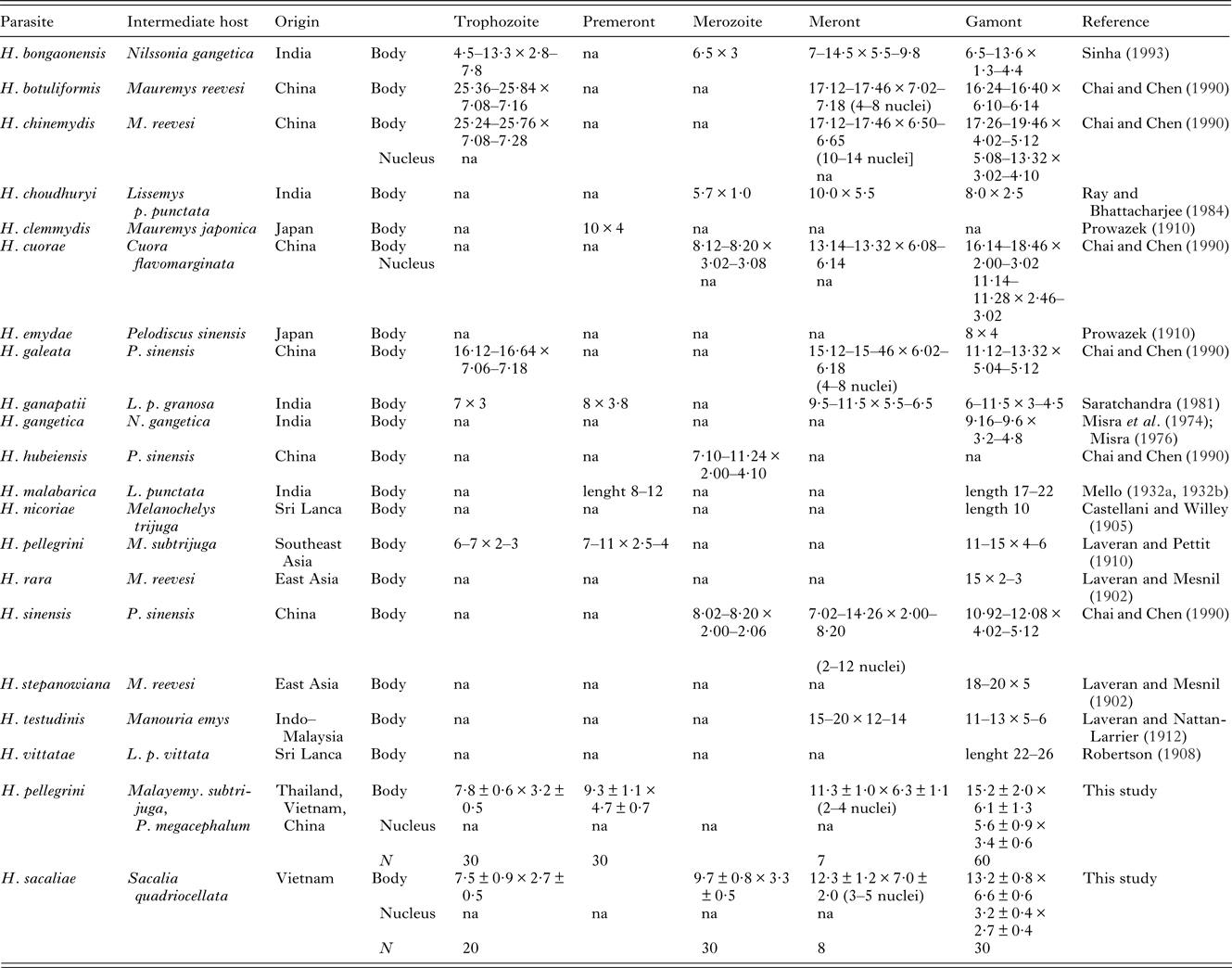
na – data not available. Dimensions of individual developmental stages of H. billeti, H. laverani, H. mesnili (Simond, Reference Simond1901) and H. xavieri (Mello, Reference Mello de1932b) were not specified in the literature. All the measurements are provided in μm.
Description of Haemogregarina sacaliae sp. n.
Trophozoites: Smallest life stages (Fig. 1a) occur individually in erythrocytes, less frequently in pairs, measure 7·5 ± 0·9 × 2·7 ± 0·5 (6·0–9·0 × 2·0–3·0; n = 20). Centrally located, distinct, dark purple nucleus covers a large part of the parasite. Cytoplasm is whitish-blue, finely vacuolated.

Fig. 1. Morphology of H. sacaliae n. sp. (a–d) and H. pellegrini (e-l). Life-stages of H. sacaliae detected in the present study – trophozoite (a), meront (b), differentiated merozoites (c), and gamont (d). Life-stages of H. pellegrini – trophozoite (e), premeront (f), early meront (g), meront (h), microgamont (i), macrogamont from M. subtrijuga for comparison (j) and from P. megacephalum (k), both micro- and macrogamont from M. subtrijuga (l). All figures are in the same scale; scale bar = 10 μm.
Meronts: Rarely detected blood stages, containing 3, 4 or 5 nuclei. Meront containing 3 nuclei measures 12 × 5, meronts with 4 nuclei 12·3 ± 1·5 × 7·7 ± 2·1 (Fig. 1b), and meront with 5 nuclei 12 × 5. Cytoplasmatic differentiation into merozoites was visible in one case (Fig. 1c).
Merozoites: Merozoites found as single, in pairs, or in groups within meronts. Their elongated, worm-like body is sometimes curved (Fig. 1c). Cytoplasm is vacuolated, usually at both poles. Dark, purple-stained nucleus is disintegrated and centrally situated. Merozoites measure 9·7 ± 0·8 (9·0–11·0) × 3·3 ± 0·5 (3·0–4·0; n = 30).
Gamonts: Most abundant life stage, occasionally occurring in pairs, is situated within spacious bean-shaped parasitophorous vacuole occupying approximately half of erythrocyte (Fig. 1d). Parasite is re-curved in vacuole into 2 branches, but division is clearly visible only in a few gamonts. Blue cytoplasm lacks vacuoles. Dark, purple-stained nucleus is formed by chromatin lumps and located near to one pole. Capsule measure 13·2 ± 0·8 × 6·6 ± 0·6 (12·0–15·0 × 6·0–8·0; n = 30), LW is 87·4 ± 10·1 μm2 (72–112) and L/W ratio is 2·0 ± 0·2 (1·7–2·5). Oval nuclei measure 3·2 ± 0·4 × 2·7 ± 0·4 (3·0–4·0 × 2·0–3·0), LW is 8·8 ± 1·9 μm2 (6·0–12·0). Host erythrocyte nucleus is displaced marginally towards edge of cell by all stages of parasites.
Stages in the vector: Unknown
Type host: Sacalia quadriocellata (Siebenrock, 1903) (Testudines: Geoemydidae).
Other hosts: Unknown.
Vector: Unknown
Type locality: Vietnam, exact locality unknown.
Other localities: Unknown
Prevalence: A single examined S. quadriocellata was parasitized (100%)
Molecular features: 18S rDNA sequence of 1418 bp (GenBank accession number KM887507), GC content 39·1%.
Material deposited: Blood film (marked as VN-34-13) and DNA sample no. 5084 are deposited in the collection of Department of Biology and Wildlife Diseases, University of Veterinary and Pharmaceutical Sciences Brno, Brno, Czech Republic.
Etymology: The specific epithet sacaliae is derived as a genitive of the host generic name Sacalia, which is grammatically a feminine.
Remarks. Host species and geography are usually considered in the identification of haemogregarines and their species. No Haemogregarina species has been so far reported from turtles of the genus Sacalia. Since the host specificity of haemogregarines to their turtle hosts is not strict, all known Haemogregarina spp. described from the Oriental zoogeographic region were considered for differential diagnosis with special attention given to the species of turtles of the family Geoemydidae (due to the similar ecology of their representatives). A comparison of the available morphological data on H. sacaliae sp. n. shows an overlap in some criteria with H. nicoriae Castellani et Willey, Reference Castellani and Willey1905, H. mesnili Simond, Reference Simond1901, H. stepanowiana Laveran et Mesnil, Reference Laveran and Mesnil1902, H. pellegrini Laveran et Pettit, Reference Laveran and Pettit1910, and H. cuorae Chai et Chen, Reference Chai and Chen1990 (Table 3; Simond, Reference Simond1901; Laveran and Mesnil, Reference Laveran and Mesnil1902; Castelani and Willey, Reference Castellani and Willey1905; Laveran and Pettit, Reference Laveran and Pettit1910; Chai and Chen, Reference Chai and Chen1990). The blood stages of H. nicoriae, H. mesnili and H. stepanowiana are similar to those of H. sacaliae. H. nicoriae differs in the significantly smaller dimensions of its gamonts and H. mesnili has a larger longitudinally situated nucleus in its gamont life stages. H. stepanowiana differs in displaying clearly compound and differentiated branches; its stages do not contain vacuoles and do not form spacious parasitophorous vacuole. Trophozoites and gamonts of H. pellegrini as well as merozoites of H. cuorae are of a similar size as those of H. sacaliae. However, H. sacaliae differs primarily in possessing crumbled nuclei and spacious parasitophorous vacuoles in its gamonts and vacuolated merozoites. These traits may be considered as unique, diagnostic features.
Haemogregarines detected in the blood films of 3 individuals of M. subtrijuga from Thailand, one M. subtrijuga from Vietnam and one P. megacephalum from China were identified as Haemogregarina pellegrini Laveran et Pettit, Reference Laveran and Pettit1910. Additional data on the developmental stages obtained by the microscopic analysis of blood smears warrant the redescription of H. pellegrini.
Redescription of Haemogregarina pellegrini Laveran et Pettit, 1910.
Trophozoites: Smallest forms of parasite (Fig. 1e), elongated and slightly curved with bounded nucleus consisting of accumulated mass of chromatin at mid-body; several vacuoles and dark purple granules are sometimes found in cytoplasm. Size is 7·8 ± 0·6 × 3·2 ± 0·5 (7·0–9·0 × 2·0–4·0; n = 30). Nucleus of infected erythrocyte is often displaced to polar position.
Premeronts: Oval sometimes elongated stages with vacuolated cytoplasm and granules (Fig. 1f). They measure 9·3 ± 1·1 × 4·7 ± 0·7 (8·0–12·0 × 4·0–6·0; n = 30). Nuclei consist of mass of chromatin located usually in central position.
Early meronts: Elongated stages measuring 12·1 ± 0·7 × 5·7 ± 0·6 (11·0–13·0 × 5·0–7·0; n = 15) occur in erythrocytes individually. Inclusions of chromatin situated mostly along periphery, sometimes scattered within parasite in stained deep blue cytoplasm (Fig. 1g).
Meronts: Binucleate and multinucleate meronts found in bloodstream, elongated, slightly recurved. Immature meronts with 2 small nuclei measured 10·8 ± 1·0 × 6·0 ± 1·4 μm (10·0–12·0 × 4·0–7·0; n = 4), 2 meronts with 3 nuclei (Fig. 1h) 12 × 6 and 12 × 7, respectively, and single meront with 4 nuclei measure 12 × 7.
Gamonts: Two distinguishable forms are detected – microgamonts (Fig. 1i) and macrogamonts (Fig. 1j,k). Estimated number of macro- and microgamonts found in individual smears is similar. Macrogamonts represent largest encapsulated life stage with significantly more stained cytoplasm compared with microgamonts. Formation of 2 branches is not clearly visible. Both forms elongated, slightly recurved (cucumber-shaped), usually place longitudinally along full length of host's erythrocytes long axis. Nucleus of young gamonts is localized significantly closer to ‘anterior’ wider end (‘head’) than to ‘posterior’ sharp end (‘tail’). Posterior end is sometimes bent.
Mostly compact and basophilic nucleus of oval, circular, or sometimes banded shape occupy full width of gamont. It is situated eccentrically, in macrogamonts, also sometimes centrally. Whitish-purple polar cap fills one-third of interior space on opposite pole to nucleus placement. Basophilic granules are sometimes visible in microgamonts.
Capsule of macrogamont averages 16·9 ± 1·1 × 7·2 ± 0·8 (15·0–19·0 × 6·0–9·0; n = 30), with LW 119·5 ± 17·7 μm2 (96–152) and L/W ratio 2·36 ± 0·26 (1·77–3·00). Their nuclei are 6·2 ± 0·7 × 3·6 ± 0·6 (5·0–7·0 × 3·0–5·0) with LW 22·3 ± 4·5 μm2 (15·0–30·0). Microgamont capsules measure 13·5 ± 1·0 × 5·0 ± 0·7 (12·0–15·0 × 4·0–6·0; n = 30), with LW 66·9 ± 8·4 μm2 (52–90) and L/W 2·79 ± 0·52 (2·00–3·75). Dimensions of nuclei are 5·0 ± 0·8 × 3·3 ± 0·6 (4·0–7·0 × 2·0–4·0), with LW 16·6 ± 4·04 μm2 (10·0–24·0). Host erythrocyte nuclei are displaced towards periphery of cell and strongly compressed.
Type host: Malayemys subtrijuga (Schlegel et Müller, 1845) (Testudines: Geoemydidae).
Other hosts: Platysternon megacephalum Gray, 1831 (Testudines: Platysternidae).
Type locality: Southeast Asia, exact locality unknown (Laveran and Pettit, Reference Laveran and Pettit1910).
Other localities (this study): Surin, Isaan province, Thailand. China and Vietnam – for both, exact locality unknown.
Prevalence: 4 of 14 (28·6%) M. subtrijuga and 1 of 2 (50%) P. megacephalum parasitized.
Molecular features: 18S rDNA sequence of 1423 bp (GenBank accession number KM887508), GC content 39·4%.
Material deposited: Blood films (marked as TH-1-06, TH-2-06, TH-3-06, VN-37-13 and CHI-33-13) and DNA samples no. 5093 and 5083 are deposited in the collection of Department of Biology and Wildlife Diseases, University of Veterinary and Pharmaceutical Sciences Brno, Brno, Czech Republic.
Remarks. H. pellegrini was described from a single specimen of M. subtrijuga by Laveran and Pettit in 1910. They described it as croissant-shaped parasite inside a capsule with rounded extremities or one tapered end. Its granulated cytoplasm contained an undifferentiated nucleus. The free parasite measured 10–14 × 2–3 μm. The authors identified small, medium and large forms. The small forms were usually spherical or oval in shape, measuring 6–7 × 2–3 μm, their nuclei consisted of more or less compact grains of chromatin. The most common forms – medium forms – were cylindrical, 7–11 × 2·5–4 μm, usually curved and with rounded ends, sometimes one slightly tapering with a pale-blue, vacuolated cytoplasm. Chromophilic granules were sometimes present; the nucleus was identical to nucleus of the small forms. Large forms measuring 11–15 × 4–6 μm were situated in the main axis of erythrocytes, rarely recurved with a nucleus consisting of chromatin particles often located in the middle of the parasite. The cytoplasm contained numerous chromophilic granules. The erythrocytic nucleus was displaced to the edge of the cell, and slightly enlarged and deformed by the surrounding pressure of the parasite. We have distinguished life stages according to Telford (Reference Telford2009), where we have considered the small forms to be trophozoites, the medium forms premeronts, and the large forms gamonts. Additionally, we found that trophozoites may contain dark purple granules. Premeronts possessed a significantly vacuolated cytoplasm with granules. In terms of gamonts (the large forms), we have discerned macro- and microgamonts (Fig. 1l); we assumed that Laveran and Pettit (Reference Laveran and Pettit1910) classified both macro- and microgamonts as a single form. Despite their descriptions being very similar, their nuclei may be located centrally and the stages may include basophilic granules. All individual developmental stages are comparable in their sizes.
Molecular characteristics and phylogeny
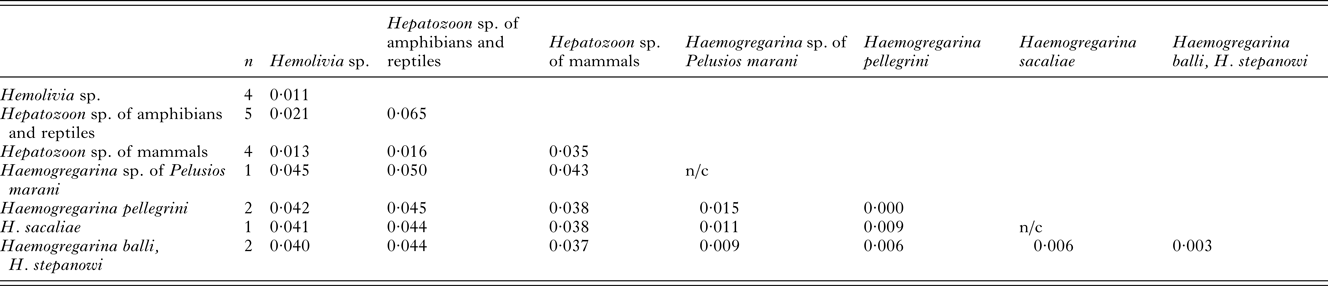
In our molecular analyses, 18S rDNA sequences of 3 samples were included. The obtained sequences of the lengths 1411 bp, 1418 bp and 1423 bp were used to calculate p-distances (see Table 4). Phylogenies were based on the alignment of 1210 bp with a total of 19 sequences. All analyses (BI, ML and MP) provided identical topologies, however, with different node supports (Fig. 2). A monophyletic cluster was formed by the 3 main branches: (1) the single Dactylosoma ranarum; (2) the well-supported Hemolivia-Hepatozoon clade comprising the Hepatozoon species of mammals, Hepatozoon spp. of amphibians and reptiles and Hemolivia spp. of turtles and skink; (3) the clade consisting of Haemogregarina species. A haemogregarine from S. quadriocellata represented a sister taxon to Haemogregarina from the African hinged terrapin, Pelusios marani, from Gabon. Their closest relatives were H. balli and H. stepanowi. Haemogregarines from M. subtrijuga and P. megacephalum were genetically identical, thus conspecific, and constituted a sister taxon to all other Haemogregarina species included in the analysis.

Fig. 2. Phylogenetic tree of Haemogregarina sp. inferred from partial 18S rDNA sequences. Numbers at the nodes show posterior probabilities under BI/ bootstrap values for ML/MP higher than 0·50 or 50%, respectively. Posterior probabilities and bootstrap that supports lower than 0·50 or 50% are marked with asterisk (*). Taxa for which new sequences were obtained in this study are printed in bold.
Table 4. P-distances based on total of 1210 positions in the final dataset
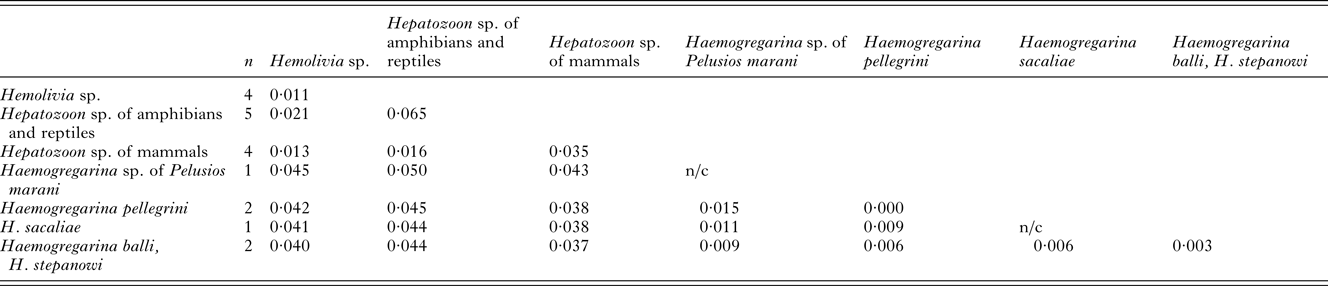
The numbers of base differences per site from an estimation of net average within each group and between groups of sequences are shown. All positions containing gaps and missing data were eliminated. The presence of n/c in the results denote cases in which only one sequence was contained.
DISCUSSION
To date, a number of Haemogregarina species has been described from numerous turtle hosts and broad geographic areas. Their differentiation based only on morphological characteristics is considerably limited (Telford et al. Reference Telford, Norton, Moler and Jensen2009). Most frequently observed developmental stages often possess a uniform morphology, which complicates the certainty of assignment of found haemogregarines to the previously described taxa. Stages from the definitive hosts, which are helpful in differential diagnosis, are known for only 2 Haemogregarina species described from studied region – H. nicoriae and H. choudhuryi (Ray and Bhattacharjee, Reference Ray and Bhattacharjee1984; Robertson, Reference Robertson1910). The conspecificity of any 2 given haemogregarines can only be hypothesized when studied isolates originate from the same area or from the same or related turtle hosts, and at that, with a degree of caution. Nevertheless, it has been proved repeatedly that a single Haemogregarina species can infect several turtle species from different families (Paterson and Desser, Reference Paterson and Desser1976; Siddall and Desser, Reference Siddall and Desser1992; Telford et al. Reference Telford, Norton, Moler and Jensen2009; Dvořáková et al. Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014). Although the employment of molecular-genetic tools is helpful in ascertaining the taxonomy of recently examined or described species, these methods are inapplicable to earlier descriptions, where sequence data are missing. In such cases, microscopy remains the only useful method, since the blood stages of the same Haemogregarina species evince only slight morphological differences when infecting the hosts of different families (Dvořáková et al. Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014). Similarly, the geographic distribution of one haemogregarine species may span thousands of kilometres, and as was suggested in the case of H. stepanowi, the occurrence of haemogregarines probably depends on the range of its definitive host – the leech (Reichenow, Reference Reichenow1910; Bielecki et al. Reference Bielecki, Cichocka, Jabłoński, Jeleń, Ropelewska, Biedunkiewicz, Terlecki, Nowakowski, Pakulnicka and Szlachciak2012; Dvořáková et al. Reference Dvořáková, Kvičerová, Papoušek, Javanbakht, Tiar, Kami and Široký2014). Hence, the risk of infection probably exists anywhere the leech vector and appropriate turtle host occur together.
The vector is not necessarily always only a single leech species; for example, H. balli is transmitted by 2 related leeches – Placobdella parasitica and P. ornata (Paterson and Desser, Reference Paterson and Desser1976). The prevalence of leeches on their turtle hosts varies depending on the microhabitat predominantly occupied by the turtle. Aerial-basking reduces leech loads through desiccation and predation (McAuliffe, Reference McAuliffe1977), while bottom-dwelling close to the substrate where leeches reside causes heavier infestation (McCoy et al. Reference McCoy, Failey, Price and Dorcas2007). Accordingly, we suggest that a single haemogregarine species may parasitize a group of turtle species occupying the same or very similar ecological niches.
Our findings were compared with available morphological data of formerly described species from the studied region, although the published descriptions of parasites were very simplistic in some cases; e.g. H. clemmydis and H. emydae (Prowazek, Reference Prowazek1910). We confirmed that the host specificity of haemogregarines is not strict, since we found life stages of a single Haemogregarina sp. in the bloodstream of 2 turtle species belonging to different families, but occurring in similar semiaquatic habitats. In general appearance, a haemogregarine infecting M. subtrijuga and P. megacephalum was identical and comparable in size to H. pellegrini recorded by Laveran and Pettit (Reference Laveran and Pettit1910) also from M. subtrijuga. The only authors reporting a survey for haemogregarines from P. megacephalum were Chai and Chen (Reference Chai and Chen1990), who examined 2 individuals, but did not detect any haemogregarines. Compared to Laveran and Pettit (Reference Laveran and Pettit1910), we were able to distinguish macrogamonts and microgamonts in our slides. Discovered haemogregarine from S. quadriocellata – H. sacaliae differs from previously described species of this region in a combination of vacuolated merozoites, crumbled nucleus and the spacious parasitophorous vacuole of gamonts. However, the parasite was detected in a single turtle, and we cannot exclude that crumbled nucleus of gamonts may represent only their younger forms. 18S rDNA sequences of H. pellegrini from both tortoise hosts, M. subtrijuga and P. megacephalum, were identical. Phylogenetic analyses based on 18S rDNA placed both H. pellegrini and H. sacaliae firmly within the Haemogregarina clade (Fig. 2). The availability of sequences of a single gene from a few Haemogregarina species did not allow for any co-evolutionary or phylogeographic analyses.
Our study provides the first Haemogregarina sequences from the region of Southeast Asia. It is surprising that despite the large number of already described Haemogregarina species from Asian turtles, none of them has been recorded repeatedly. Descriptions of new species are published continuously instead of trials to confirm the presence of already known species, to specify their host spectra, and to characterize their geographic ranges. Under such circumstances, a considerable synonymy of haemogregarines in this region is very probable (Siddall, Reference Siddall1995). Hence, the applications of molecular-genetic characteristics (barcodes) together with rigorous morphological analysis are necessary prerequisites for avoiding further taxonomic inflation in this group.
ACKNOWLEDGEMENTS
Thank L. Hojný, J. Moravec, and P. Petrás for the help with sampling. Thank Christopher Steer for language correction.
FINANCIAL SUPPORT
This work was supported by the Czech Science Foundation (project no. P506/11/1738), by the project ‘CEITEC – Central European Institute of Technology’ (no. CZ.1.05/1.1.00/02.0068) from the European Regional Development Fund, and by the IGA VFU grant (project no. 6/2014/FVHE).