Introduction
Maize (Zea mays L.) has been transformed by introducing truncated genes of the entomopathogenic bacteria, Bacillus thuringiensis (Bt), the so called Bt maize. In 2010, Bt maize was grown on 46 million ha in the world (James, Reference James2010). In Europe, it is mostly grown in Spanish areas where maize lepidopteran borers are serious pests, such as Lleida (Catalonia, NE Spain), where the present study was performed. Bt is selectively toxic for some insects, many of them belonging to the Lepidoptera, in which the degree of susceptibility to the toxin differs according to the species (Ibargutxi et al., Reference Ibargutxi, Estela, Ferre and Caballero2006; Bird & Akhurst, Reference Bird and Akhurst2007). The only Bt maize allowed for cultivation in the EU contains the transformation event MON810 (Monsanto Company), which confers a good degree of resistance to the two main maize borers present in the EU, the European corn borer (ECB) and the Mediterranean Corn Borer (MCB) [Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) and Sesamia nonagrioides Lefèbvre (Lepidoptera: Noctuidae), respectively] (Hellmich et al., Reference Hellmich, Albajes, Bergvinson, Prasifka, Wang, Weiss, Romeis, Shelton and Kennedy2008). However, it has a much lower efficacy for controlling other lepidopteran pests, such as the true armyworm, Mythimna unipuncta, and the corn earworm, Helicoverpa armigera (Lepidoptera: Noctuidae). These two species cause occasional but severe losses to maize in the study area (Lleida, Spain). The introduction of corn borer-resistant plants based on the event MON810 could favour the occurrence of secondary pests such as M. unipuncta and H. armigera that are less susceptible to Bt, are not controlled effectively with conventional pesticides and could, therefore, become severe pests.
Mythimna unipuncta is a polyphagous insect and an important pest of graminaceous crops in Europe (Bues et al., Reference Bues, Poitout, Anglade and Robin1986) and North America (McNeil et al., Reference McNeil, Miller, Laforge and Cusson2000). Helicoverpa armigera is an important cosmopolitan insect pest whose larvae are quite polyphagous (Fitt, Reference Fitt1989), and it has a high capacity to develop resistance to insecticides (Torres-Vila et al., Reference Torres-Vila, Rodriguez-Molina, Lacasa-Plasencia, Bielza-Lino and Rodriguez-del-Rincon2002). Eizaguirre et al. (Reference Eizaguirre, Madeira and López.2010) observed in the study area that larvae of both species can survive and complete their development feeding on Bt maize and, therefore, ingesting Bt toxins.
Bacillus thuringiensis var. kurstaki is a bacterium whose spores produce crystal proteins that are protoxins called δ-endotoxins, including Cry toxins, which major component is the protein of 130-kDa (Bulla et al., Reference Bulla, Kramer, Cox, Jones, Davidson and Lookhart1981). After ingestion, the toxicity of Cry toxins is the result of a complex process that requires multiple steps occurring in the midgut (Whalon & Wingerd, Reference Whalon and Wingerd2003). Once ingested, the spore crystal proteins are dissolved into protoxins and cleaved proteolytically in the midgut lumen of the larvae to produce toxic fragments that are resistant to further proteolysis. The activated toxins, with insecticidal action, bind to specific receptors present on the microvilli of the insect midgut and form pores in the apical membranes of columnar cells of the midgut (Gill, Reference Gill1992; Schnepf et al., Reference Schnepf, Crickmore, Van Rie, Lereclus, Baum, Feitelson, Zeigler and Dean1998). These small pores of the epithelial cells cause an osmotic imbalance leading to cell lysis (Knowles, Reference Knowles1994; Bravo et al., Reference Bravo, Soberón and Gill2005). Several Cry proteins have been identified. Differences in insect susceptibility to Bt may be caused by differential efficacy of Cry toxins to perform any of the above steps and also by the amount of Bt that is ingested, because large differences in toxin ingestion among species have been reported in the literature (Head et al., Reference Head, Brown, Groth and Duan2001; Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006). Recently, Broderick et al. (Reference Broderick, Raffa and Handelsman2006, Reference Broderick, Robinson, McMahon, Holt, Handelsman and Raffa2009) stated that the susceptibility to the Bt toxins of the larvae of several Lepidoptera depends on the indigenous gut bacteria composition of each species. One or more of these mechanisms may be involved in the differences in susceptibility of insect species to Cry toxins or in the acquisition of resistance to them, one of the most serious risks of genetically modified Bt crops. Therefore, it is important to determine the fate of Bt toxins from their ingestion by the insect until they are metabolized before or after reaching action sites.
The aims of the present study were to determine the fate of Cry1Ab, the Bt toxin that is expressed in the Bt varieties currently cultivated in EU, when it is ingested in sublethal concentrations by two maize pests that are poorly susceptible to Bt. Cry1Ab content was measured in tissues of the larvae of the two species fed on a diet with lyophilized Bt maize leaves, and the recovery of the larvae fed on Bt diet after they had changed to a non-Bt diet was also measured. Additionally, the present study aimed to describe the morphological effects of the Cry1Ab toxin on the midgut of H. armigera larvae using light and electron microscope methodologies.
Material and methods
Insects and rearing
The larvae of M. unipuncta and H. armigera came from the culture maintained in the entomological laboratory at the UdL-IRTA research centre, renewed every three or four generations with insects collected in the field in the area of Lleida. Mythimna unipuncta larvae were reared on a semi-artificial diet according to López et al. (Reference López, Madeira, Pons and Eizaguirre2008), a modification of Shorey & Hale (Reference Shorey and Hale1965), and H. armigera larvae were reared on a semi-artificial diet according to Eizaguirre & Albajes (Reference Eizaguirre and Albajes1992). The adults were supplied with a sugar/water solution (10% sugar) and maintained at 25 °C and high humidity (>60%) under a 16:8h light:dark photoperiod.
Bioassays
Bioassays were carried out using sixth instar (L6) larvae of M. unipuncta and H. armigera fed on a diet with lyophilized Bt or non-Bt maize leaves. Previously, the experimental larvae had been fed on a semi-artificial diet with non-Bt lyophilized maize leaves (Pérez-Hedo et al., Reference Pérez-Hedo, Marques, López, Albajes and Eizaguirre2012) until the day of the moult to sixth instar (L6d0) larvae. The maize leaves were freeze-dried, milled to a homogeneous fine powder and stored at −80 °C until use. The varieties of maize leaves assayed were Bt corn PR33P67 containing the transformation event MON810 and its corresponding near-isogenic line PR33P66 (non-Bt corn), both from Pioneer Hi-Bred® International Inc. (Iowa, USA). Larvae were fed on Bt, non-Bt or a mixed diet after moult to sixth instar (L6d0) for five days. Two types of mixed diet were tested: (i) Bt for one day and then on non-Bt diet for four days; and (ii) Bt diet for three days and then on non-Bt for two days. The different treatments were compared at three time periods: L6d0–L6d1, L6d1–L6d3 and L6d3–L6d5. Before being submitted to the treatments, the larvae were weighed. After the treatments, the larvae were weighed again and some tissues were extracted. An index of larval weight gain during each period was calculated by dividing the weight of the larvae at the end by their weight at the beginning of the period. At least 20 larvae were used for each group.
Tissue extraction
The content of the Cry1Ab toxin was measured in tissues of the treated larvae. The tissues analyzed were the hemolymph, the peritrophic membrane and its contents, and the midgut epithelium. Hemolymph was extracted from live larvae by cutting a proleg with microscissors, the peritrophic membrane and its contents, and midgut epithelium of the same larva were dissected under insect saline solution. Five samples were prepared for each tissue, each one obtained from three different larvae and then pooled to obtain 60μl of hemolymph, 90mg of peritrophic membrane and its contents and 90mg of midgut epithelium per sample.
Cry1Ab quantification
Cry1Ab toxin was analyzed with an ELISA kit (AGDIA-BIOFORDS, 5 rue Henri Desbruères 91030 EVRY Cedex-France, catalogue number PSP 06200). Cry1Ab standards at concentrations 0, 0.25, 0.5, 1, 1.5, 2, 4, 6 and 8 ppb were used as calibrators. The amount of the Cry1Ab protein was quantified by following the protocol of the manufacturer. Tissues were homogenized in 500μl, and the hemolymph in 300μl of extraction buffer was provided in the kits and centrifuged at 10,000rpm for 5min; the supernatants were stored in a freezer at –80 °C until analysis. All wells of the ELISA plates were coated with the peroxidase enzyme conjugate and the samples, and incubated for 2h at 25 °C. Subsequently, the plates were emptied and washed seven times with wash buffer, a TMB substrate solution provided in the kits was added, and the plates were incubated for 20min at room temperature. Absorbance was read at 650nm, using a VICTOR3 Multilabel Plate Counter (PerkinElmer Life and Analytical Sciences, Madrid, Spain). Absorbance of each sample was transformed into ng Cry1Ab mg–1 fresh material using the regression graph of Cry1Ab standards.
Light and transmission electron microscopy analysis
The midgut epithelium of L6 larvae of H. armigera, fed for three days on a Bt, non-Bt or a mixed diet of Bt diet for three days and then on non-Bt for two days, was dissected. The tissues were isolated and fixed in phosphate buffer (0.1 M, pH 7.2) with 2.5% glutaraldehyde, washed three times with phosphate buffer and post-fixed with 1% osmium tetroxide for 2h, dehydrated in a graded ethanol solution (30min per stage) at 4 °C and embedded in EMBED812/Araldite resin (Electron Microscopy Sciences, Fort Washington, PA, USA). For light microscopy, semi-thin sections were stained with Richardson blue, while for the electron microscopy studies ultra-thin sections were stained with uranyl acetate and lead citrate (Reynolds, Reference Reynolds1963) and examined in a Zeiss EM-910 electron microscope at 80 Kv.
Statistical analysis
A one-way ANOVA was used to analyze the effects of the Bt and non-Bt diet on the concentration of Cry1Ab in each tissue and on the growth gain index on days 1, 3 and 5 of the L6 instar, using the SAS package (SAS Institute, 2001) with P ≤ 0.05. In cases of significant differences between treatments, the Duncan multiple range test was used to compare means.
Results
Growth gain
The growth gain index of H. armigera and M. unipuncta shown in fig. 1 indicates some differences between the two species. Statistical analyses were performed to compare growth gain of larvae fed on a Bt, non-Bt or mixed diet at three time periods: L6d0–L6d1, L6d1–L6d3 and L6d3–L6d5.

Fig. 1. Mean growth gain index of H. armigera (grey columns) and M. unipuncta (black columns) fed on Bt or non-Bt diet for one, three or five days after moult to L6 or fed on the Bt diet for one or three days and then on the non-Bt diet (Bt1d-NoBt and Bt3d-NoBt, respectively). Error bars represent ±SE. Different letters over the bars within a period indicate significant differences (P < 0.05) in the growth gain index between the treatments according to Duncan comparisons.
The growth gain index of H. armigera larvae fed for one day on Bt diet was significantly lower than that of the larvae fed on non-Bt diet (F = 94.13; df = 1, 118; P < 0.0001). Although larvae of H. armigera, fed on the Bt diet for three days, increased their weight significantly (L6d3 compared to L6d1) (F = 20.49; df = 1, 139; P < 0.0001), their growth gain index was lower than that of H. armigera larvae fed on the non-Bt diet for three days (L6d1–L6d3) (F = 25.09; df = 2, 85; P < 0.0001). Larvae fed first on the Bt diet and then on the non-Bt diet (Bt1d-NoBt, Bt3d-NoBt) showed the highest growth gain index two days after the transfer from the Bt to the non-Bt diet, but the weight gain index four days after the transfer (Bt1d-NoBt) was similar to that of the rest of larvae. There were no differences in the growth gain index of the larvae of M. unipuncta fed on the Bt and the non-Bt diet for one day (L6d0–L6d1) (F = 1.14; df = 1, 129; P = 0.2874) and three days (L6d1–L6d3). Again, the larvae fed first on the Bt diet and then on the non-Bt diet (Bt1d-NoBt, Bt3d-NoBt) showed a higher weight gain index than the other larvae (F = 6.49; df = 2, 99; P = 0.0023 and F = 17.52; df = 3, 56; P < 0.0001, respectively). The growth gain index of the last period tested (L6d3–L6d5) was lower for the larvae of both species fed on the non-Bt and the Bt diet, particularly in the case of the larvae of M. unipuncta fed on the non-Bt diet, indicating that the pupation process had started.
Concentration of the Cry1Ab toxin
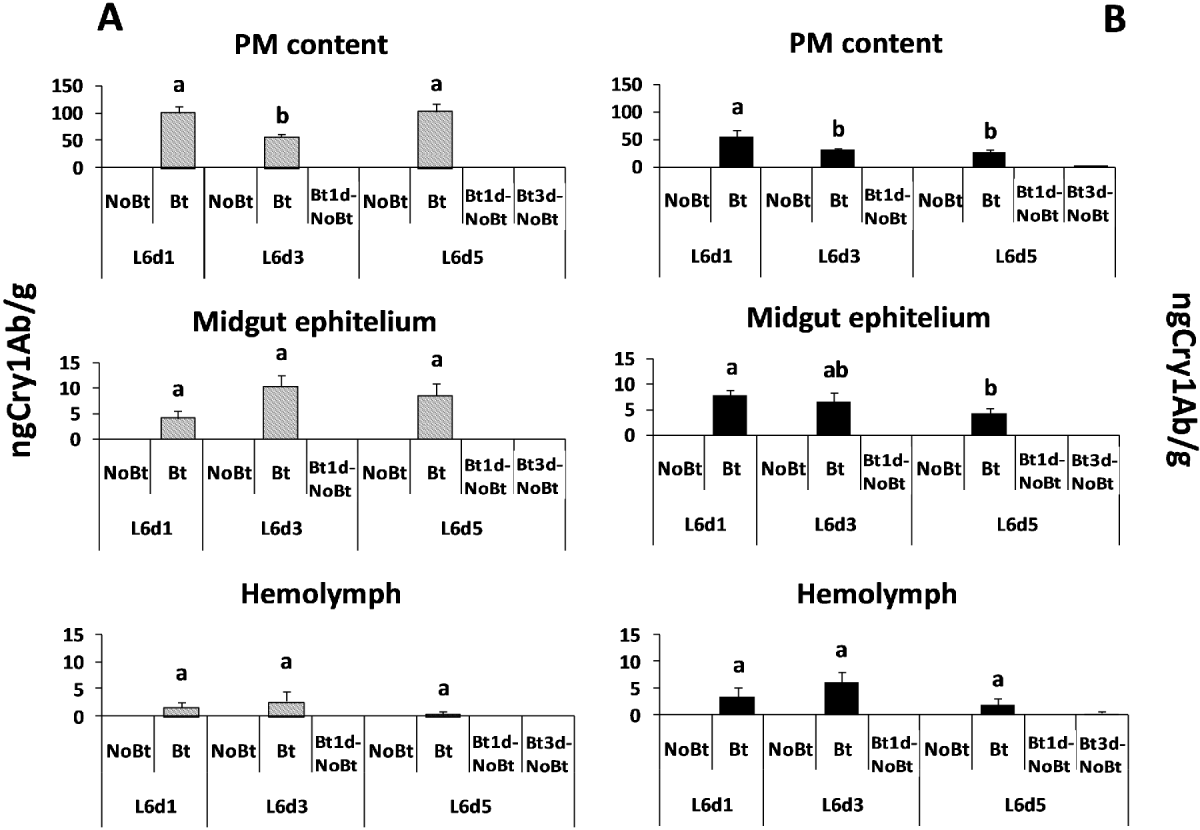
The Bt toxin was only detected in the larvae fed on the Bt diet. Larvae of H. armigera did not show differences in the Bt toxin content in the midgut epithelium (F = 2.40; df = 2, 14; P = 0.1328) or in the hemolymph (F = 0.83; df = 2, 14; P = 0.4592) according to the number of days they fed on the Bt diet (fig. 2A) In contrast, the amount of the Bt toxin found in the peritrophic membrane and its contents was lower in the larvae fed for three days on the Bt diet than in those fed on this diet for one or five days (F = 5.52; df = 2, 13; P = 0.0219). The results were different for the M. unipuncta larvae (fig. 2B), which showed a higher amount of Cry1Ab in the peritrophic membrane and its contents or in the midgut epithelium on the first day after feeding on the Bt diet than two or four days later (F = 3.64; df = 2, 14; P = 0.0580 and F = 2.69; df = 2, 14; P = 0.0520, respectively), whereas there were no significant differences in the hemolymph (F = 1.48; df = 2, 14; P = 0.2655) according to the number of days that the larvae fed on the Bt diet.
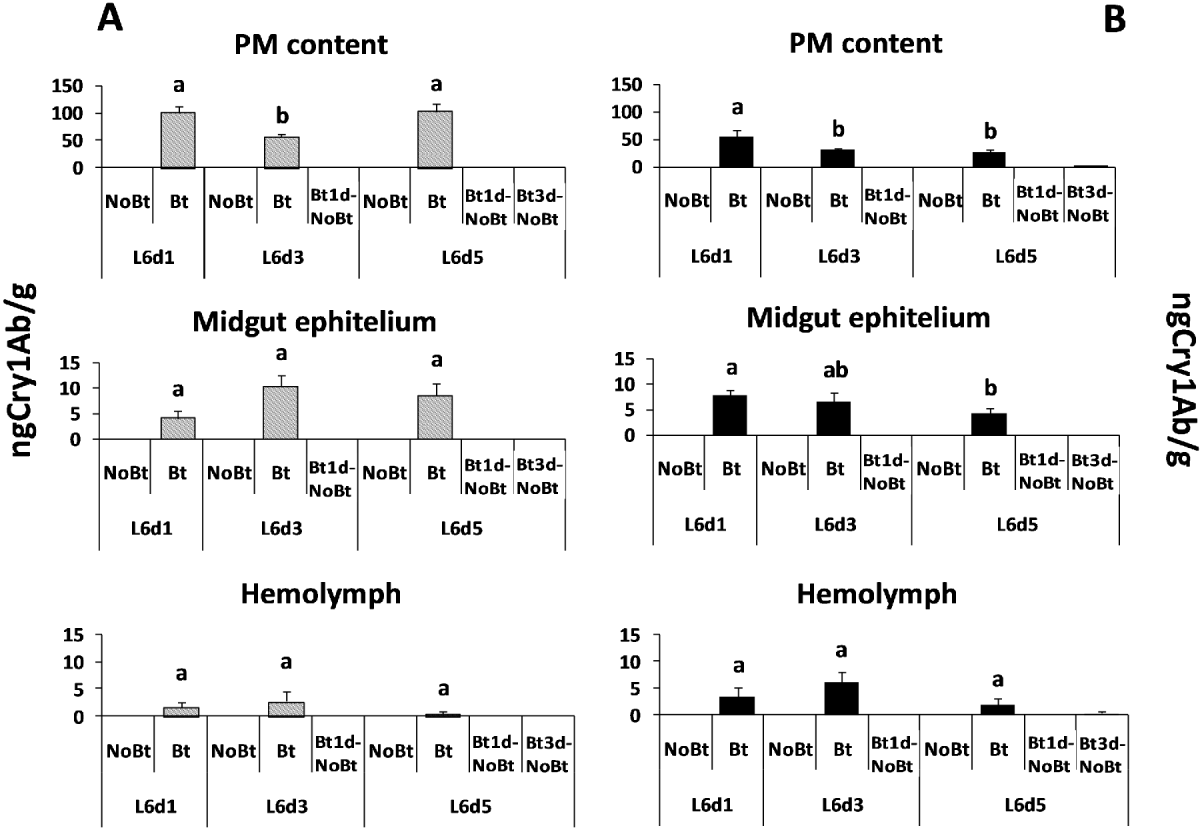
Fig. 2. Mean Cry1Ab toxin content measured in the peritrophic membrane and its contents, midgut epithelium and hemolymph of larvae of H. armigera (A, grey columns) and M. unipuncta (B, black columns) fed on the Bt and the non-Bt-diet for one, three or five days after moult to L6 and fed on the Bt diet for one or three days and then on the non-Bt diet (Bt1d-NoBt and Bt3d-NoBt, respectively). Error bars represent +SE. Different letters over the bars indicate significant differences (P < 0.05) in the Cry1Ab toxin content between the treatments according to Duncan comparisons.
Bt toxin was not detected in any of the tissues of M. unipuncta and H. armigera larvae two days after they were transferred from the Bt to the non-Bt diet; traces of the toxin were only found in M. unipuncta larvae fed for three days on the Bt diet and then on the non-Bt diet.
The amount of Cry1Ab toxin in the diet with lyophilized Bt maize leaves supplied to the larvae was 704.15 ± 119.78ng g–1, an amount ten times higher than that of the peritrophic membrane and its contents, whereas the toxin titre in the peritrophic membrane and its contents was again ten times higher than that in the midgut epithelium and the latter was higher than that in the hemolymph.
Morphology and histology of the midgut of H. armigera
The midgut wall of H. armigera three days after the moult to the last instar shown in fig. 3A has similar features to those described in other lepidopterans (Levy et al., Reference Levy, Falleiros, Moscardi, Gregorio and Toledo2004; Sousa et al., Reference Sousa, Santos, Wanderley-Teixeira, Teixeira, de Siqueira, Alves and Torres2010).

Fig. 3. Midgut epithelium of H. armigera larvae (light microscopy) fed on (A) the non-Bt diet and (B) the Bt diet for three days. SC, stem cells; CC, columnar cells; MV, microvilli of the columnar cells; CCE, columnar cell extrusion; GC, Globet cells; *, goblet-shaped cavity. Note in (B) the irregular and shorter microvilli (arrow), the columnar cell extrusion in the lumen and the change in the shape of the goblet cells.
The cellular midgut pathology observed in larvae fed on a Bt diet is shown in figs 3B and 4B. The main effects of Bt included the following features: some microvilli shorter and irregular; formation of localized columnar cell membrane extrusion, which goes into the midgut lumen and alterations in the morphology in the columnar and goblet cells; the columnar cells were vacuolated, elongated and became thin, whereas the goblet cells were swollen and the membrane of the goblet-shaped cavity showed alterations and degenerated lateral cytoplasmatic projections.
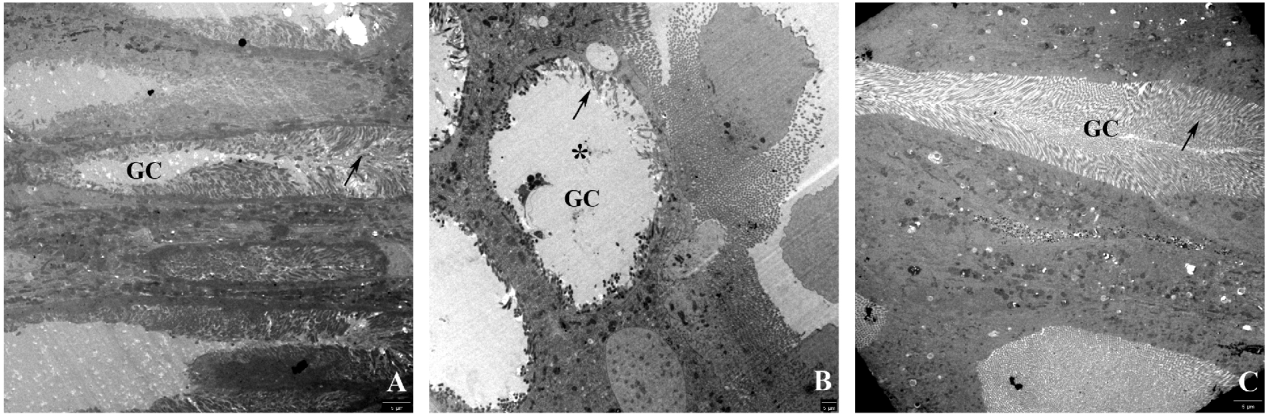
Larvae fed on the Bt diet for three days and then on the non-Bt diet for two days appeared normal; the cells were regenerated, and the lateral cytoplasmatic projections of the goblet cells were similar to those of the control ones (fig. 4A, C).
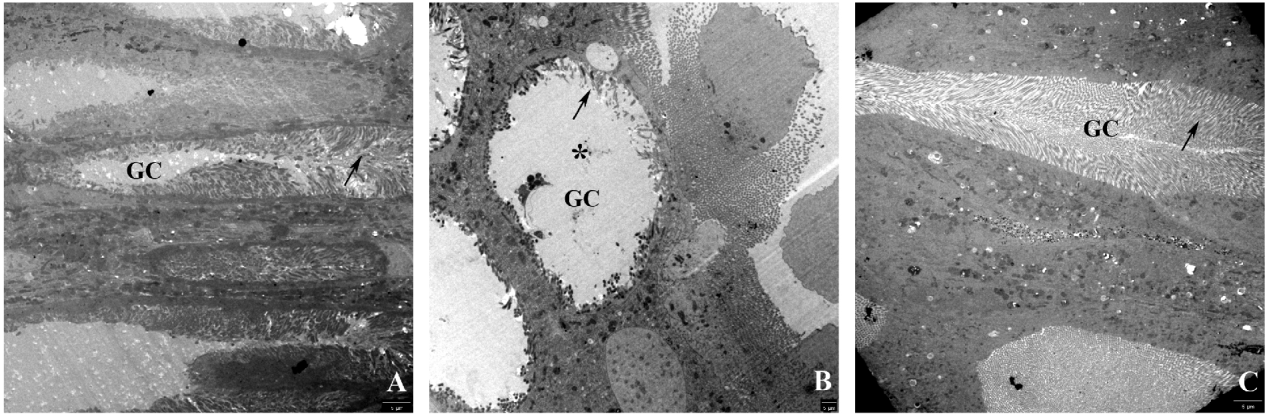
Fig. 4. Goblet cells (electron microscopy) of the midgut epithelium of H. armigera larvae fed on (A) a non-Bt diet, (B) a Bt diet for three days, and (C) a mixed regime of a Bt diet for three days and then a non-Bt diet for two days. Note strongly altered lateral cytoplasmatic projections (arrow) and the swollen goblet-shaped cavity (*) in larvae fed on the Bt diet (B) and the goblet cells recovered two days after feeding on the Bt diet (C).
Discussion
The two noctuids studied in this paper are common lepidopteran pests of maize in the Mediterranean Basin. They are little susceptible to Bt toxins but can be affected by the widespread adoption of transgenic Bt maize. Eizaguirre et al. (Reference Eizaguirre, Madeira and López.2010) indicated that larvae of M. unipuncta and H. armigera can survive when feeding on Bt maize. Knowing the mechanisms that may cause their low susceptibility to Bt toxins can help to select the most effective Bt toxins and to prevent or delay the occurrence of resistance in the field. The low susceptibility of some species to Bt toxins can be explained through a variety of mechanisms, several of which could occur in the species studied. The first one is avoidance of feeding on the Bt crop. The results of this study confirm that both H. armigera and M. unipuncta feed continuously on the Bt diet although their feeding behaviour differed. While H. armigera larvae showed a lower weight gain when fed on diet containing Bt maize, which could suggest that they consumed less diet, M. unipuncta larvae showed a similar weight gain when fed on the Bt or non-Bt diet; however, the effects were the same in the two species, so the low susceptibility to the Bt toxin is presumably not due to a lower ingestion of the Bt diet. As found by Broderick et al. (Reference Broderick, Robinson, McMahon, Holt, Handelsman and Raffa2009) in other Lepidoptera larvae, differences of susceptibility to Bt toxin also may be due to differences in the gut microbiota composition, an aspect that has not been studied in the present work. It seems that the two species show a kind of homeostatic overcompensation response, as was defined by Cohen (Reference Cohen2006) because their weight gain index increased significantly when they were changed from the Bt to the non-Bt diet. This sharp increase in weight gain may indicate that the individuals identified the Bt toxin as toxic and reacted to it, although the low Bt concentration in the diet had a low effect on the final growth gain.
Eizaguirre et al. (Reference Eizaguirre, Madeira and López.2010) and Pérez-Hedo et al. (Reference Pérez-Hedo, Albajes and Eizaguirre2011) observed that the Bt toxin produces an extended larval development in M. unipuncta and in other noctuids such as S. nonagrioides and suggested that this phenomenon could be explained as a defence mechanism against the Bt toxin. This prolonged development in the M. unipuncta larvae fed on the Bt diet might be the cause of the increased weight index observed here in those larvae in comparison with the decreased weight index that announces the purging process in the larvae fed on the non-Bt diet.
Shao et al. (Reference Shao, Cui, Liu, Yi, Ji and Yu1998) for H. armigera, and Whalon & Wingerd (Reference Whalon and Wingerd2003) and Heckel et al. (Reference Heckel, Gahan, Baxter, Zhao, Shelton, Gould and Tabashnik2007) for other Lepidoptera indicated that excessive or increased rates of toxin degradation could be other biochemical mechanisms of insensitivity to Bt toxins in insects. In the present study, this mechanism could also be present because the toxin concentration in the peritrophic membrane and its contents just one day after feeding on the Bt diet, quantified using ELISA, was ten times lower than the concentration in the Bt diet supplied to the larvae. While H. armigera larvae showed a similar low level of Bt toxin throughout the experiment, larvae of M. unipuncta showed a decreasing content of the Bt toxin during the experiment despite displaying a similar growth gain index to that of the larvae fed on the non-Bt diet. In addition to protein degradation, toxin excretion that is now being investigated in both species could be another mechanism to explain the lower levels of Bt toxin in the peritrophic membrane and its contents, as Rees et al. (Reference Rees, Jarrett and Ellar2009) indicated for a number of Lepidoptera larvae. According to Granados et al. (Reference Granados, Fu, Corsaro and Hughes2001), the peritrophic membrane is important for protecting the midgut epithelium from abrasion and also acts as a size-selecting ultrafilter that could be involved in the low permeability to Bt toxins entering the ectoperitrophic space. The low concentration of the toxin in the midgut epithelium suggests a low permeability of this membrane or, according to Whalon & Wingerd (Reference Whalon and Wingerd2003) and Ballester et al. (Reference Ballester, Granero, Tabashnik, Malvar and Ferre1999), either a low binding affinity or fewer binding sites or, again, the activity of digestive proteases. Finally, fast regeneration of midgut cells after exposure to sublethal doses of Bt toxin (Loeb et al., Reference Loeb, Martin, Hakim, Goto and Takeda2001; Ferre & Van Rie, Reference Ferre and Van Rie2002) could be the reason why the larvae needed only two days to recover from the ingestion of Bt. This rapid regeneration is confirmed with the images of the light electron and microscopy of the H. armigera larval midgut tissues.
Several structural changes were observed in the histopathological study of the midgut epithelium of the sixth instar larvae of H. armigera fed on the Bt diet. As midgut cells are the primary target tissue affected by the Bt toxin, the effects on them are a valid model system for assessing the mode of action of this toxin (Baines et al., Reference Baines, Schwartz, Sohi, Dedes and Pang1997). Cells of the midgut of the larvae intoxicated by ingestion of the Bt protein were altered in comparison with the control; columnar cells showed a modified shape and degradation of the microvilli (brush border) and goblet cells were swollen. These effects have also been found in other Lepidoptera provided with Bt protein, such as Manduca sexta (Spies & Spence, Reference Spies and Spence1985), Alabama argillacea (Sousa et al., Reference Sousa, Santos, Wanderley-Teixeira, Teixeira, de Siqueira, Alves and Torres2010), Spodoptera frugiperda (Knaak et al., Reference Knaak, Franz, Santos and Fiuza2010) and in Diptera, Simulium pertinax (Cavados et al., Reference Cavados, Majerowicz, Chaves, Araujo-Coutinho and Rabinovitch2004), and confirm the susceptibility of H. armigera to the toxin assayed.
The recovered larvae, fed first with the Bt diet and then with the non-Bt diet, showed midgut cell features similar to those of the control larva that had not ingested Bt protein. Similarly, Loeb et al. (Reference Loeb, Martin, Hakim, Goto and Takeda2001) reported that the ratios of cell types in the culture of midgut cells of Heliothis virescens larvae returned to approximate control levels two days after the toxin was provided, and Spies & Spence (Reference Spies and Spence1985) reported that midgut tissues of M. sexta larvae damaged by Bt toxin treatment recovered completely after six days, allowing normal development.
In summary, the results of this work indicate that multiple mechanisms could be involved in the low susceptibility of M. unipuncta and H. armigera to the Bt toxin. Although larvae of both species fed on a Bt diet, the low content of the toxin inside the peritrophic membrane and its contents 48h after ingestion indicate a high rate of toxin degradation in this space, a fast toxin excretion or both. Moreover, the larvae of M. unipuncta fed on the Bt diet that displayed a similar growth gain index to the larvae fed on the non-Bt diet showed an increasing elimination rate during the experiment. Although the effect of the toxin in the midgut epithelium cells was detected by light and electron microscopy, a low amount of toxin reached the epithelium, thus indicating a low permeability of the peritrophic membrane, a low affinity at the binding sites or the activity of the digestive proteases. Larvae of both species fed on the Bt toxin recovered very quickly, showing overcompensation mechanisms, and the recovery was detected not only in the weight gain but also in the midgut epithelium.
Acknowledgements
We thank Joan Safont and Aurora Ribes for their technical assistance. This study was partially funded by the Spanish R&D Agency (Comisión Interministerial de Ciencia y Tecnología) through project AGL2008-02355.