Introduction
Despite a ‘germ plasm’ having been reported as typical for oocytes of many metazoan animals it is currently recognized that mouse oocytes have none of this determinative substance (for a review see Matova & Cooley, Reference Matova and Cooley2001). A previous investigation (Reunov, Reference Reunov2004) showed that in laboratory mice Mus musculus the population of compact electron-dense bodies comparable with ‘germinal bodies' or ‘dense bodies' (for a review see Eddy, Reference Eddy1975) arose in the Graafian oocytes by condensation of some ‘mitochondrial derivatives' that in turn originated from mitochondria (Fig. 1). Much remains to be determined from a cytological perspective about the development of these mitochondrion-originated bodies (MB) in successive stages of the mouse life cycle.

Figure 1 Representation of germinal body formation in a Graafian oocyte of Mus musculus summarized from ultrastructural data (Reunov, Reference Reunov2004). Mch, mitochondrion; Cmd, condensing mitochondrial derivatives; Mb, mitochondrion-originated germinal-body-like structure. Arrows indicate the direction of MB development.
Because the early embryonic cells of mouse have also been reported as lacking inherited germ line determinants (Saffman & Lasko, Reference Saffman and Lasko1999; Matova & Cooley, Reference Matova and Cooley2001; Yoshimizu et al., Reference Yoshimizu, Obinata and Matsui2001), it would be interesting to determine whether MB might be found in them. Rather than oogenesis, where the germ plasm function and origin obviously overlap, spermatogenesis is the perfect model for the terminal stages of the germ plasm pathway restricted by determinative activity only. Thus, the spermatogenic cells are very suitable for studying possible morphological variability of MB during sex cell differentiation.
The aim of this study was to provide an ultrastructural description of MB in the 4.5-day embryonic cells and spermatogenic cells of M. musculus. Since such germ-plasm-related structures (GPRS) as ‘nuage', ‘chromatoid bodies' and ‘mitochondrial clusters' are known in mouse and other mammals (for a review see Eddy, Reference Eddy1975) these structures were also searched for possible affinity with MB. Ultrastructural study combined with morphometric analysis could provide interesting morphofunctional insight into the structure of sex cell determinants in mice.
Materials and methods
Transmission electron microscopy
The tissues of male gonads were prepared for electron microscopy as described previously (Reunov, Reference Reunov2004).
The 4.5-day mouse embryos were obtained, fixed, embedded and kindly placed at our disposal by Drs E.A. Kizilova and N.M. Matveeva of the Institute of Cytology and Genetics (Novosibirsk, Russia). The materials were delivered to our laboratory by Dr A.I. Shukalyuk. Sections were cut on an Ultracut-E (Reichert) ultramicrotome by a diamond knife, stained with uranyl acetate and lead citrate, and examined with a JEM 100 B transmission electron microscope. Before ultrathin sectioning the required parts of the materials were histologically identified using a Polyvar light microscope.
Morphometric study
4.5-day embryo cells
Five blocks containing one embryo each were sectioned for transmission electron microscopy. One technically perfect section through each embryo was studied, mounted on a slot grid coated with Formvar film stabilized with carbon. Thus, five sections were studied. On each section both the trophoblast cells and the inner cell mass of embryos were investigated. On the sections the embryos consisted of 25, 21, 23, 24 and 23 cells, respectively. Thus, sections of 116 cells were investigated. The MB and GPRS were identified and the frequency of the latter per cell section was calculated. The results were analysed by the Microsoft XL program using Student's t-test.
Spermatogenic cells
The identification of successive stages of male gametes was carried out accordingly to previous descriptions of mammalian spermatogenesis (Clermont, Reference Clermont1960; Roosen-Runge, Reference Roosen-Runge1962; Stefanini et al., Reference Stefanini, Conti, Geremia, Ziparo, Metz and Monroy1985; Johnson, Reference Johnson1995). Spermatogonia appeared to be in direct contact with the testis wall whereas spermatocytes were located above spermatogonia. Primary spermatocytes in zygotene–pachytene stages were distinguishable from spermatogonia in having more highly condensed chromatin containing synaptonemal complexes. The early spermatids were recognized by the presence of the proacrosomal vesicles.
The gonad particles from five male individuals were embedded. One block from each individual was randomly selected. Thus, five blocks were sectioned for transmission electron microscopic study. The sections were mounted on slot grids coated with Formvar film stabilized with carbon. One section from each of five blocks was studied. Thus, observation of five sections was done. Ten spermatogenic cells of each type (spermatogonia, zygotene–pachytene spermatocytes, early spermatids, late spermatids, spermatozoa) were investigated on each section. Thus, 50 spermatogonia, 50 zygotene–pachytene spermatocytes, 50 early spermatids, 50 late spermatids and 50 spermatozoa were investigated on these sections. In each cell type the MB and GPRS were identified and the frequency of the latter per cell section was counted. The results was analysed by the Microsoft XL program using Student's t-test.
Results
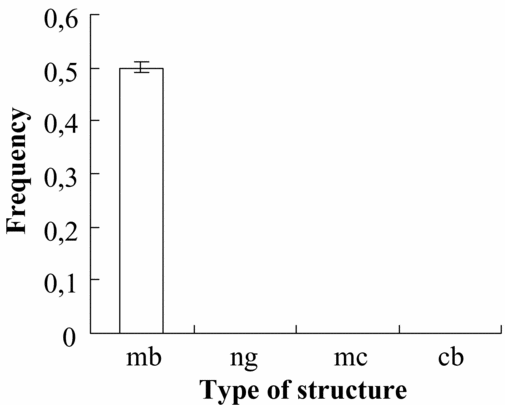
During examination of the 4.5-day embryo sections, bodies ultrastructurally similar to MB (Fig. 2A) were observed, though nuage, mitochondrial clusters and chromatoid bodies were not found (Fig. 3).

Figure 2 The mitochondrion-originated germinal-body-like structure (MB) and other germ-plasm-related structures (GPRS) in M. musculus. (A) MB in the cytoplasm of a 4.5-day embryo cell. (B) MB in the cytoplasm of a spermatogonium. (C) Fragmented MB or ‘nuage' in the cytoplasm of a spermatogonium. (D) Nuage fragment in contact with a mitochondrion in the cytoplasm of a spermatogonium. (E) Mitochondria aggregated by nuage fragments in the cytoplasm of a spermatogonium. (F) Mitochondria aggregated into a mitochondrial cluster by nuage fragments in the cytoplasm of a spermatogonium. (G) Membraneous conglomerate containing mitochondria, typical of mitochondrial clusters of zygotene–pachytene spermatocytes. (H) Membraneous conglomerate emerging from an ‘open' mitochondrion. Mb, mitochondrion-originated germinal-body-like structure; Ng, nuage; Ngf, nuage fragment; Mch, mitochondrion; Nu, nucleus; Mcg, membraneous conglomerate. Scale bars represent: (A)–(H), 0.5 μm.

Figure 3 Frequency of MB and GPRS per 4.5-day embryo cell section in M. musculus. mb, mitochondrion-originated germinal-body-like structure; ng, nuage; mc, mitochondrial cluster; cb, chromatoid body.
In spermatogonia compact MB were common(Fig. 2B). However, some MB were obviously fragmented and appeared as ‘nuage' (Fig. 2C). The nuage fragments were frequently found in contact with mitochondria (Fig. 2D) and the groups of mitochondria tended to be connected by these materials (Fig. 2E). Mitochondrial clusters consisting of several mitochondria were typically formed, being aggregated by nuage fragments (Fig. 2F). In spite of the MB, nuage and mitochondrial clusters being found quite frequently, chromatoid bodies were not found (Fig. 4A).

Figure 4 Frequency of MB and GPRS per spermatogenic cell section in M. musculus. (A) Spermatogonia. (B) Zygotene–pachytene spermatocytes. (C) Early spermatids. (D) Late spermatids. mb, mitochondrion-originated germinal-body-like structure; ng, nuage; mc, mitochondrial cluster; cb, chromatoid body.
On the sections of zygotene-pachytene spermatocytes compact MB were rarely found, though the nuage content was higher (Fig. 4B). The amount of mitochondrial clusters was higher than in spermatogonia (Fig. 4B). Immature chromatoid bodies, which presumably arise by coalescence of electron-lucent vesicles (Fig. 5A), were rarely observed in some spermatocytes (Fig. 4B). The mitochondria aggregated by nuage were typically found with large vacuoles containing membraneous conglomerate (Fig. 2G). Some of these mitochondria were ‘open' in vacuolar area and membraneous conglomerates were observed in the cytoplasm (Fig. 2H).

Figure 5 Chromatoid bodies in spermatogenic cells of M. musculus. (A) Chromatoid body that is forming in a zygotene–pachytene spermatocyte. (B) Mature chromatoid body in an early spermatid. (C) Chromatoid body located near the basal part of the nucleus of an early spermatid. (D) Nuage that has presumably arisen from a chromatoid body in the posterior part of a late spermatid. (E) Membraneous conglomerate in the vicinity of an ‘open' mitochondrion in a late spermatid.(F) Concave mitochondria lacking membraneous conglomerates. (G) Mitochondria located along the flagellum in a late spermatid. Cb, chromatoid body; V, vesicles that presumably form a chromatoid body; Nu, nucleus; A, acrosomal vesicle; Ng, nuage presumably formed from chromatoid bodies; Mch, mitochondria; Fl, flagellum; Mcg, membraneous conglomerate. White star indicates an ‘open' mitochondrion; black stars indicate concave mitochondria lacking membraneous conglomerates. Scale bars represent: (A)–(D), 1 μm; (E), 0.3 μm; (F) and (G), 0.5 μm.
Observation of the sections of early spermatids showed that MB, nuage and mitochondrial clusters are no longer seen, but chromatoid bodies occurred with comparatively high frequency (Fig. 4C). These quite large electron-dense membrane-free structures marked by electron-lucent cavities in their central portion presumably represent mature chromatoid bodies (Fig. 5B). During acrosome formation the chromatoid body is typically located near the basal part of the nucleus (Fig. 5C).
On the late spermatid sections the nuage that presumably originated from chromatoid bodies by dispersion of the latter was observed, located in the posterior part of the cell (Fig. 5D). Though the content of nuage fragments in the late spermatids was high, MB, mitochondrial clusters and compact chromatoid bodies were not been found (Fig. 4D). In late spermiogenesis the mitochondria typically seemed to undergo transformation similar to that in spermatocytes. The membraneous conglomerates were quite often found both inside mitochondria as well as in the vicinity of ‘open' mitochondria (Fig. 5E). The organelles lacking the membraneous conglomerates first appeared concave (Fig. 5F), but when located along the flagellum were observed with a restored shape (Fig. 5G).
On the sections of spermatozoa no signs of any MB or GPRS were found.
Discussion
In mouse embryogenesis the primordial germ cells appear in the 8-day embryo (Wassarman & Josefowicz, Reference Wassarman and Josefowicz1978) and compact structures comparable to germinal granules were described in these cells (Spiegelmann & Bennett, Reference Spiegelmann and Bennett1973). However, oocytes and the earlier stages of embryogenesis have been reported as lacking inherited germ line determinants (Matova & Cooley, Reference Matova and Cooley2001). Nevertheless, since germinal-body-like structures (MB) were found in Graafian oocytes (Reunov, Reference Reunov2004) and 4.5-day mouse embryo (present study), the existence of germ line determinants in oocytes and early embryogenesis seems a possibility. It is difficult to say whether the MB-containing cells of the 4.5-day embryo are future primordial sex cells or whether the presence of MB is universal for early embryonic cells. To help answer this question more detailed ultrastructural study of mouse early embryogenesis is required. Because MB were found without any ultrastructural alterations in 4.5-day embryo cells (present study) and primordial cells of later embryos (Spiegelmann & Bennett, Reference Spiegelmann and Bennett1973) MB could be suspected to be inactive during embryogenesis.
It is very tempting to speculate that in spermatogonia and spermatocytes MB disintegrate into nuage (Fig. 6A, B). In support of this notion, is the fact that the frequency of MB decreased whereas that of nuage increased during the transition from spermatogonia to spermatocyte (Fig. 4A, B). In addition, in accordance with Mahowald's suggestion, the polar or germinal granules formed in late oocytes during embryogenesis transform into nuage that is present in germ cells (Mahowald, Reference Mahowald1977). In fact, it seems as though ‘Mahowald's rule', formulated for Drosophila, also holds for mouse, though in M. musculus the germinal-body-like structures presumably pass through embryogenesis without being dispersed and become nuage in meiotic cells only.

Figure 6 Representation of MB differentiation connected with mitochondria in spermatogonia and zygotene–pachytene spermatocytes of M. musculus. (A) Mitochondrion-originated germinal-body-like structure. (B) Fragmented germinal body-like structure or nuage. (C) Mitochondrial cluster formed by nuage fragment. (D) Mitochondrial cluster with mitochondria containing membraneous conglomerates. (E) Mitochondrial cluster with mitochondria excreting membraneous conglomerates. Mb, mitochondrion-originated germinal body; Ng, nuage; Ngf, nuage fragment (or intermitochondrial cement); Mch, mitochondrion; Mcg, membraneous conglomerate.
The amount of mitochondrial clusters increased during the development of spermatogonia into primary spermatocytes (Fig. 4A, B) and probably the nuage originated from MB plays a key role in the formation of these structural complexes. The nuage fragments were observed in contact with single mitochondria as well as among the mitochondria of mitochondrial clusters (Fig. 6C). Mitochondrial clusters or mitochondria aggregated by ‘intermitochondrial cement’ (Flores & Burns, Reference Flores and Burns1993) have been recorded from a wide range of invertebrates and vertebrates (Eddy, Reference Eddy1975; Aizenstadt & Gabaeva, Reference Aizenstadt and Gabaeva1987; Williams, Reference Williams1989; Inoue & Shirai, Reference Inoue and Shirai1991; Wilsch-Brauninger et al., Reference Wilsch-Brauninger, Schwarz and Nusslein-Volhard1997), though the origin of the cement is not clear. It seems possible that in mouse spermatogenic cells the intermitochondrial cement arises by fragmentation of compact MB.
Although the formation of nuage–mitochondrial clusters has been recorded in the early gametogenic cells of many metazoan animals, the explanation of this phenomenon has yet to be elucidated. In mouse spermatocytes the mitochondria of mitochondrial clusters underwent excretion of membraneous conglomerates (Fig. 6D, E). This finding is not unique for mouse. The release of mitochondrial substance into the cytoplasm was first suggested for spermatogonia of the sea urchin Anthocidaris crassispina (Reunov et al., Reference Reunov, Isaeva, Au and Wu2000). According to the data of Villegas et al. (Reference Villegas, Araya, Bustos-Obregon and Burzio2002) the 16S mitochondrial rRNA is transferred from the organelles to the nucleus of spermatogonia before the onset of meiosis. As stated by these authors the translocation of mitochondrial rRNA to the nucleus occurs by an unknown mechanism. Maybe this mechanism is conducted by excretion of mitochondrial matter into the cytoplasm followed by penetration of mitochondrial molecules into the nucleus. It seems that detailed cytological and molecular investigation of excreted membraneous conglomerates as well as their possible attraction to the nucleus is desirable to highlight the role of mitochondrial clusters in early spermatogenic cells of the mouse.
Chromatoid bodies have previously been recorded for spermatids of mouse and rat (Fawcett, Reference Fawcett1972; Eddy, Reference Eddy1975; Clermont & Rambourg, Reference Clermont and Rambourg1978). In agreement with our observations, these structures first arose in spermatocytes (Fig. 4B) as a consequence of the aggregation of electron-lucent vesicles and are observed more often on the sections of early spermatids (Fig. 4C). According to Fawcett (Reference Fawcett1972) the chromatoid bodies participate in spermatid tail differentiation and our data are in agreement with this opinion. Indeed, in the middle part of late spermatids the chromatoid bodies were seen to be dispersed into nuage. This is probably a reason for the chromatoid body replacement by nuage that occurs during differentiation of early spermatids into late spermatids (Fig. 4C, D). Given the absence of chromatoid-body-originated nuage in spermatozoa one may speculate that this substance disappeared by being intermingled with cytoplasm of the posterior part of the spermatid during late spermiogenesis. Though in spermatids the mitochondria do not aggregate into clusters, the excretion of membraneous conglomerates similar to that in spermatocytes was found and the significance of this feature is not clear. In any case, the excretion of membraneous conglomerates was noticed to be parallel to chromatoid body dispersion. No studies have determined the possible influence of chromatoid body matter on mitochondria, and future experimental work is required to clarify this suggestion. Although the mechanism by which the chromatoid body arises and its role in spermatid differentiation have yet to be elucidated in more detail, it seems obvious that this material is not implicated in the MB track.
Acknowledgements
Many thanks go to my wife, Ms Y.A. Reunova, and to Dr O.V. Yurchenko and Ms Y.N. Alexandrova, for their kind help. I am very grateful to Drs E.A. Kizilova, N.M. Matveeva and A.I. Shukalyuk for mouse embryos. Thanks go also to Mr D.V. Fomin for his help in the TEM units of the Institute of Marine Biology. This work was supported by grants from the Far East Branch of the Russian Academy of Sciences (06-III-A-06-170; 06-III-A-06-162), by the Russian Fund of Basic Research (N 06-04-48744) and by the Fund of Help for Native Science for A. Reunov.