Significant outcomes
-
∙ The uptake rate of 18F-FDG in the forced swim group showed a significant decrease in the prefrontal cortex (0.86±0.20%ID/g, p<0.01) and thalamus (0.77±0.17%ID/g, p<0.05).
-
∙ The uptake rate of 123I-IMP in the forced swim group remained unchanged in all regions.
-
∙ Our finding suggested that a forced swim stress can cause mismatch change of rCBF and rCMRglc.
Limitations
-
∙ As the uptake rate of 18F-FDG and 123I-IMP were measured in the different subjects, the results did not indicate the direct correlation between rCBF and rCMRglc.
-
∙ The blood levels of lactate and glucose were measured only in animals injected with 18F-FDG but not in those with 123I-IMP.
-
∙ Therefore, it was not possible to test whether the expected higher lactate is present in animals with 123I-IMP.
Introduction
A close correlation of regional cerebral blood flow (rCBF) and regional cerebral metabolic rate of glucose (rCMRglc) was found in autoradiographic studies in rats (Reference McBean, Ritchie, Olverman and Kelly1).
However, altered coupling of rCBF and rCMRglc was reported in patients with depression. Dunn et al. (Reference Dunn, Willis and Benson2) were the first to show the uncoupling of rCBF and rCMRglc in patients with depression, although rCBF and rCMRglc are closely correlated in controls and in patients with bipolar disorder. Depression is considered as a stress-related disorder understanding the role of stress as a key determinant in disease aetiology. Therefore, the mismatch change of rCBF and rCMRglc could be a stress-related phenomenon.
Similarly, inconsistency of rCBF and rCMRglc has been observed in an animal model of depression. Van Donkelaar et al. (Reference van Donkelaar, Ferrington, Blokland, Steinbusch, Prickaerts and Kelly3) measured the rCBF using [14C]-iodoantipyrine and the rCMRglc using [14C]-2-deoxyglucose by autoradiography in acute tryptophan depletion rats. In that model, the rCBF was significantly decreased in the hippocampus, amygdala, thalamus and parietal cortex, but there was no overt alteration of the rCMRglc in any brain region. Although the Van Donkelaar study was the first report showing a mismatch in the changes of the rCBF and rCMRglc in an animal model of depression, the possibility cannot be excluded that pharmacological effects produced by tryptophan depletion were the cause of the selective decrease of the rCBF without alteration of the rCMRglc. Indeed, tryptophan depletion causes serotonin deficiency in the brain (Reference Delgado, Miller and Salomon4, Reference Booij, van der Does, Haffmans, Spinhoven and McNally5), and serotonin is known to possess vasoactive properties (Reference Vecsei, Szalardy, Fulop and Toldi6).
In the present study, to test whether the inconsistency of rCBF and rCMRglc related to stress, we examined rCBF and rCMRglc in rats exposed to forced swim stress, which has long been used as an animal model for assessing the effects of antidepressants activity (Reference Porsolt, Le Pichon and Jalfre7). And the uptake values were measured in prefrontal cortex, hippocampus and thalamus, which were associated with stress and easy to be separated from rat brain. The uptake rate in cerebellum which is not associated with stress was as a reference value for other region.
Materials and methods
Animals
All experiments were performed in accord with the Guide for Animal Experimentation at the Hamamatsu University School of Medicine. Forty-three male Sprague-Dawley rats (6 weeks old, 180–200 g; Japan SLC, Hamamatsu, Japan) were used. The rats were divided into four groups: 123I-IMP + control: n=11, 123I-IMP+forced swim stress: n=11, 18F-FDG+control: n=10, 18F-FDG+forced swim stress: n=11. They were housed in cages (three rats in each cage) in a temperature (25°C)-controlled room on a 12-h light, 12-h dark cycle (lights on from 07:00 am to 19:00 pm) with free access to food and water. The rats were habituated to the environment with daily handling for 5 days, and were fasted but allowed access to drinking water on the day before the experiment. The body weights of the rats were monitored every day.
Forced swim stress
A single rat was placed into a clear acrylic cylinder (dia. 25 cm, height 60 cm) containing water controlled to a temperature of 25±1°C. The water depth was adjusted to 40 cm so that the rat must swim or float without its hind limbs or tail touching the bottom. The rat was forced to swim for 15 min. Its behaviours were recorded with a video camera and scored at 5-s intervals, assigned as one of three categories: swimming, climbing and immobility. As soon as possible after the forced swimming, the rat was placed in a cage with a warm mat for 30 min for its body temperature to recover. For the control groups, each rat was placed into the same cylinder without water for 15 min and then kept in the cage without a warm mat for 30 min.
Evaluation of rCBF and rCMRglc
As the tracer for the rCBF, we used N-isopropyl-4-[123I] iodoamphetamine (123I-IMP) (Perfusamine® Nihon Medi-Physics, Tokyo, Japan), and as the tracer for the rCMRglc, we used 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) (automatic synthesiser F-200; Sumitomo Heavy Industries, Tokyo, Japan). At 30 min after forced swimming, its rectal temperature was measured and a tracer was injected through the tail vein at a constant rate over the first 30 s of the experiment (123I-IMP: 0.74 MBq per rat in 0.5 ml saline, 18F-FDG: 5.55 MBq per rat in 0.5 ml saline).
The rats in the two IMP groups were decapitated at 5 min after the injection of 123I-IMP, and the rats in the two FDG groups were decapitated at 45 min after the injection of 18F-FDG, which is the time to peak of each tracer concentration. A total of 2 ml of blood from the neck stump was collected in counting vials to measure the radioactivity in blood. According to the atlas of Paxinos and Watson (Reference Paxinos and Watson8), the tissues were sampled bilaterally from four brain regions: the prefrontal cortex, hippocampus, thalamus and cerebellum. The radioactivity of 123I (T 1/2=13.27 h) and 18F (T 1/2=1.83 h) in each brain sample and the blood was measured in an automatic scintillation gamma counter (Wizard 1480; Perkin-Elmer, Yokohama, Japan). The radioactivity was measured in bilateral samples as a whole in each brain region. The radioactivity of counter per minutes (cpm) was converted to becquerel (Bq), and the uptake rates of 123I-IMP and 18F-FDG are expressed as the percentage injected dose per gram of body weight (%ID/g), which was calculated as [the radioactivity in each tissue (Bq)/weight of the tissue (g)]/[injected dose of the tracer (Bq)/weight of the animal (g)]. The 18F-FDG uptake rate was compensated for by the blood concentration of glucose.
Measurement of lactate and glucose
A total of 2 ml of blood was also collected from each rat’s neck stump to determine whether the blood levels of lactate and glucose were altered after forced swimming. The concentrations of lactate and glucose in the blood were measured with a blood gas analyser (i-STAT; Abbott Point of Care, Princeton, NJ, USA).
Statistical analyses
All statistical analyses were performed using GraphPad Prism software (version 5.00; GraphPad Software, San Diego, CA, USA) and SPSS software (version 18.0 J; IBM, Tokyo, Japan). We tested the main effect of the forced swim stress on the uptake rates of 123I-IMP and 18F-FDG derived from four brain regions using a two-way repeated analysis of variance (ANOVA) followed by a post hoc Bonferroni’s test. The two different categorical independent variables were Control/FS and Brain region with the continuous dependent variable being uptake of 123I-IMP and 18F-FDG, respectively, and statistical significance was set at p<0.05. All quantitative data in the text are expressed as mean values±standard error of the mean (SEM).
The correlation coefficients between the uptake rates and the measured values including body weight, body temperature, lactate, glucose and behavioural scores were obtained using Spearman’s rank correlation coefficient after a distribution analysis of Shapiro–Wilk normality test.
Results
The body weights and body temperatures did not differ significantly between the control group and FS group in both the 123I-IMP and 18F-FDG treatments (Table 1). There were no significant differences in the blood levels of lactate or glucose in the 18F-FDG analysis (Table 1).
Table 1 Characteristics of the rats in the control and forced swim groups

FDG, fluorodeoxyglucose; FS, forced swim; IMP, N-isopropyl-4-iodoamphetamine.
The uptake rate of 123I-IMP in the control group was 8.70±2.64 for the prefrontal cortex, 5.86±2.01 for the hippocampus, 6.20±1.85 for the thalamus and 5.55±1.68%ID/g for the cerebellum, whereas that in the FS group was 9.18±1.00 for the prefrontal cortex, 5.76±0.51 for the hippocampus, 6.36±0.45 for the thalamus and 5.66±0.37%ID/g for the cerebellum. Therefore, both groups showed similar uptake rates of 123I-IMP in each of the brain areas tested (Fig. 1a).
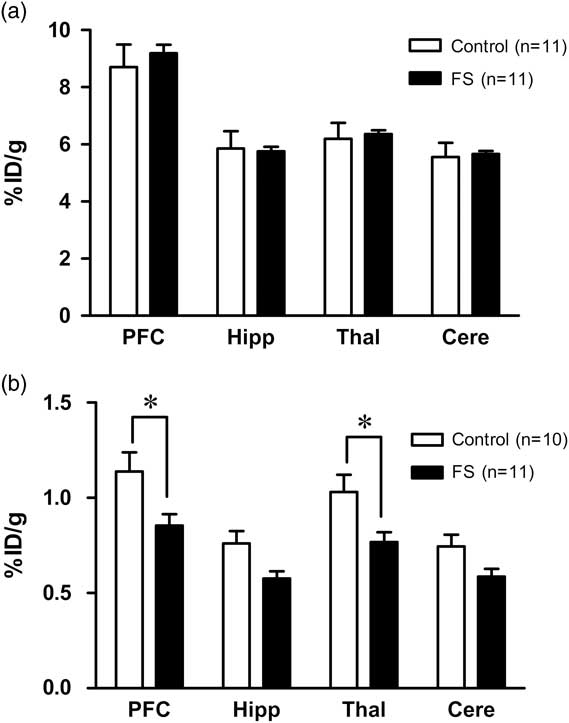
Fig. 1 (a) There was no significant difference in the 123I-IMP uptake rate in any region of the brain between the control group (n=11) and the FS group (n=11). (b) Compared to the control group (n=10), the uptake rate of 18F-FDG in the FS group (n=11) showed a significant reduction in the prefrontal cortex and thalamus, but not in the hippocampus or cerebellum. Data are mean±SEM. *p<0.05. Cere, cerebellum; FS, forced swim; Hipp, hippocampus; PFC, prefrontal cortex; Thal, thalamus.
In contrast, a significant effect of the forced swimming on the regional uptake rates of 18F-FDG was observed [F(1,19)=6.09, p=0.023]. The post hoc Bonferroni’s test revealed that the regional uptake rates of 18F-FDG in the forced swim group was significantly lower than that of the control group in both the prefrontal cortex (1.14±0.32%ID/g for the control group vs. 0.86±0.20%ID/g for the forced swim group, p<0.05) and the thalamus (1.03±0.29%ID/g for the control group vs. 0.77±0.17%ID/g for the forced swim group, p<0.05). Although the differences did not reach the statistical significance, the uptake rate of 18F-FDG tended to be lower in the forced swim group than the control group in the hippocampus (0.76±0.20%ID/g for the control group vs. 0.58±0.13%ID/g for the forced swim group) and the cerebellum (0.74±0.20%ID/g for the control group vs. 0.59±0.13%ID/g for the forced swim group) (Fig. 1b).
There were no significant correlations between either tracer’s uptake rate and the measured values including body weight, body temperature, serum levels of lactate and glucose, and the behavioural scores (data not shown).
Discussion
When we measured the uptake rate of 123I-IMP in the rats’ prefrontal cortex, hippocampus, thalamus and cerebellum 30 min after the forced swim stress, there was no overt change in any of these brain areas. However, the uptake rate of 18F-FDG measured in the brain was globally decreased. In particular, the uptake rate of 18F-FDG for the prefrontal cortex and thalamus was significantly decreased, whereas that for the hippocampus and cerebellum did not reach a significant difference.
The uptake values were measured 35 min after swimming for 123I-IMP and 75 min for 18F-FDG, because the longer period was required for the clearance of the latter. One may argue that the two indices, rCBF and rCMRglc, might have not represented exactly the same time point after the forced swim. Although there is no easy solution for this issue, the timing of the injection was adjusted between the two tracers in this study. Therefore, rCBF and rCMRglc could have reflected close time periods after the injection of each tracer.
A forced swim in water can induce hypothermia, which cause alterations of the rCMRglc and the blood level of glucose (Reference Arai, Tsuyuki, Shiomoto, Satoh and Otomo9–Reference Abel11). In the present study, however, the decrease in the rCMRglc was independent of hypothermia; the rCMRglc was measured after the recovery of body temperature. Our results therefore suggest that forced swim stress could induce inconsistency of the rCMRglc and rCBF in certain brain regions, including the prefrontal cortex and thalamus.
Under physiological conditions, there is a close correlation in the mammalian brain between the rCMRglc and the rCBF (Reference McBean, Ritchie, Olverman and Kelly1, Reference Dunn, Willis and Benson2). In the present study, however, the rCMRglc was decreased after the forced swim stress, although the rCBF remained unchanged in all regions. Blood-borne glucose is the main fuel for the brain and can support its energy demand. However, since the blood concentration of glucose remained unchanged after the forced swim stress in the present experiment, it may well be that the decrease in the rCMRglc after the forced swim is not due to the alteration of the blood glucose level. Recent studies have shown the potential role of lactate as a supplemental fuel for the brain (Reference Simpson, Carruthers and Vannucci12). Therefore, the increase in the blood lactate concentration could make lactate available to the brain, and the brain switches from a net producer of lactate to an importer (Reference Videbech13) and glucose uptake is reduced (Reference Kemppainen, Aalto and Fujimoto14). However, this possibility is not applicable to our finding of inconsistency of the rCMRglc and rCBF, because the rats’ blood lactate concentrations were not altered. Although the mechanism for decreased rCMRglc, without no change in rCBF, is not known, forced swim stress can increase plasma levels of corticosterone (Reference Rogoz, Kabzinski, Sadaj, Rachwalska and Gadek-Michalska15), which have inhibitory effects on glucose transport in rats (Reference Garcia-Bueno, Caso, Perez-Nievas, Lorenzo and Leza16).
In the present study, the forced swim stress induced the decreased rCMRglc in the prefrontal cortex and thalamus. The result is not contrast with those by Jang et al. (Reference Jang, Lee, Lee, Park, Cho and Kim17), who have shown reduced rCMRglc in the hippocampus, inferior colliculus, orbital cortex and insula during forced swim stress in rats by using a 18F-FDG micro-PET imaging. However, rCMRglc in the striatum and cerebellum was simultaneously increased (Reference Jang, Lee, Lee, Park, Cho and Kim17). The previous study have also demonstrated that rCMRglc measured by [14C]-2-deoxyglucose uptake during forced swim stress is increased in prefrontal cortex, motor cortex, and lateral septum, associated with increased fos-like-immunoreactivity in these areas (Reference Duncan, Johnson and Breese18). Taken together, forced swim stress can cause alteration in rCMRglc, which is dependent on the time of its assessment: increased or decreased rCMRglc in some brain areas during forced swim, and decreased rCMRglc after forced swim. It is not known whether or how function of glucose transport is involved in the rCMRglc during and after forced swim stress. The blood levels of lactate and glucose were measured only in animals injected with 18F-FDG but not in those with 123I-IMP. Therefore, it was not possible to test whether the expected higher lactate is present in animals with 123I-IMP.
In conclusion, a forced swim stress can cause mismatch change of rCBF and rCMRglc, which is reflect a stress-related phenomenon.
Acknowledgements
The authors thank Ms. Tae Takahashi, Ms. Erina Sakamoto, and Ms. Mika Oyaizu for their excellent technical assistance. They thank KN International (USA) for assistance with English usage.
Authors Contributions: Y.K. and H.M. carried out animal experiments, performed the statistical analysis, participated in the design of the study and drafted the manuscript. Y.I., S.T., K.I. and K.S. performed the statistical analysis and participated in the sequence alignment and drafted the manuscript. K.T. and T.W. carried out animal experiments, participated in the design of the study. M.Y. and N.M. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Financial Support
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (to Y.K.), a Grant-in-Aid for Scientific Research (B) from MEXT (to N.M.), and a Grant-in-Aid for Young Scientists (B) from MEXT (to Y.K.).
Conflicts of Interest
The authors declare no conflict of interest.





