Introduction
Anemone nemorosa L. (wood anemone) is a member of the Ranunculaceae subfamily Ranunculoideae. The Ranunculaceae consists of 2525 species in 62 genera, divided into five subfamilies (Stevens, Reference Stevens2005). A. nemorosa is a perennial herbaceous geophyte with dormant underground rhizomes. It is found across northern temperate Europe and western Asia. Habitat preferences for A. nemorosa include woodlands and damp calcareous pastures; it is also found on scree, wastelands, and shaded mires and cliffs (Grime et al., Reference Grime, Hodgson and Hunt1988). Seeds germinate in the spring after a period of moist, cool conditions in which embryo growth occurs; germination percentages are particularly high after a severe winter (Grime et al., Reference Grime, Hodgson and Hunt1988). Shoots emerge between March and May and flowering occurs 2 weeks after emergence. Flowers are typically white, hermaphrodite, self-incompatible and insect pollinated. Fruits ripen and disperse between May and June. Seeds do not form a persistent seed bank (Stehlik and Holderegger, Reference Stehlik and Holderegger2000), and reproduction is thought to be vegetative, with some reports of seedling recruitment (Eriksson, Reference Eriksson1995; Holderegger, Reference Holderegger1996; Holderegger et al., Reference Holderegger, Stehlik and Schellner1998).
The Ranunculaceae is a family reported to have morphological dormancy (MD) (Baskin and Baskin, Reference Baskin and Baskin1998), with rudimentary or linear embryos (Martin, Reference Martin1946). Typically, morphologically dormant seeds are defined as having small, underdeveloped embryos (the embryo may only occupy 1% of the seed) that may or may not be differentiated (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin, Baskin, Smith, Dickie, Linington, Pritchard and Probert2003). Seeds defined as exhibiting MD typically need a period of time, the ‘dormancy period’, for the embryos to grow to a species-critical length before germination can occur. When seeds are shed with undifferentiated embryos, both differentiation and growth occur before radicle emergence. If seeds require longer than 30 d for radicle protrusion, they are defined as having morphophysiological dormancy (MPD) (Baskin and Baskin, Reference Baskin, Baskin, Smith, Dickie, Linington, Pritchard and Probert2003). Seeds with MPD have both underdeveloped embryos and exhibit physiological dormancy (failure to germinate due to an inhibition mechanism within the embryo or embryo-covering structures). Different types of MPD are overcome by different warm and/or cold stratification periods.
Engell (Reference Engell1995) reported that spring-dispersed A. nemorosa embryos were undifferentiated at the time of dispersal; embryo maturation occurred over a cultivation period of a few months. However, the conditions under which the achenes were cultivated were not described, nor how long it was before embryo differentiation began. Previous work (1976) carried out at Wakehurst Place looked at the germination requirements of A. nemorosa seeds collected from a local population (R.H. Sanderson, unpublished). Freshly harvested seeds were sown at temperatures between 2 and 31°C; optimal germination was obtained at 11°C, with no germination at 21°C and above. The slow germination observed (>250 d) would suggest that A. nemorosa would be categorized as having MPD. Following 16 weeks of warm stratification (21°C), germination at 11°C was more rapid and uniform. However, since warm stratification was not absolutely necessary before germination occurred, this behaviour does not fit any of the Baskin et al. (1995) types of MPD. Similarly, Shirreffs (Reference Shirreffs1985) found that germination of A. nemorosa did not occur at 18 or 21°C, but the seeds would germinate without stratification between 11 and 17°C. Seeds initially sown at 18 or 21°C germinated when transferred to 14°C, but germinated poorly when transferred to 17°C. Shirreffs (Reference Shirreffs1985) also found that cold stratification at 4°C for 16 weeks, followed by subsequent germination at 15°C, increased the rate of germination, and that storing seeds in dry storage for a year reduced viability.
The present study was prompted by the fact that two conservation collections of A. nemorosa held at the Millennium Seed Bank failed to germinate in routine germination tests after just 1 year of storage [ − 18°C after drying to equilibrium at 15% relative humidity (RH)]. Very low viability was confirmed by a tetrazolium test. As well as having undifferentiated embryos, A. nemorosa seeds are dispersed when they are still green, further indicating that they may not be fully mature at this point; even if this species can be categorized as desiccation tolerant (orthodox), immaturity at the time of natural dispersal may result in desiccation sensitivity and/or poor longevity (Hay and Smith, Reference Hay, Smith, Smith, Dickie, Linington, Pritchard and Probert2003). Thus, we tested the hypothesis that the poor viability of these A. nemorosa seeds was caused by immaturity, and hence desiccation sensitivity, of the embryos at the time of collection. Post-harvest/post-dispersal differentiation and growth of embryos was also examined in the context of desiccation tolerance, storability and the classification of seed dormancy.
Materials and methods
Seed collection and post-harvest treatments
Achenes (hereafter referred to as seeds) were collected at the time of natural dispersal (off heads from which some seeds had already dispersed) from a population of A. nemorosa in Wickham Woods, Haywards Heath, West Sussex [Ordnance Survey (OS) grid reference TQ 333 267] in May 2003 and May 2004. In 2003, bulk lots of seeds were placed either on plain water agar (10 g l− 1) at 20°C in 50 × 120 × 170 mm (H × W × D) clear plastic boxes (‘laboratory conditions’) or in nylon (1 mm mesh) bags buried in the leaf litter at the collection site (‘field conditions’) for 14, 28, 56 or 112 d. In 2004, seeds under laboratory conditions were stored for 0 (control), 5, 11, 14, 18, 22 or 28 d, while seeds under field conditions were left for 56, 84, 112, 140 or 168 d. Temperature data for field conditions were recorded every 30 min, using a Tiny Tag PT 100 temperature logger (Gemini, Chichester, West Sussex, UK) placed in the leaf litter along with the nylon sachets throughout the course of the experiment. Heat sum (°C d) was calculated for field and laboratory treatments, assuming a base temperature of 0°C, following Daws et al. (Reference Daws, Cleland, Chmielarz, Gorian, Leprince, Mullins, Thanos, Vandvik and Pritchard2006):
![Heat \,\,\,sum = {{ {\sum [temp (\deg C)\,at\,logging\,interval\times logging\,interval\,(h)]}}\over{{24}}}](https://static.cambridge.org/binary/version/id/urn:cambridge.org:id:binary:20151024042128241-0100:S0960258507783149_eqnU1.gif?pub-status=live)
Seed development and quality
A number of measurements were carried out on the control seeds and after each post-harvest treatment period. Seed equilibrium relative humidity (eRH) was measured on each control or post-harvest treated seedlot using an AW Water Activity Monitor in conjunction with a Hygropalm 3 (Rotronic, Crawley, West Sussex, UK). Fresh seed germination tests were carried out by sowing four replicates of 25 seeds each on plain water agar (10 g l− 1) held in 90-mm Petri dishes (Bibby Sterilin, Stone, Staffordshire, UK); these were warm stratified at 20°C for up to 112 d depending on the length of the post-harvest treatment, e.g. seeds that had been given a 28 d post-harvest treatment were warm stratified for 84 d to give a total of 112 d before being transferred to fresh plain water agar (10 g l− 1 at 10°C). Seeds were scored for germination (radicle emergence) weekly for 504 d (2003) or 490 d (2004).
Desiccation tolerance was assessed for each seedlot by drying 100 seeds for 21 d in a dry-room maintained at 15% RH and 15°C. Germination tests were carried out as for germination tests of fresh seeds, with the duration of post-harvest treatment taken into account with respect to the period of warm stratification. Non-germinating seeds were confirmed as non-viable by a cut-test at the end of the germination test.
In 2003, additional seeds were included for water content determination and controlled ageing tests. Gravimetric water content was determined on control seeds, on seeds at the time of removal from the treatments, and after drying in the dry-room. For each seedlot, three replicates of 50 seeds were dried in aluminium crucibles at 103°C for 17 h in a ventilated oven (ISTA, 1985). Seed water content (WC) is expressed as a proportion of the dry weight (DW). Controlled ageing was carried out on control and field- or laboratory-treated seeds that had been dried for 21 d in the dry-room after retrieval. Seeds were rehydrated as 12 subsamples of 50 seeds in an electrical enclosure box (300 mm H × 300 mm W × 102 mm D; Ensto Briticent, Parvoo, Finland) at 47% RH and 20°C for 14 d, before ageing in a similar box at 60% RH and 45°C (Davies and Probert, Reference Davies and Probert2004). Non-saturated LiCl solutions were used to create the desired RH. Subsamples were removed at regular intervals, and germination tests were carried out as before.
Embryo development
In 2004, in order to examine embryo changes during the laboratory post-harvest treatment, additional seed samples were included for later sectioning and examination. Since there was lower seed set at the collection site in 2004 compared with 2003, it was not possible to collect enough seeds to make the same measurements for field-treated samples. At the time of retrieval, 25 seeds were fixed in formalin–acetic–alcohol (formaldehyde, glacial acetic acid and denatured ethanol, 10 : 5 : 85 v/v). Seeds were stored at 5°C for at least 14 d and then dehydrated through a denatured ethanol series (70–100%). LR white medium-grade acrylic resin (London Resin Company, Theale, Berkshire, UK) was progressively introduced to samples over a total period of at least 14 d, using ratios of 3 : 1, 1 : 1, 1 : 3 and 0 : 1 denatured EtOH : resin at 5°C. The resin was then polymerized in gelatine capsules (size 000; Agar Scientific, Wetzlar, Germany) in a vacuum oven (Binder, Tuttlingen, Germany) at 58–60°C and 440 mmHg for 24 h. Three 5 μm sections were made from each of 10 seeds per seedlot using a microtome (1140 Autocut, Reichert-Jung, Wetzlar, Germany). Sections were temporarily mounted on 25 × 75 × 1 mm slides (Menzel-Glaser SuperFrost Plus, Braunschweig, Germany). Sections were stained with 0.5% Toluidine Blue-O (Electrophoresis Grade; FisherBiotech, Slangerup, Germany) for 15 min and rinsed with distilled water. After surface drying, sections were dehydrated through an absolute EtOH series (50%, 70%, 90%, 4 × 100%). Histo-Clear solution (Fisher Scientific, Schwerte, Germany) was applied to the slides for 5 min prior to permanent mounting in Eukitt Mounting Medium (O. Kindler GmbH & Co., Baden-Nürttemberg, Germany). Images of sections for each seedlot were taken at 1.6, 5, 10 and 20 × magnifications using a Stemi SV 11 Microscope (Carl Zeiss, Welwyn Garden City, Herts, UK). Images were used to measure the area of both the endosperm and embryo from samples of up to 10 seeds to quantify aspects of embryo development during post-harvest treatment.
Statistical analysis
All analyses, including analysis of variance (ANOVA) and probit analysis (of seed longevity data), were carried out using GenStat for Windows, Version 5 (VSN International Ltd, Hemel Hempstead Herts, UK).
Results
Field temperature
In 2003, the mean temperature of the soil and leaf litter surrounding the field samples was 10.2°C, with a maximum temperature reading of 20.0°C in August and a minimum of − 1.5°C in January (Fig. 1). In 2004, the mean temperature of the soil and leaf litter surrounding the field samples was 13.3°C; the maximum temperature reached was 19.0°C soon after collection in May and the minimum temperature recorded was 3.0°C in November when the last seedlot was retrieved from the leaf litter.

Figure 1 Field temperatures during the post-harvest treatments of Anemone nemorosa seeds in (a) 2003 and (b) 2004.
Seed dry weight and water content
In 2003, the water content of the seeds in the laboratory remained high [average of 1.97 g (g DW)− 1] throughout the post-harvest treatment (Fig. 2). In contrast, the water content of seeds in the field started to fall after 28 d and had dropped to ≥ 0.25 g (g DW)− 1 after 112 d. Even though there had been a decline in water content, the eRH of these seeds was still high (>98%; data not shown). Similarly, in 2004, there was a small ( < 3%) reduction in eRH of field-treated seeds after 168 d, while laboratory-treated seeds did not show any reduction in eRH over the 28 d (data not shown). Mean seed dry weight declined by about 20% over the first 56 d, from 0.125 mg down to c. 0.1 mg (2003; Fig. 2). There was no further change in dry weight up to 112 d.

Figure 2 Changes in mean dry weight (circles) and water content (squares) of Anemone nemorosa seeds during laboratory (open symbols) and field (closed symbols) post-harvest treatments in 2003.
Desiccation tolerance
In 2003, fresh, non-dried seed viability remained high (c. 80%) throughout the post-harvest treatments (Fig. 3a). Seed viability after drying in the dry-room (15% RH, 15°C; to 0.059 g H2O (g DW)− 1 changed significantly over time (P < 0.001) for both laboratory- and field-treated seeds. At the time of harvest, 28% of the sample survived drying; after 14 d post-harvest treatment, the proportion of seeds tolerating desiccation to this level had increased to 68% and 76% for laboratory- and field-treated seeds, respectively, by which point the heat sum experienced by the seeds was 280 and 190°C d, respectively (Fig. 3a, inset). The proportion of seeds that were desiccation tolerant subsequently declined to < 20% for laboratory- and 42% for field-treated seeds after 112 d post-harvest treatment (heat sums of 2240 and 1681°C d, respectively).

Figure 3 Changes in viability of non-dried, fresh (circles) and dry (squares) Anemone nemorosa seeds during laboratory (20°C, open symbols) and field (closed symbols) post-harvest treatments in (a) 2003 and (b) 2004. Seeds were warm stratified for up to 112 d (see Materials and methods for details) and then transferred to 10°C for 504 d (2003) or 490 d (2004) on water agar. The inset graphs show the same data plotted against heat sum. Field heat sum data for 2004 were not available beyond 130 d, when the heat sum was 2139°C d.
In 2004, in order to determine the timing of acquisition and loss of desiccation tolerance more precisely, laboratory post-harvest treatments were sampled more frequently up to 28 d. Germination was significantly higher for fresh, non-dried seeds than dry seeds at each sampling time (P < 0.001), although there was still a clear increase in the proportion of desiccation-tolerant seeds in the dried-seed treatment, from 22 to 78% over the first 5 d of the post-harvest treatment (heat sum 100°C d; Fig. 3b, inset). Desiccation tolerance was subsequently lost in dried, laboratory stored seeds, with a decline between 5 and 11 d; only 60% of the sample survived drying after a 28-d post-harvest treatment (heat sum 560°C d).
In 2004, field post-harvest treatments were sampled at 56, 84, 112, 140 and 168 d. In contrast to 2003, there was some evidence of a decline in fresh seed viability, falling to 69% after 140 d in the field. This may be partly due to germination in the field; after 168 d, 71% of seeds in the field had already germinated at the time of retrieval (data not shown). The remainder of the seeds in the field at 168 d had generally been predated (i.e. were empty). After 56 d in the field (heat sum 768°C d), the proportion of seeds that tolerated desiccation was 60% (92% of fresh seeds germinated at this time) falling to 11% at 140 d (heat sum >2139°C d). Fresh and dry seed germination was not significantly affected by the year of collection (P = 0.317).
Seed longevity
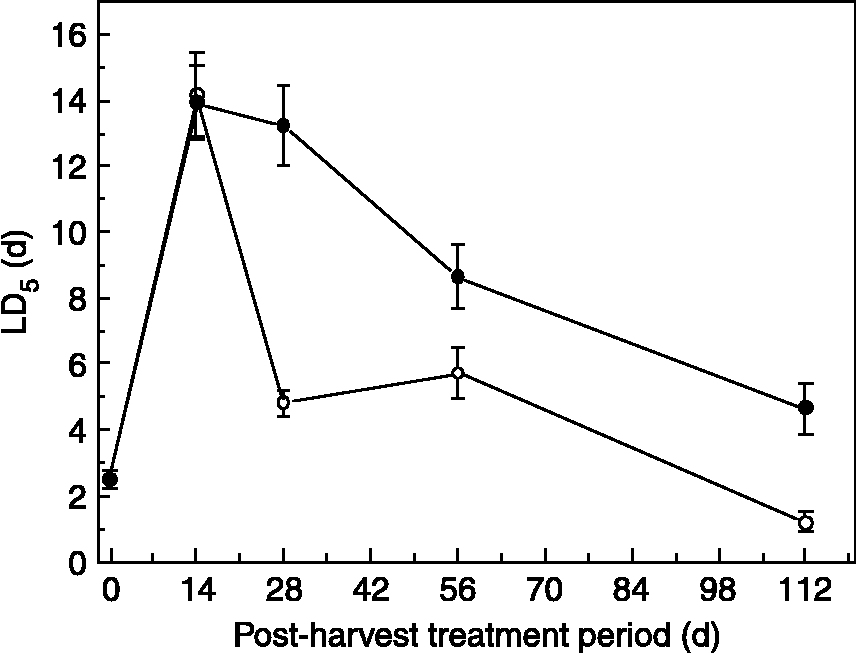
Changes in seed longevity during the post-harvest treatments in 2003 reflected the changes in desiccation tolerance. Some seedlots were not 50% viable at the start of the comparative longevity experiments, due to desiccation intolerance; consequently the time taken for viability to fall to 5% (LD5) was estimated by the probit analysis. LD5 increased from 2.5 to 14 d over the first 14 d of post-harvest treatment (Fig. 4). Subsequent decline in LD5 occurred at a faster rate in laboratory samples compared with field-treated seeds, falling to 1.2 and 4.6 d after 112 d for laboratory- and field-treated seeds, respectively.
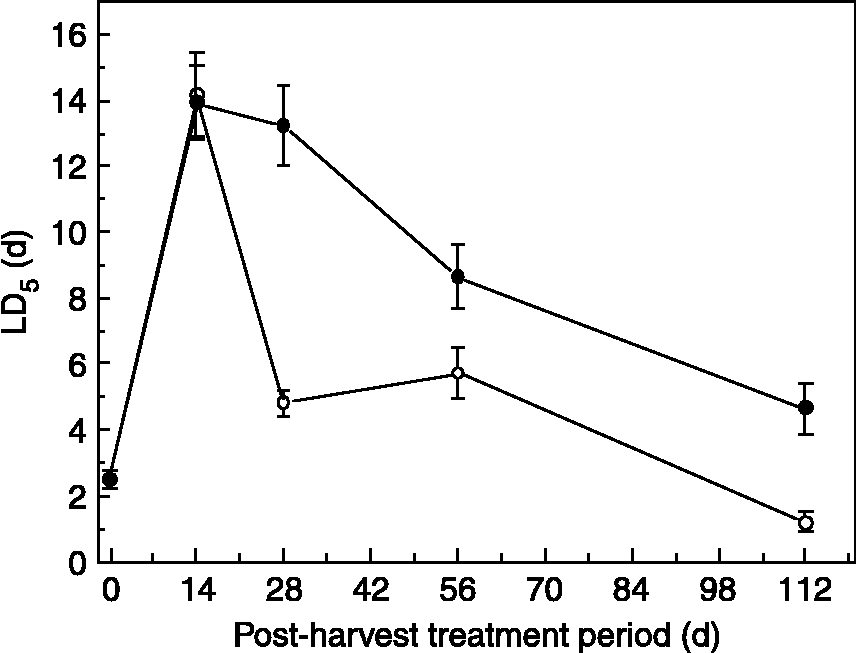
Figure 4 Changes in seed longevity (time for viability to fall to 5% ± SE) for Anemone nemorosa seeds given different lengths of laboratory (20°C on water agar, open symbols) or field (closed symbols) post-harvest treatments before drying. Seeds were dried for 21 d (15% RH, 15°C), rehydrated for 14 d (47% RH, 20°C) and then aged at 60% RH and 45°C.
Embryo growth and differentiation
Embryos steadily increased in size during the 28-d laboratory, post-harvest treatment (Fig. 5). Globular embryos subtended by a short suspensor were visible in all seeds sampled at the time of natural dispersal. Embryo growth was evident as early as 5 d of the laboratory post-harvest treatment, with mean embryo area (embryos from 5–10 seeds) increasing from 0.05 to 0.14 mm2. After 28 d, embryos had grown to 0.6 mm2 and occupied nearly 60% of the mucilaginous endosperm within which the embryo develops. This early embryo growth was accompanied by differentiation, and the transition from the globular to the heart stage coincided with the acquisition of desiccation tolerance in a large proportion of the population. After just 5 d, embryos had developed from the globular to the heart stage, during which the cotyledons became discernible. By 11 d, some torpedo-shaped embryos (i.e. embryos with extending cotyledons) were evident. The greatest variability with respect to embryo size and stage of development was observed at this time, with at least three distinct stages evident (Figs 5 and 6). Variability in embryo size and maturity among individual seeds continued to be observed after 11 d, but growth was progressive, and there was no evidence of developmental arrest at any stage. After 112 d in the field, the embryo entirely filled the mucilaginous endosperm area in most of the seeds, and the first visible germination occurred. After 140 d, there was more uniform radicle emergence, and after 168 d post-harvest treatment in the field, 83% of the seeds had germinated (data not shown).

Figure 5 Changes in Anemone nemorosa embryo area (mean ± SE of 5–10 individuals; closed squares) and proportion of seeds tolerating drying at 15% RH and 15 °C (closed circles) for 21 d, following laboratory post-harvest treatments in 2004.

Figure 6 Changes in embryo development during laboratory (A–G) and field (H–J) post-harvest treatments in 2004. Images are representatives from a sample size of (A) 4 seeds; (B) 3 seeds; (C) 1 seed; (D) 4 seeds; (E) 6 seeds; (F) 4 seeds; (G) 6 seeds; (H) 5 seeds; (I) 5 seeds; and (J) 5 seeds. Scale bars: (A)–(G), 100 μm; (H)–(I), 100 μm; (J), 1 mm.
Discussion
In seed development of orthodox species, most seeds acquire the ability to survive rapid, enforced desiccation (desiccation tolerance) around the time that the vascular connection with the maternal plant is broken. There then follows a period of post-abscission water loss in situ. During this stage of seed development, there are often improvements in seed-quality traits, such as longevity. However, it also coincides with reduced metabolic activity (e.g. Farrant et al., Reference Farrant, Pammenter, Berjak and Walters1997) and entry into either a dormant (requiring a specific treatment before germination occurs) or a quiescent state (merely requiring water and an appropriate temperature and/or light regime). Desiccation tolerance is subsequently lost when germination occurs (for review, see Bewley and Black, Reference Bewley and Black1994; Vertucci and Farrant, Reference Vertucci, Farrant, Kigel and Galili1995). In contrast, desiccation-sensitive seeds are shed at high water contents and tend not to be dormant; metabolism continues and the seeds usually germinate quickly (Farrant et al., Reference Farrant, Pammenter, Berjak and Walters1997; Berjak and Pammenter, Reference Berjak and Pammenter2001).
The A. nemorosa seeds harvested in 2003 still had a high water content [1.97 g (g DW)− 1] at the point of natural dispersal (Fig. 2), i.e. they had not been through a phase of post-abscission water loss that is typical of orthodox species. It is also clear that the seeds were still metabolically active at the time of natural dispersal, since embryo growth and differentiation continued immediately after seed collection, with the transformation of a globular embryo into a heart-shaped embryo (Figs 5 and 6). The seeds did not enter a state of dormancy sensu stricto or quiescence.
Nonetheless, this collection of A. nemorosa did acquire desiccation tolerance, albeit transiently (Fig. 3). At the time of collection only 20–30% of the population germinated following drying to 0.059 g H2O (g DW)− 1, and the maximum proportion of desiccation-tolerant seeds observed in any of the samples was 80%. Similarly, germination trials on seeds collected from more northerly populations (around Sheffield) that were dried for 1–2 d at laboratory temperatures, stored in darkness at 5°C for 12 months, and followed by 20°C for 5 weeks, showed low viability when germinated at 20/15°C (Grime et al., Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neal and Shaw1981). We may assume that all the seeds in the population did acquire desiccation tolerance at some point, either prior to or after this length of time in the post-harvest treatment. While we attempted to collect seeds at the time of natural dispersal, the seeds were still on the maternal plant, although they did dehisce readily when touched. Since the acquisition and loss of desiccation tolerance appears to occur within a matter of days (Fig. 3), desiccation tolerance in situ may be acquired only a short time before the point of actual release of individual seeds.
Being dispersed in late spring, and unlikely to be exposed to dry conditions in the field [seed water content fell only to 0.25 g (g DW)− 1 after 112 d in the field – on the 17 September; Fig. 2], it would appear that seeds of A. nemorosa (at least in the UK) have not been subject to selection pressure to ensure that seeds withstand desiccation for a prolonged period prior to germination. Loss of desiccation tolerance in individual seeds clearly coincides with growth and differentiation of the embryo, although it was not possible to pinpoint the embryo stage at which desiccation tolerance was lost [e.g. advancement to the torpedo stage is not a precondition for the loss of desiccation tolerance since more than 60% of the seed population tolerated desiccation at 28 d, when all the sampled seeds had torpedo-shaped embryos (Fig. 5)].
Our evidence clearly points to the absence of developmental arrest in A. nemorosa. The damp woodland habitat where the seeds fall ensures that seed eRH remains high, and conditions are therefore conducive to the continuation of development. At the time of natural dispersal, embryos are largely undifferentiated and are desiccation sensitive. However, embryo differentiation accompanied by the acquisition of desiccation tolerance occurs quickly, followed in turn by embryo growth, loss of desiccation tolerance, and eventual radicle emergence.
Earlier studies of seed germination in A. nemorosa (R.H. Sanderson, unpublished) showed that seeds would germinate after about 250 d when incubated at a narrow range of constant temperatures around 10°C, but germination was faster and more synchronous if the seeds were initially stratified at warmer temperatures for up to 112 d. Although Shirreffs (Reference Shirreffs1985) noted that stratification is not required for germination of A. nemorosa seeds, our field observations confirmed that seeds do not germinate under natural conditions until temperatures fall in the autumn, i.e. A. nemorosa seeds germinate after a period of warm stratification (during which there is embryo development) followed by cooler temperatures (radicle emergence). Using the familiar system of dormancy classification (Baskin and Baskin, Reference Baskin, Baskin, Smith, Dickie, Linington, Pritchard and Probert2003), seeds of A. nemorosa would therefore be classified as having morphophysiological dormancy. However, under laboratory conditions, A. nemorosa seeds will germinate slowly (>250 d) at cooler temperatures without stratification (R.H. Sanderson, unpublished). Therefore, it is difficult to reconcile our data with the concept of dormancy when we find no evidence of developmental arrest. Embryo development continues seamlessly, ultimately resulting in radicle emergence (germination) after 112 d under natural conditions. The loss of desiccation tolerance as the embryo develops is similar to the loss of desiccation tolerance seen in orthodox seeds upon radicle emergence.
While we conclude that these seeds did not exhibit dormancy, observations of seedlings after radicles had emerged showed that cotyledons and plumules did not appear, even during prolonged incubation at 10°C. This evidence, combined with reports that seedlings do not appear until the spring, raises the possibility that A. nemorosa displays epicotyl dormancy. There are clearly also some temperature-related effects on the rate of germination; embryo growth appears to be greater under warmer temperatures, although the seeds will not actually germinate at higher temperatures (R.H. Sanderson, unpublished). This suggests a narrower optimum temperature range for radicle emergence than for embryo growth.
While the two treatments gave similar trends in viability before and after drying, loss of desiccation tolerance appeared to occur at a slightly faster rate in seeds in the laboratory treatment, compared with the field-treated seeds (Fig. 3a). However, when the viability data are plotted against heat sum, the difference between the two treatments is reduced (Fig. 3a, inset). Furthermore, the appearance of embryos after 56 d in the field was similar to observations of some laboratory-treated seeds after 14 d (Fig. 6). By 56 d in the field, the heat sum experienced by the seeds was 768°C d, which was considerably more than the 280°C d experienced by the laboratory seeds at 14 d (Fig. 3b, inset). Variable temperatures and some reduction in the water content of seeds in the field (Fig. 1) may have further slowed embryo development.
Even though a largely (c. 70%) desiccation-tolerant seed population was apparent after 14 d post-harvest treatment in 2003 (Fig. 3a), when these seeds were aged at 60% RH and 45°C, it took 14 d for the viability of these seeds to fall to 5%. Comparing this result with seeds from many other species aged under identical conditions (Bird, Reference Bird2006; Hay et al., Reference Hay, Klin and Probert2006), A. nemorosa seeds are extremely short-lived. Indeed, extrapolating to seed-bank conditions, we estimate that a seedlot of A. nemorosa with a similar level of initial viability would lose all viability within a year when stored under conventional seed-bank conditions ( − 18°C after drying to equilibrium with 15% RH, 15°C).
Roberts (Reference Roberts1973) defined orthodox seeds as those that survive drying to low moisture contents and whose longevity is increased (within broad limits) with decreases in moisture content and eRH. This population of A. nemorosa seeds did tolerate drying, and we would predict that their longevity would have been increased by reducing moisture content and temperature, i.e. the seeds could be described as orthodox. However, the fact that it is unlikely that longevities of more than a year could be achieved, even under standard seed-banking conditions, means that they might best be described as non-orthodox. Furthermore, Berjak et al. (Reference Berjak, Farrant, Pammenter and Taylorson1989) hypothesized that recalcitrance is a consequence of a continuous transition from development to germination. Thus, apart from the fact that seeds of A. nemorosa transiently develop desiccation tolerance, in many respects they resemble recalcitrant seeds.
Previous studies (e.g. Hay and Probert, Reference Hay and Probert1995; Hay and Smith, Reference Hay, Smith, Smith, Dickie, Linington, Pritchard and Probert2003) have highlighted variation among species in the timing of the acquisition of desiccation tolerance in relation to mass maturity and seed dispersal. In A. nemorosa, we have shown that embryo differentiation and the acquisition of desiccation tolerance may not be acquired until after seeds have been naturally dispersed. While it is clear from gene-bank records that species with seeds dispersed with underdeveloped embryos are fully desiccation tolerant and reasonably long lived at the time of harvest (close to the point of natural dispersal), the frequency of species displaying behaviour similar to that of A. nemorosa needs to be assessed urgently. Anecdotal evidence of poor storage behaviour suggests that other moist, temperate woodland species may also be only weakly or transiently desiccation tolerant. If this is the case, practical treatments will need to be developed for such species to maximize desiccation tolerance and storability if effective long-term ex situ conservation is to be achieved. Further studies of the relationship between embryo development and the acquisition of seed desiccation tolerance across a broad range of species will also facilitate a better understanding of the evolution of this critically important seed trait in relation to ecology and plant distribution.
Acknowledgements
We thank Lydia White of the Plant Micromorphology Group, Royal Botanic Gardens Kew for assistance. Financial support was provided by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew receives grant-aided support from Defra, UK.