Introduction
Eudialyte-group minerals (EGM) are complex Na-Ca-Zr-cyclosilicates with the general formula Na15(Ca,REE)6(Fe,Mn)3Zr3Si(Si25O72)(O,OH,H2O)3(Cl,OH)2 (Johnsen et al., Reference Johnsen, Ferraris, Gault, Grice, Kampf and Pekov2003) characteristic of evolved peralkaline [molar (Na2O + K2O)/Al2O3>1] SiO2-undersaturated syenites, also called agpaitic syenites. This mineral is generally considered as diagnostic of agpaicity, i.e. those rocks in which high-field-strength elements (HFSE, such as Zr, Hf, Nb, Ta, U and Th) and rare-earth elements (REE) are incorporated into complex Na-K-Ca-Zr-silicates, rich in volatiles (Cl, F and H2O) (Sørensen, Reference Sørensen1992, Reference Sørensen1997; Khomyakov, Reference Khomyakov1995; Le Maitre et al., Reference Le Maitre, Streckeisen, Zanettin, Le Bas, Bonin, Bateman, Bellieni, Dudek, Efremova, Keller, Lameyre, Sabine, Schmid, Sørensen and Woolley2002; Marks et al., Reference Marks, Hettmann, Schilling, Frost and Markl2011). By contrast, when HFSE are incorporated in more common minerals (e.g. zircon, titanite), rocks are termed miaskitic. EGM can contain important amounts of REE and are considered as a potentially valuable ore of REE and Zr (e.g. the Kringlerne deposit in the Ilímaussaq complex, the Norra Kärr complex in Sweden and the Kipawa complex in Canada; Goodenough et al. (Reference Goodenough, Schilling, Jonsson, Kalvig, Charles, Tuduri, Deady, Sadeghi, Schiellerup, Müller, Bertrand, Arvanitidis, Eliopoulos, Shaw, Thrane and Keulen2016) and Saucier et al. (Reference Saucier, Noreau, Casgrain, Coté, Larochelle, Bilodeau, Al Hayden, Poirier, Garon, Bertrand, Kissiova, Mailloux, Rougier, Camus and Gagnon2013)). Because EGM crystallize through a range of magmatic to hydrothermal conditions, their compositional variability can been used to monitor the conditions of the environment in which they crystallized (Mitchell and Liferovich, Reference Mitchell and Liferovich2006; Schilling et al., Reference Schilling, Marks, Wenzel and Markl2009; Marks et al., Reference Marks, Lindhuber, Ratschbacher, Giehl, Nowak and Markl2015; Ratschbacher et al., Reference Ratschbacher, Marks, Bons, Wenzel and Markl2015). Very commonly, EGM are partly-to-completely replaced by secondary phases, at late magmatic-to-hydrothermal stages (Salvi et al., Reference Salvi, Fontan, Monchoux, Williams-Jones and Moine2000; Mitchell and Liferovich, Reference Mitchell and Liferovich2006; Karup-Møller et al., Reference Karup-Møller, Rose-Hansen and Sørensen2010; Sheard et al., Reference Sheard, Williams-Jones, Heiligmann, Pederson and Trueman2012; Karup-Møller and Rose-Hansen, Reference Karup-Møller and Rose-Hansen2013).
Although EGM were considered initially to be characteristic of highly differentiated SiO2-undersaturated rocks, these minerals have also been reported in a few SiO2-oversaturated peralkaline rocks. The only known examples, to our knowledge, are: quartz syenite from the Carlingford complex, Ireland (Nockolds, Reference Nockolds1950); the Pajarito mountain, Texas (McLemore, Reference McLemore2015); granitic dykes from the Ascension Island in the South Atlantic (Harris et al., Reference Harris, Cressey, Bell, Atkins and Beswetherick1982); the Straumsvola complex in Antarctica (Harris and Rickard, Reference Harris and Rickard1987); the Windy Fork pluton in Alaska (Gunter et al., Reference Gunter, Johnson, Knowles and Solie1993); pegmatitic granitic dykes from the Ambohimirahavavy complex in Madagascar (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a); the Dara-i-Pioz massif in Tajikistan (Grew et al., Reference Grew, Belakovskiy, Fleet, Yates, Mcgee and Marquez1993); Rockall Island in the North Atlantic (Sabine, Reference Sabine1957). However, in studies of most of these occurrences, EGM are only mentioned as an accessory mineral and are not thoroughly described in terms of texture and composition, although in their recent compilation, Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011) characterized the EGM from Ascension Island and Straumsvola.
In place of EGM, other magmatic complex Zr-silicates are usually described in peralkaline granites and pegmatites (Table 1). The most commonly reported Zr-minerals include dalyite (K2ZrSi6O15), wadeite (K2ZrSi3O9), elpidite (Na2ZrSi6O15·3H2O), catapleiite (Na2ZrSi3O9·2H2O) and, more rarely, gittinsite (CaZrSi2O7) and armstrongite (CaZrSi6O15·2H2O). It is noteworthy that, among these six minerals, none contain Cl or F, whereas three contain H2O (elpidite, catapleiite and armstrongite). Several Zr-minerals are usually reported in the same peralkaline rocks and their complex textural relationships have often led to multiple interpretations (e.g. Strange Lake, Birkett et al., Reference Birkett, Miller, Roberts and Mariano1992; Roelofsen and Veblen, Reference Roelofsen and Veblen1999). Moreover, in many cases, post-magmatic processes have destabilized the primary assemblages, substantially complicating identification of the original magmatic mineralogy.
Table 1. List of the main Zr-silicates in agpaitic peralkaline granite and pegmatite.

References: [1] Birkett et al. (Reference Birkett, Miller, Roberts and Mariano1992); [2] Estrade et al. (Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a); [3] Fleet and Cann (Reference Fleet and Cann1967) [4] Grew et al. (Reference Grew, Belakovskiy, Fleet, Yates, Mcgee and Marquez1993); [5] Harris et al. (Reference Harris, Cressey, Bell, Atkins and Beswetherick1982); [6] Harris (Reference Harris1983); [7] Harris and Rickard (Reference Harris and Rickard1987); [8] Kempe et al. (Reference Kempe, Möckel, Graupner, Kynicky and Dombon2015); [9] Khomyakov et al. (Reference Khomyakov, Dusmatov, Ferraris, Gula, Ivaldi and Nechelyustov2003); [10] Kynicky et al. (Reference Kynicky, Chakhmouradian, Xu, Krmicek and Galiova2011); [11] Mariano, Pers. Comm.; [12] Marks et al. (Reference Marks, Lindhuber, Ratschbacher, Giehl, Nowak and Markl2015); [13] Nockolds (Reference Nockolds1950); [14] Roelofsen and Veblen (Reference Roelofsen and Veblen1999); [15] Salvi and Williams-Jones (Reference Salvi and Williams-Jones1995); [16] Schmitt et al. (Reference Schmitt, Trumbull, Dulski and Emmermann2002); [17] Sherer (Reference Sherer1990).
The main factors controlling the saturation of EGM in a melt are its peralkalinity and high HFSE (mainly Zr) and volatile contents (mainly Cl, F and H2O) (Sørensen, Reference Sørensen1997). These conditions are usually met in highly-evolved undersaturated melts derived from the differentiation of magmas under low oxygen fugacity (Markl et al., Reference Markl, Marks and Frost2010). Conversely, the problem of scarcity of EGM in peralkaline granites has, to date, not been discussed in the literature.
This study focuses on the occurrence of EGM in peralkaline granitic pegmatites in the Ambohimirahavavy alkaline complex, northwest Madagascar. On the basis of textural and compositional data for EGM, other agpaitic minerals, clinopyroxene and amphibole, we assess the magmatic conditions required for the stability of EGM in peralkaline SiO2-oversaturated rocks and compare them to their undersaturated counterpart.
Geological setting and petrography
The Ambohimirahavavy alkaline complex (24.2 ± 0.6 Ma for nepheline syenite and 23.5 ± 6.8 Ma for peralkaline granite; in situ U–Pb radiometric age determination of zircon, Estrade et al., Reference Estrade, Béziat, Salvi, Tiepolo, Paquette and Rakotovao2014b) is part of the Cenozoic alkaline province located in the north-western part of Madagascar, in the Ampasindava peninsula. The complex consists of silica-undersaturated and silica-oversaturated syenites and granites as well as volcanic units of alkaline affinity, including basalt, phonolite, trachyte and rhyolitic obsidian (Fig. 1). A network of granitic and pegmatitic dykes intruded the Isalo sedimentary units along the external flank of the south syenitic ring-dyke. The dykes are characterized by an alkaline mineralogy (i.e. contain sodic clinopyroxene and sodic amphibole) and by the presence of minerals rich in HFSE and REE. Their local intrusion in limestone resulted in the formation of a REE-rich skarn (Estrade et al., Reference Estrade, Salvi, Béziat and Williams-Jones2015).
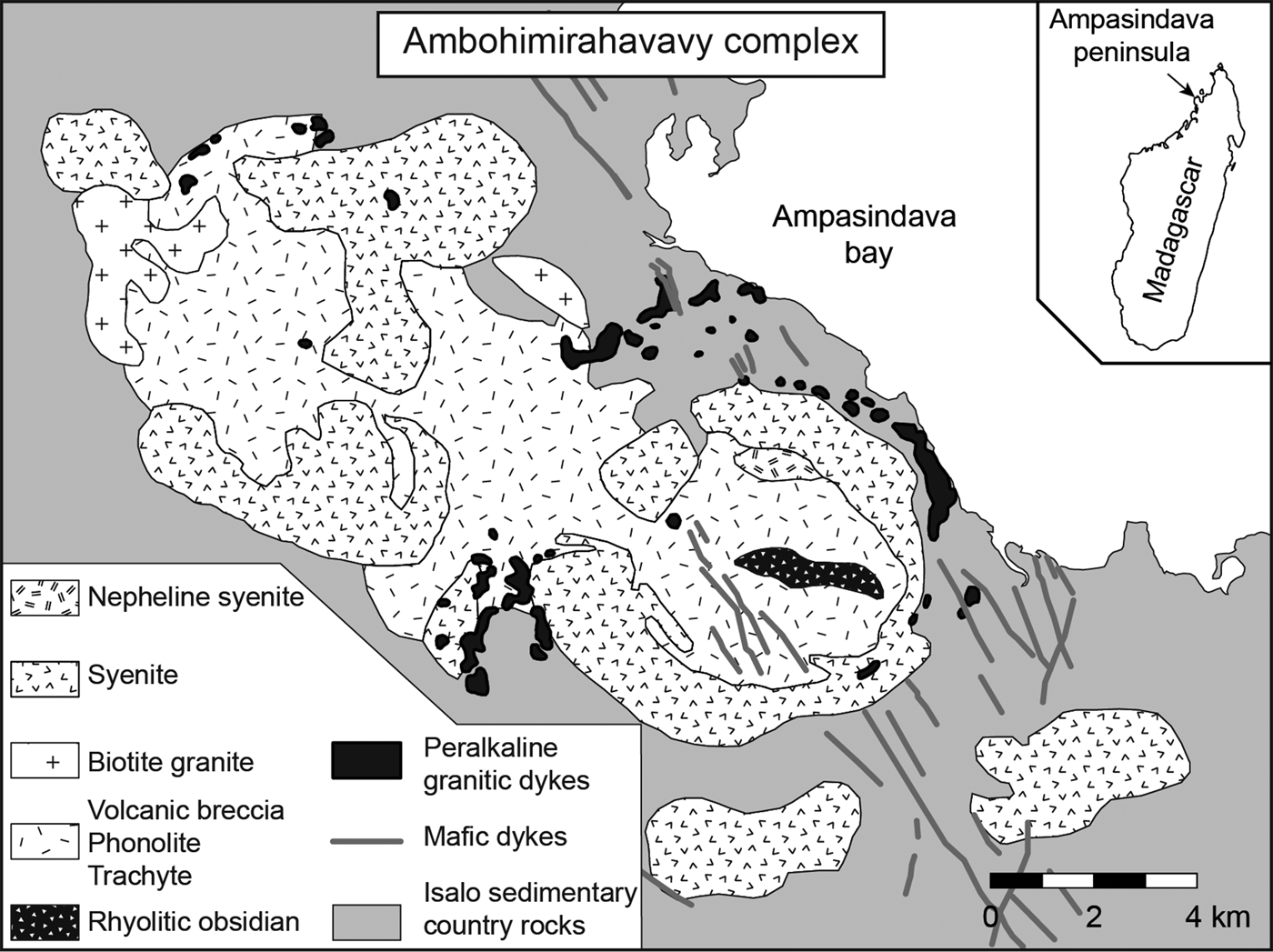
Fig. 1. Geological map of the Ambohimirahavavy complex, modified after Donnot (Reference Donnot1963).
This complex is currently being explored by Tantalus Rare Earth AG, with a focus on the ion-adsorption REE mineralization in clays associated with laterites. The company has also evaluated the economic potential of the bedrock, as most of the HFSE and REE occur as primary mineralization in peralkaline granite and pegmatite dykes. In a previous study, we distinguished three types of dykes according to their textures and major mineralogy (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a): (1) A coarse-grained granitic type (GR-I), characterized by an allotriomorphic granular texture, composed of quartz, perthitic alkali feldspar, Na-amphibole and Na-clinopyroxene. The main HFSE-bearing minerals form a miaskitic assemblage consisting of zircon, pyrochlore-group minerals, monazite-(Ce) and chevkinite-(Ce). (2) A pegmatitic granitic type (GR-II), consisting of quartz, perthitic alkali feldspar, Na-clinopyroxene, Na-amphibole, and containing an agpaitic to miaskitic accessory assemblage consisting of EGM, mostly altered to a secondary miaskitic assemblage that is dominated by zircon and quartz (Fig. 2a,b,c). This rock is termed transitional because of this feature and because EGM only occur as accessory minerals. (3) A pegmatitic agpaitic granitic type (GR-III) that consists of K-feldspar, albite, Na-clinopyroxene, Na-amphibole and quartz, plus EGM as rock-forming minerals (Fig. 2d–e). Other agpaitic minerals include nacareniobsite-(Ce) (Na3Ca3(Ce,La)(Nb,Ti)(Si2O7)2OF3) and turkestanite (Th(Ca,Na)2K1–x□x(Si8O20)·nH2O).
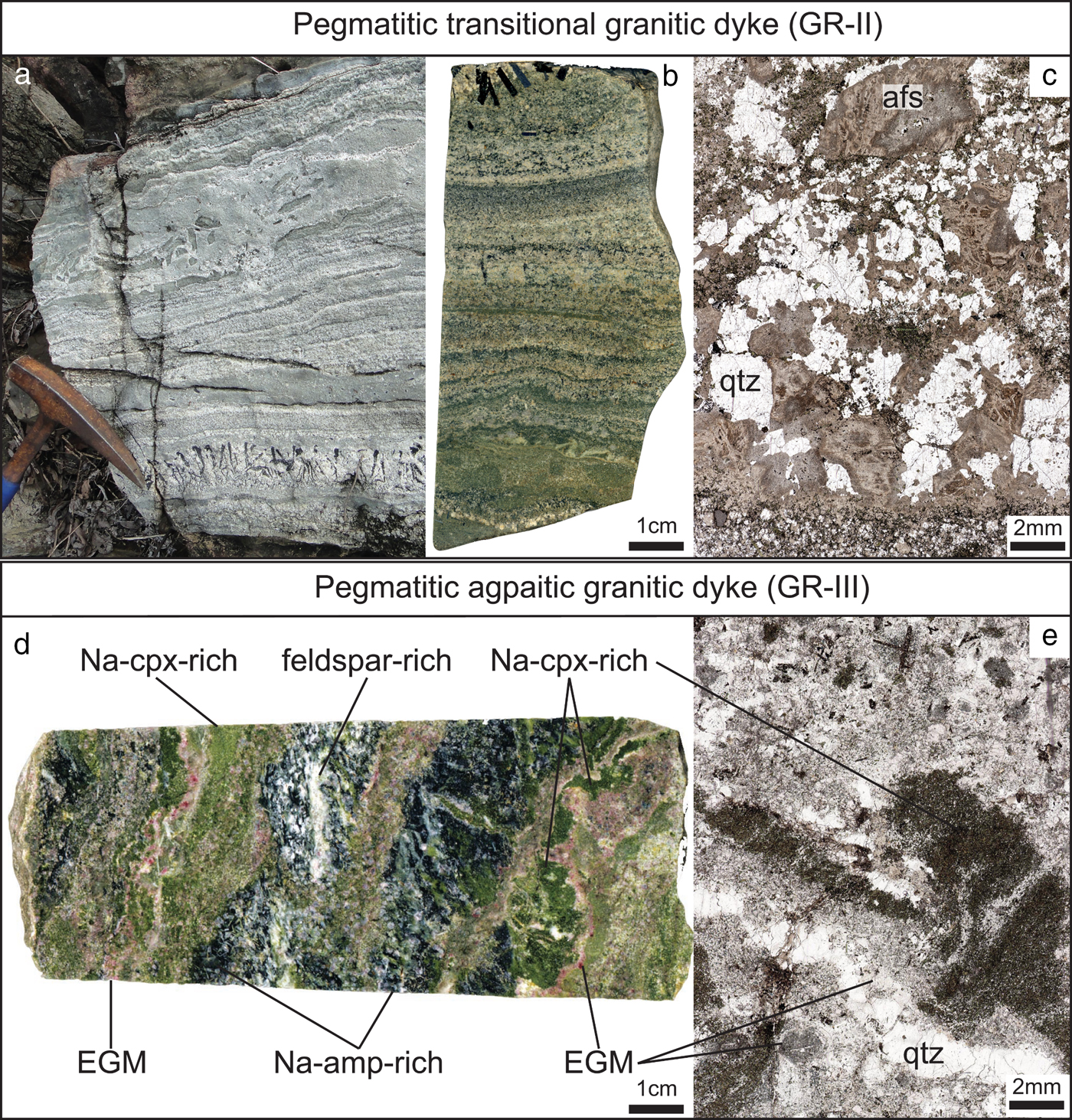
Fig. 2. Outcrop photograph (a), scanned polished rock slab (b–d) and scanned thin sections (c–e) of the pegmatitic transitional granitic dyke (GR-II) (a, b, c) and pegmatitic agpaitic granitic dyke (GR-III) (d, e). Both rock types show pronounced layering and variable grain sizes typical of pegmatite. Abbreviations: afs: alkali feldspar, amp: amphibole, cpx: clinopyroxene and qtz: quartz.
In the following sections, we describe in detail the EGM and the complex mineralogy of both the pegmatitic transitional (GR-II) and the pegmatitic agpaitic (GR-III) dykes. These dykes form a more-or-less continuous belt around the ring-dyke of syenite. They vary in thickness from a few centimetres to a few metres. Although no relationships have been observed between GR-II and GR-III dykes, their similar mode of emplacement suggests a contemporaneous origin. GR-II dykes are far more common in the complex than GR-III, which have been only found in boreholes. However, Lacroix (Reference Lacroix1915, Reference Lacroix1923) and Ganzeev and Grechishchev (Reference Ganzeev and Grechishchev2003) have described similar rocks to GR-III dykes containing more than 40% of EGM, suggesting that they outcrop somewhere in the flank of the complex.
Analytical methods
The mineralogy and textural relationships were investigated using optical microscopy and back-scattered electron (BSE) imaging using a JEOL JSM 6360LV scanning electron microscope (SEM) equipped with a silicon drift detector analysis system. The instrument was also used to obtain energy-dispersive X-ray phase maps.
Major and minor element concentrations in minerals were determined using a Cameca-SX-50 electron probe microanalyser (EPMA) with SAMx automation, at the Géosciences Environment Toulouse (GET) laboratory at the University of Toulouse. Operating conditions were an accelerating voltage of 15 kV and a beam current of 20 nA. Standardization was obtained using, periclase (Mg), wollastonite (Ca and Si), corundum (Al), pyrophanite (Mn and Ti), hematite (Fe), baryte (Ba), albite (Na), sanidine (K), zircon (Hf and Zr), graftonite (P), UO2 (U), ThO2 (Th), Pb2P2O7 (Pb), sphalerite (Zn), orthophosphate for REE and yttrium, Nb metal (Nb), Ta metal (Ta), topaz (F) and tugtupite (Cl).
In situ trace-element concentrations were determined by laser ablation inductively coupled plasma mass-spectrometry (LA-ICP-MS) at the University of Montpellier. The instrument used for analysis was a Geolas Q excimer CompEx 102 laser ablation system coupled to a ThermoFinnigan Element XR ICP-MS. We used a beam diameter of 60 µm for all analyses, which allowed a high detection threshold even for very low-level trace elements. For each analysis, the signal was recorded for 180 s, consisting of 60 s of background measurement followed by 120 s of ablation. To calibrate the instrument, we used NIST SRM 610 glass as an external standard and NIST SRM 612 as a secondary standard. SiO2 was used as an internal standard for the correction of different ablation rates between sample and standard. Silica concentration had been determined previously by EPMA in the ablation area. Data processing was carried out with the SILLS software (Guillong et al., Reference Guillong, Meier, Allan, Heinrich, Yardley and Sylvester2008). Typical concentration uncertainties are ± 5% for concentrations >10 µg/g and ± 10% for concentration <10 µg/g.
Mineral textures and alteration
Eudialyte-group minerals
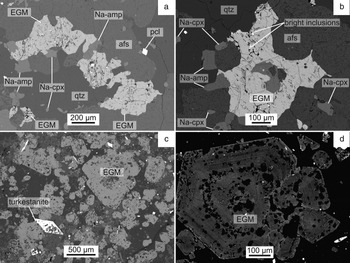
In GR-II, EGM are accessory minerals (<1 vol.%) occurring as interstitial, late-formed magmatic phases surrounded by Na-clinopyroxene, Na-amphibole, perthitic alkali feldspars and quartz (Fig. 3a–b). In most samples, EGM underwent partial-to-complete alteration and have been replaced by a complex assemblage of secondary minerals, among which zircon and quartz have been identified as the main replaced phases (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a). Although most of the EGM grains are altered, rare, entirely unaffected grains may occur adjacent to altered ones. The latter crystals lack the oscillatory growth or sector zoning usually observed in EGM from other alkaline complexes, although high-contrast BSE images show common small bright phases and dark grey patches, scattered along fractures.

Fig. 3. BSE images of EGM textures from GR-II (a–b) and GR-III (c–d): (a–b) Late-magmatic interstitial EGM partially altered along fractures. (c–d) Oscillatory-zoned early-magmatic euhedral EGM with mineral inclusions. Abbreviations: afs: alkali feldspar; amp: amphibole; cpx: clinopyroxene; qtz: quartz; pcl: pyrochlore.
In GR-III, EGM are rock-forming minerals (>10 vol.%) occurring as euhedral, early magmatic crystals together with Na-clinopyroxene, Na-amphibole, nacareniobsite-(Ce), turkestanite, albite, K-feldspar and quartz (Fig. 3c–d). Except for quartz, all these minerals occur as individual grains and as inclusions in cores of the EGM, as well as along concentric layers that follow growth zones. Quartz mainly occurs as late anhedral crystals and as layers flanked by euhedral eudialyte. Unlike in GR-II, most of the EGM are pristine; locally-altered EGM grains occur in narrow bands, together with calcite, altered nacareniobsite-(Ce) and turkestanite. All other silicates were not altered.
Other agpaitic minerals
In addition to EGM, the agpaitic minerals nacareniobsite-(Ce) and turkestanite occur in significant modal amounts (1–2 vol.%) in GR-III, whereas EGM are the only agpaitic minerals in GR-II. Nacareniobsite-(Ce) is also a mineral typical of agpaitic SiO2-undersaturated rocks, but has been only rarely described in SiO2-saturated rocks (Vilalva et al., Reference Vilalva, Vlach and Simonetti2013). In GR-III, it occurs as early magmatic elongated euhedral crystals that enclose small chadacrysts of albite, K-feldspar, Na-clinopyroxene and Na-amphibole (Fig. 4a–b). In altered portions of GR-III, nacareniobsite-(Ce) is replaced by a complex assemblage of secondary Ca-rich minerals including calcite and titanite. Turkestanite is an extremely rare mineral that has been reported in only four other alkaline complexes (Pautov et al., Reference Pautov, Agakhanov, Sokolova and Kabalov1997; Kabalov et al., Reference Kabalov, Sokolova, Pautov and Schneider1998; Vilalva and Vlach, Reference Vilalva and Vlach2010). In GR-III, it is an early magmatic phase, occurring as diamond-shaped euhedral crystals that enclose small chadacrysts of albite, K-feldspar, Na-clinopyroxene and Na-amphibole (Fig. 4c). In BSE images, these crystals commonly show zoning and alteration textures consisting of a heterogeneous core containing a network of very thin U-rich veinlets, surrounded by a BSE brighter homogeneous rim (Fig. 4d).

Fig. 4. BSE images of agpaitic minerals in GR-III: (a–b) euhedral laths of nacareniobsite-(Ce) containing mineral inclusions. (c–d) zoned euhedral turkestanite containing mineral inclusions. The core of the turkestanite shows bright U-rich veinlets and inclusions. Abbreviations: amp: amphibole; cal: calcite; K-fsp: K-feldspar; qtz: quartz.
Na-clinopyroxene and Na-amphibole
Sodic clinopyroxene is the main mafic constituent in GR-II and GR-III. In GR-II, it forms two texturally distinct generations: Na-clinopyroxene-I (cpx-I) is relatively uncommon and forms large euhedral phenocrysts (up to 1 mm across), locally showing distinct cores and rims; Na-clinopyroxene-II (cpx-II) is more abundant and occurs as homogeneous small grains or thin acicular crystals. In GR-III, Na-clinopyroxene occurs as small euhedral crystals and as chadacrysts in EGM, nacareniobsite-(Ce) and turkestanite.
Sodic amphibole is the second main mafic constituent after Na-clinopyroxene. In GR-II, its habit varies considerably and, depending on its location in the dyke, can form large euhedral pegmatitic crystals of up to 20 cm or small anhedral grains. In GR-III, Na-amphibole occurs only as small euhedral crystals.
Mineral composition
Eudialyte-group minerals
Representative compositions of EGM are shown in Table 2. Structural formulae of EGM are based on Σ (Si + Al + Zr + Ti + Hf + Nb) = 29 atoms per formula unit (apfu) and were calculated using the Excel spreadsheet of Pfaff et al. (Reference Pfaff, Wenzel, Schilling, Marks and Markl2010). EGM from GR-II and GR-III have different compositions, mainly in Mn, Ca and REE, and minor variations in Si, Fe, Na and Nb. Zirconium and chlorine contents of EGM are similar in both rock types. Binary plots of Fe vs. Mn and (La + Ce + Nd + Y) vs. Ca, expressed as atoms per formula unit, are shown in Fig. 5, where they are compared with EGM from other peralkaline granites (Straumsvola and Ascension Island) and foid syenites (Ilímaussaq, Mont Saint-Hilaire, Tamazeght, Pilansberg, Kipawa, Khibiny, Lovozero, Saima, Pocos de Caldas, Langesundfjord and Norra Kärr) using the data of Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). The Mn content of early-magmatic EGM from GR-III is lower than that of late-magmatic EGM from GR-II and is consistent with data from the other complexes, showing that, in a single intrusive complex, the Mn/Fe ratio increases in later-formed EGM (Schilling et al., Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). Calcium correlates negatively with the sum of the major REEs: La, Ce, Nd and Y; this trend is consistent with their preferential incorporation in the same structural site (M1; Johnsen and Grice, Reference Johnsen and Grice1999). In addition, most of the EGM from quartz-bearing rocks (GR-II, Straumsvola and Ascension Island) have lower Ca and higher La + Ce + Nd + Y contents than EGM from foid-bearing rocks. Most EGM with high La + Ce + Nd + Y content from foid-bearing rocks (Mont Saint-Hilaire and Pilansberg) have a post-magmatic origin.
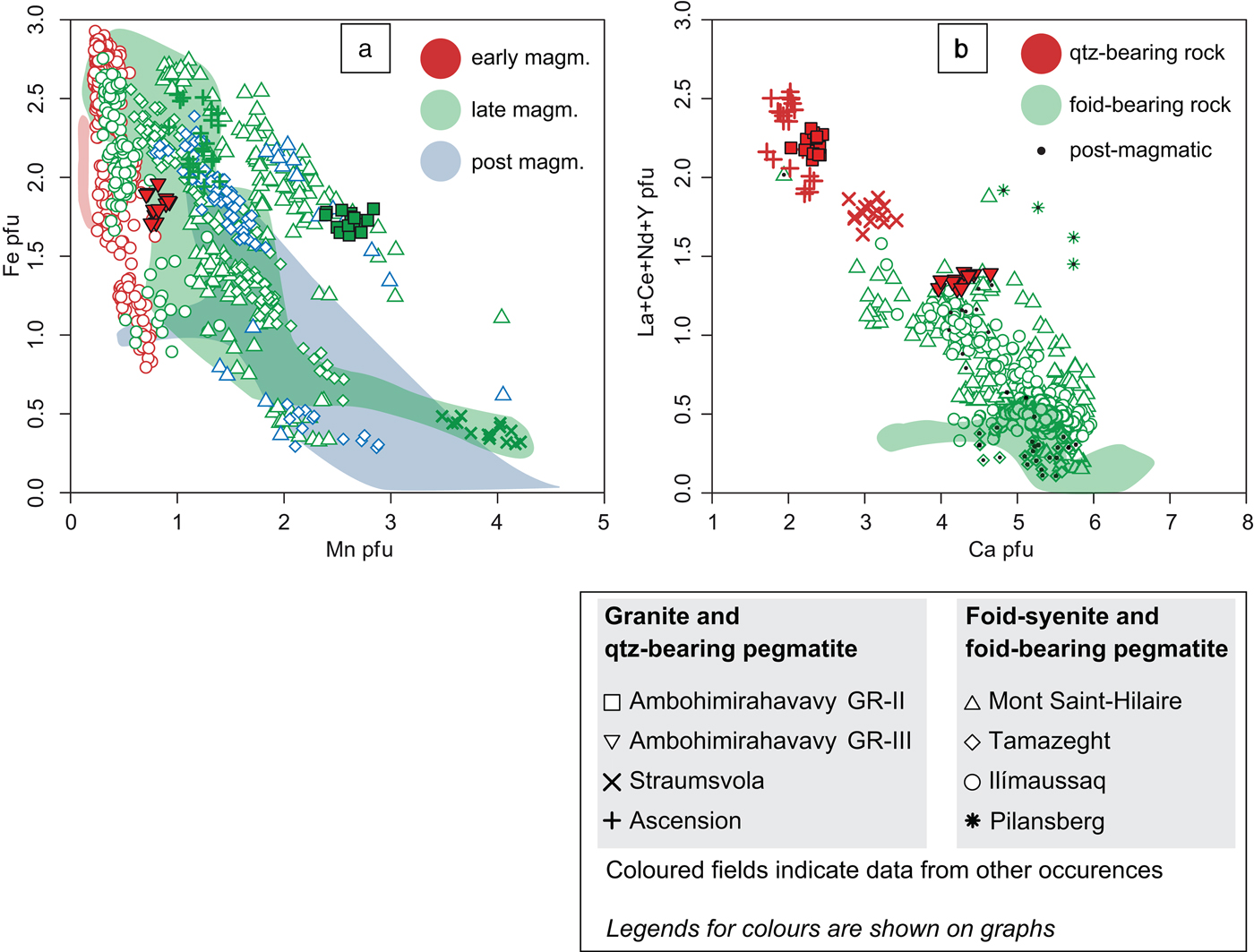
Fig. 5. (a) A plot of Fe vs. Mn in atom per formula units of EGM from the Ambohimirahavavy complex (filled symbols) compared with EGM from other alkaline complexes taken from Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). (b) A plot of La + Ce + Nd + Y vs. Ca in atom per formula units of EGM from the Ambohimirahavavy complex (filled symbols) compared with EGM from other alkaline complexes taken from Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011).
Table 2. Average compositions of EGM and their replacing phases in pegmatitic transitional (GR-II) and pegmatitic agpaitic (GR-III) dykes.

Abbreviations: EGM = eudialyte-group minerals, b.d.l. = below detection limit, n.a. = not analysed, σ = standard deviation
*NA – number of analyses
Trace-element analyses for EGM, agpaitic minerals, clinopyroxene and amphibole are presented in an electronic supplement. Rare-earth elements and trace-element distribution patterns of EGM, nacareniobsite-(Ce), turkestanite, clinopyroxene and amphibole are shown in Fig. 6. Rare-earth element and trace-element patterns of EGM are compared with patterns of EGM from other EGM-bearing granites (Straumsvola and Ascension Island) and foid-syenites using the data of Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). We only present the patterns from the most REE-rich EGM (from Ilímaussaq and Mont Saint-Hilaire), as EGM from other complexes plot far below the REE distribution patterns of GR-II and GR-III. We also compare the concentrations of the light REE (LREE; La to Sm) and the heavy REE (HREE; Gd to Lu) in EGM from different complexes in Fig. 7 using the data of Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). Among all EGM, those from quartz bearing-rocks have the highest REE concentrations and are generally enriched by up to four orders of magnitude relative to chondrite (McDonough and Sun, Reference McDonough and Sun1995) (Fig. 6a). Concentrations of the LREE in EGM from quartz bearing-rocks (>40,000 ppm) are similar to the most LREE-rich EGM from foid-syenites in Ilímaussaq and Mont Saint-Hilaire, whereas concentrations of the HREE are significantly higher in EGM from GR-II and Ascension Island (Fig. 7). All patterns show a strong negative Eu anomaly which is also a characteristic of the whole-rock REE distribution patterns of the dykes; this is consistent with the model for the origin of the melt in the Ambohimirahavavy complex (Estrade et al., Reference Estrade, Béziat, Salvi, Tiepolo, Paquette and Rakotovao2014b), i.e. differentiation of an alkali basaltic parental melt by fractionation of plagioclase. Trace-element distribution patterns have similar overall shapes, except for U and Pb, which show a negative and positive anomaly, respectively, in GR-II, and an opposite pattern in GR-III. Such opposed trends are absent in the whole-rock patterns, which show similar concentrations for these two elements.
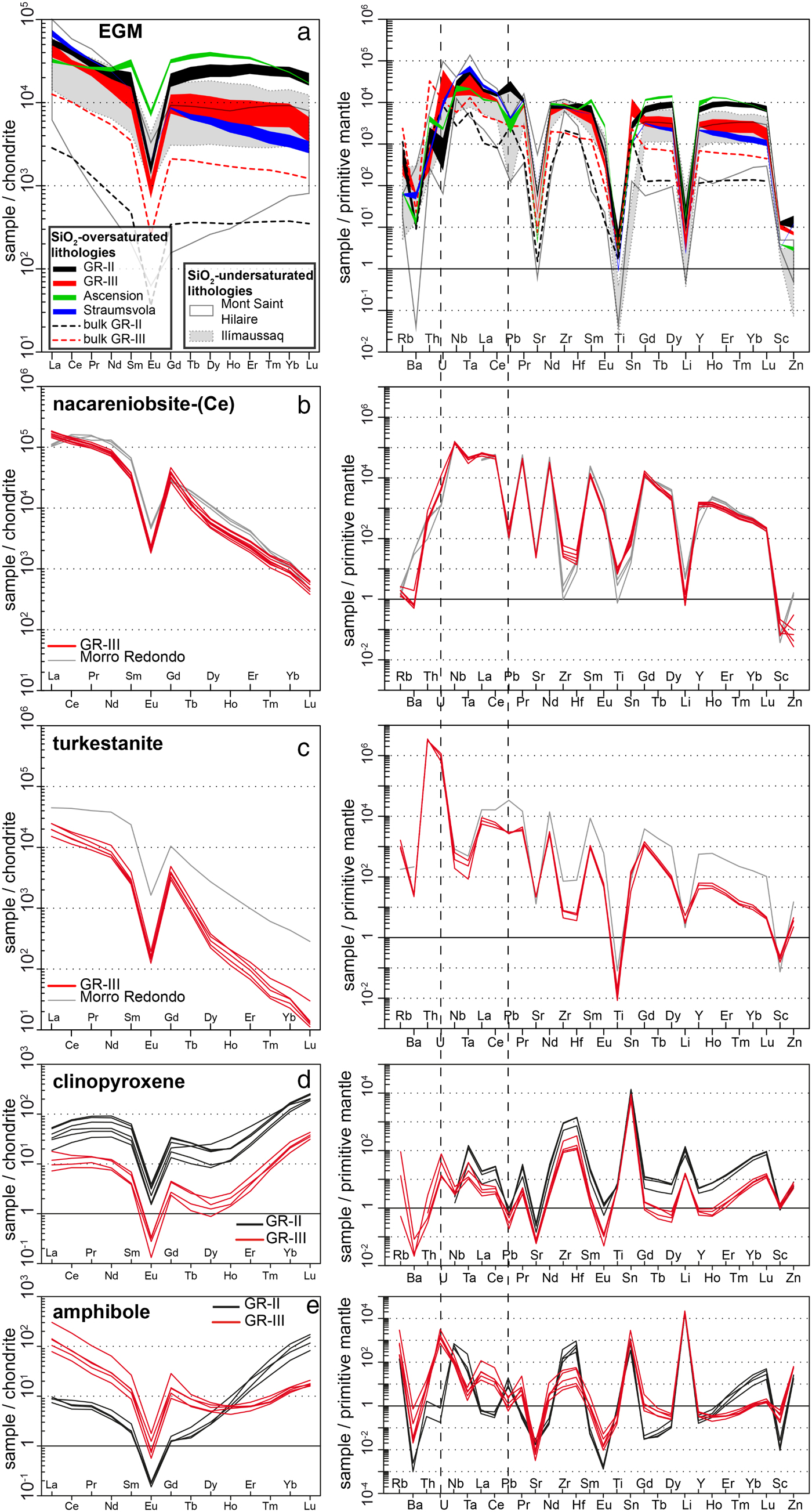
Fig. 6. Chondrite normalized REE (left) and trace-element (right) distribution patterns from (a) EGM, (b) nacareniobsite-(Ce), (c) turkestanite, (d) clinopyroxene and (e) amphibole. EGM patterns from GR-II and GR-III are compared with whole-rock patterns from GR-II and GR-III (dashed lines) using data from Estrade et al. (Reference Estrade, Béziat, Salvi, Tiepolo, Paquette and Rakotovao2014b; GR-II dyke type sample AM113A) and Estrade et al. (Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a; GR-III dyke type sample EU01). EGM patterns are also compared with EGM data from other alkaline complexes taken from Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011) (cf. legend). Nacareniobsite-(Ce) and turkestanite patterns are compared with data from the Morro Redondo complex (Brazil) taken from Vilalva and Vlach (Reference Vilalva and Vlach2010) and Vilalva et al. (Reference Vilalva, Vlach and Simonetti2013).
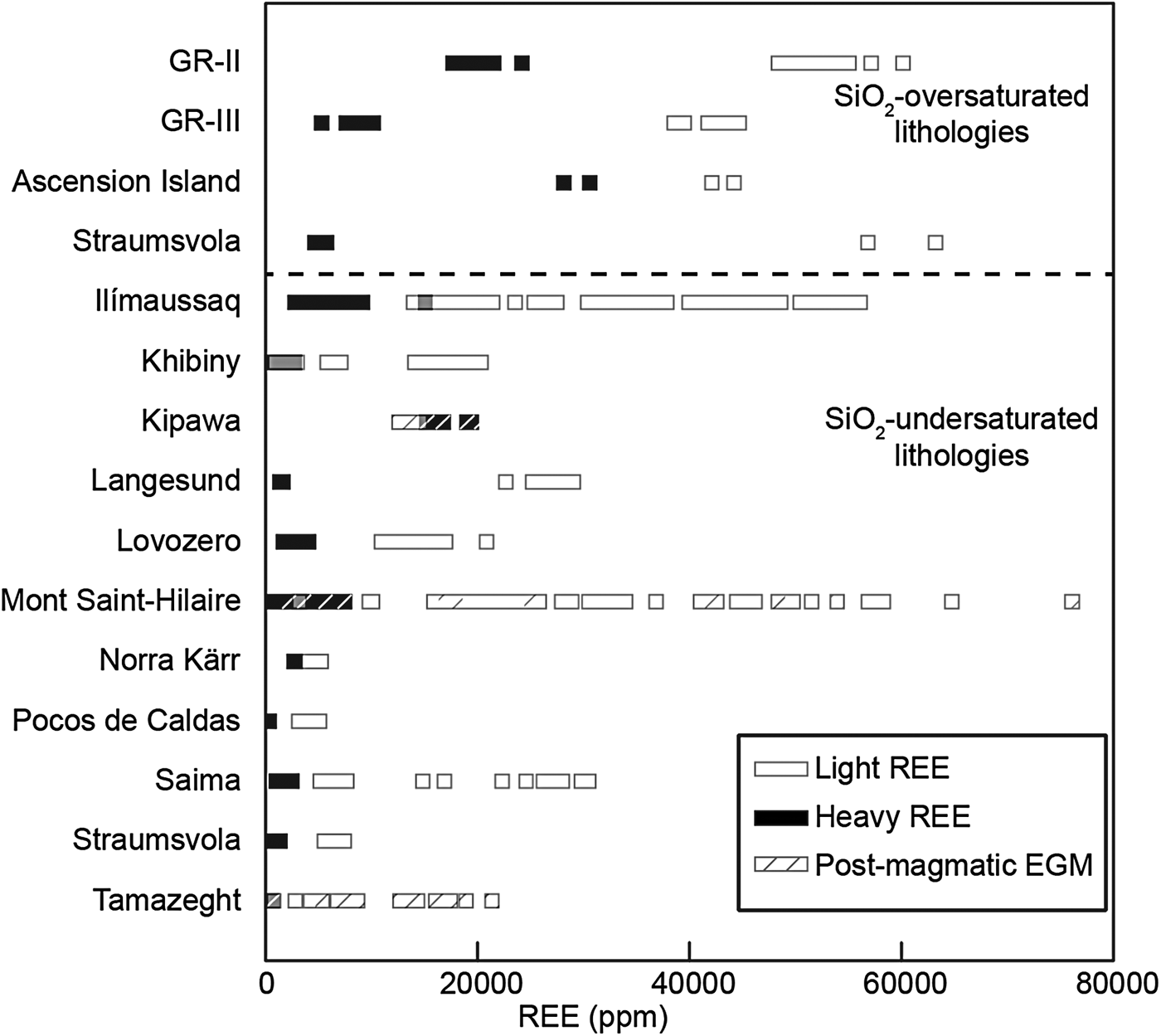
Fig. 7. Range of light (La to Sm) and heavy (Gd to Lu) REE concentrations in EGM from the Ambohimirahavavy complex and other alkaline complexes. Data for other complexes are from Schilling et al. (Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011). The grey colour indicates overlap of the white and black patterns in the legends.
Alteration paragenesis of EGM
Most of the EGM in GR-II samples are replaced entirely by a secondary paragenesis, consisting ultimately of zircon and quartz. Locally, however, EGM are partly altered and replaced by an assemblage of Zr-Ca-bearing phases and REE-Ca-F-bearing phases with no zircon (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a). In the present work, we have identified EGM that show partial replacement by zircon, and we consider this texture as representative of the alteration pattern of EGM in GR-II. An X-ray element map of partially-replaced EGM is shown in Fig. 8 and the compositions of the phases present are reported in Table 2. In this Figure, EGM are replaced ultimately by zircon and quartz through an intermediate step consisting of an unidentified Ca-zirconosilicate with higher Ca and Zr and lower Fe and Mn contents than the original EGM. All Na, Cl, Nb and most of the REE have been lost. REE-rich and Zr-rich phases co-crystallized with the Ca-zirconosilicate but were not analysed with EPMA due to their very small size. Secondary zircon is characterized by a low ZrO2 content (57.8 wt.%), a low sum of oxides (91.5 wt.%) and the presence of Fe and Ca. In GR-III, replacement of EGM involved an intermediate phase slightly enriched in Si (Fig. 9a–b). The replacing phase is an unidentified Ca-Na zirconosilicate lacking Cl, REE, Mn and Fe.
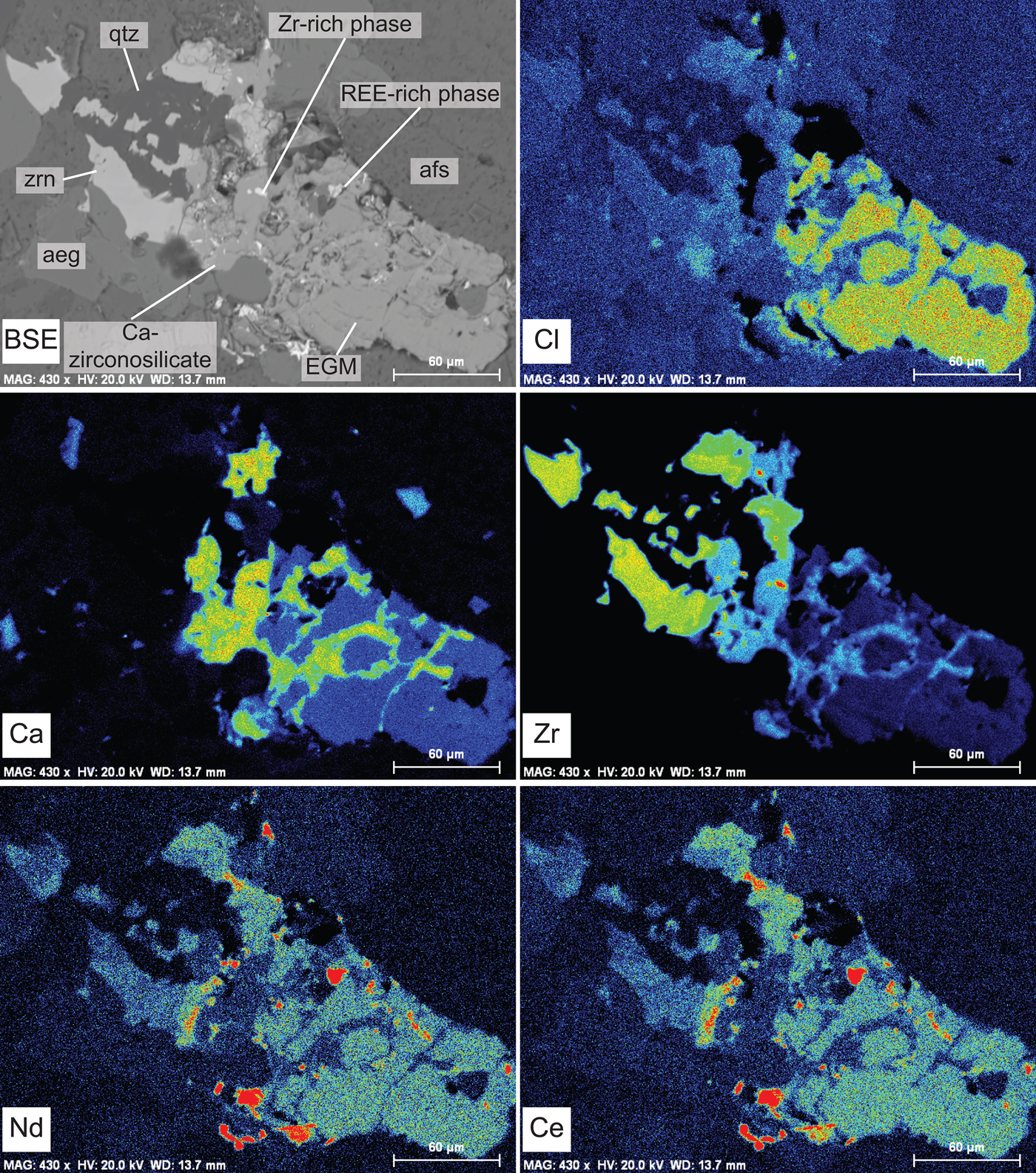
Fig. 8. BSE image and X-ray maps depicting the distribution of chlorine (Cl), calcium (Ca), zirconium (Zr), neodymium (Nd) and cerium (Ce), showing partial replacement of EGM (BSE image lower right hand side) by zircon and quartz (BSE image left hand side) in GR-II. Replacement takes place through formation of an intermediate Ca-zirconosilicate with Zr-rich and REE-rich phases. Abbreviations: aeg: aegirine; afs: alkali feldspars; qtz: quartz; and zrn: zircon.
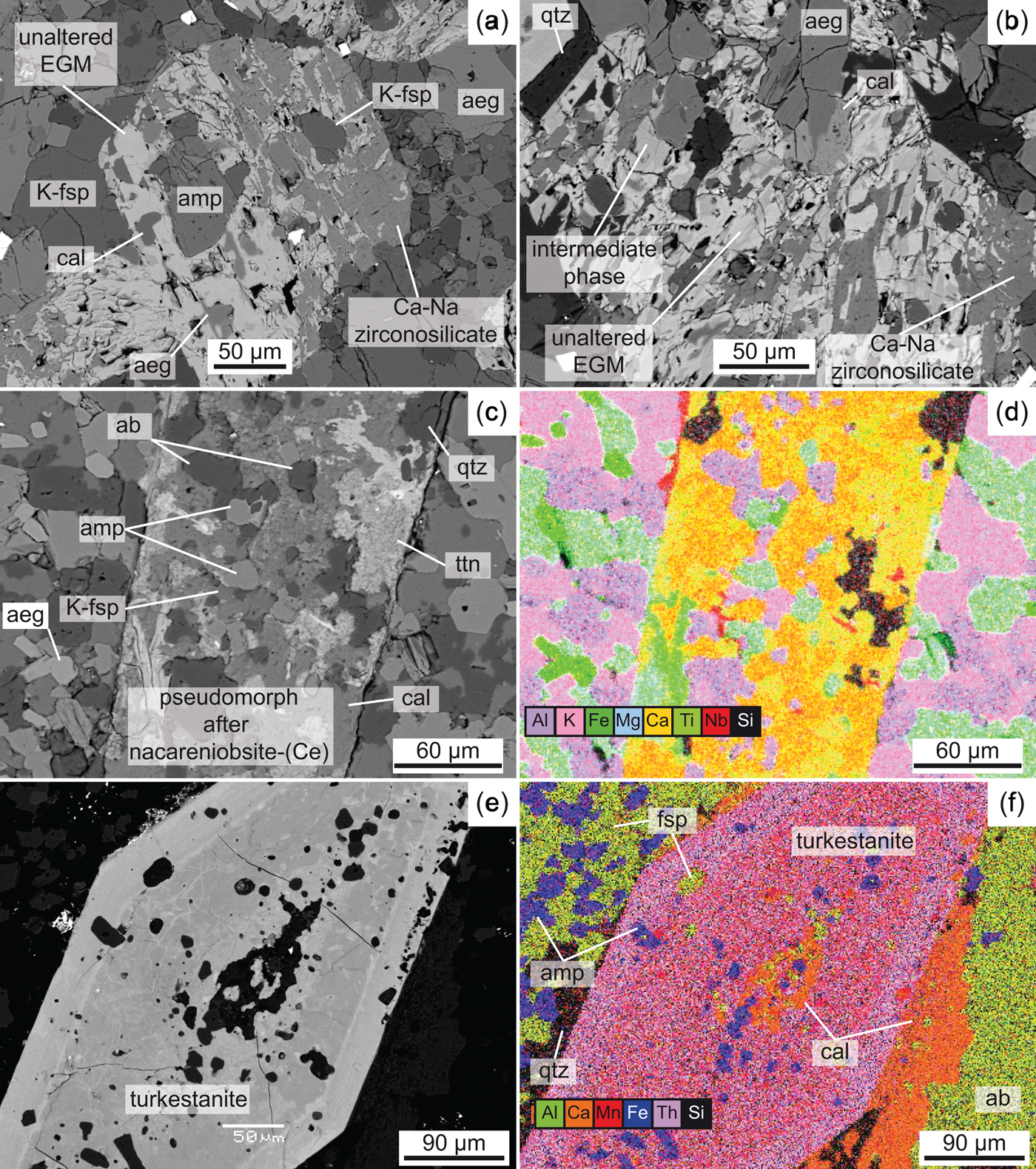
Fig. 9. BSE images (a, b, c and e) and false-coloured X-ray maps (d, f) of altered agpaitic minerals in GR-III: (a–b) altered EGM replaced by Ca-Na zirconosilicate, an intermediate phase and calcite; (c–d) altered nacareniobsite-(Ce) replaced mainly by calcite and titanite; and (e–f) zoned altered turkestanite with calcite. Abbreviations: ab: albite; aeg: aegirine; amp: amphibole; cal: calcite; K-fsp: K-feldspar; qtz: quartz; ttn: titanite.
Other agpaitic minerals
Representative compositions of nacareniobsite-(Ce) and turkestanite are given in Table 3. The compositions of different grains of nacareniobsite-(Ce) are similar and comparable to that of nacareniobsite-(Ce) published for other complexes (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2008; Vilalva et al., Reference Vilalva, Vlach and Simonetti2013; Borst et al., Reference Borst, Friis, Andersen, Nielsen, Waight and Smit2016). Fluorine content is higher than in the ideal formulae, as the calculated cations at ~3.7 apfu exceed the ideal value of 3 cations. The REE distribution patterns show a steep negative slope from LREE to HREE, similar to published patterns (Vilalva et al., Reference Vilalva, Vlach and Simonetti2013) (Fig. 6b). Trace-element distribution patterns reproduce the strong negative anomalies for Sr, Ti and Li that characterize EGM. Alteration products consist mainly of calcite, titanite and quartz (Fig. 9c–d).
Table 3. Average compositions of nacareniobsite-(Ce) and turkestanite in pegmatitic agpaitic (GR-III) dykes.

Abbreviations: b.d.l. = below detection limit, n.a. = not analysed, σ = standard deviation, n = number of analyses.
Turkestanite is characterized by a low sum of oxides (88–95 wt.%) and by compositional differences between crystals and between core and rim of altered crystals (Fig. 9e–f). The low total oxide sum is consistent with most of the compositions reported in the literature, and is probably related to structural H2O, not analysed with EPMA (Pautov et al., Reference Pautov, Agakhanov, Sokolova and Kabalov1997; Vilalva and Vlach, Reference Vilalva and Vlach2010). However, the very low sum of oxides of altered cores could result from porosity created by alteration processes and subsequent loss of volume. Altered cores are characterized by lower Si, Th, Na and K and higher Mn content compared to their rims and the unaltered crystals. Similarly to nacareniobsite-(Ce), REE distribution patterns show a steep negative slope from LREE to HREE, not unlike the patterns given by Vilalva and Vlach (Reference Vilalva and Vlach2010) (Fig. 6c).
Na-clinopyroxene and Na-amphibole
Further insights into the crystallization processes can be obtained by studying the detailed chemical changes of other rock-forming, non-agpaitic minerals, which are also liable to incorporate HFSE and REE. Representative compositions of Na-clinopyroxene and Na-amphibole in GR-II and GR-III are reported in Tables 4 and 5. Structural formulae of clinopyroxene are based on four cations and six oxygens and those of amphibole have been calculated using the spreadsheet of Locock (Reference Locock2014). All Na-clinopyroxenes analysed in both dyke types are aegirine. In GR-II, the core of cpx-I is richer in Ca and Zr (1.0 wt.% for both CaO and ZrO2) than the rim (0.6 wt.% CaO and 0.2 wt.% ZrO2) whereas the Ti content increases slightly in the rim from 1.1 to 1.6 wt.%. The composition of cpx-II is similar to that of the cpx-I rim. The REE distribution patterns have the same appearance in both dyke types although the REE concentrations are slightly higher in aegirine from GR-II than in GR-III (Fig. 6d). Trace-element distribution patterns are similar, except for Rb, Ba, Th and U, which are all below detection limits in GR-II, and above them in GR-III.
Table 4. Average compositions of aegirine in pegmatitic transitional (GR-II) and pegmatitic agpaitic (GR-III) dykes.

Abbreviations: aeg = aegirine, cpx = clinopyroxene, b.d.l. = below detection limit, (c) = calculated, σ = standard deviation, n = number of analyses.
Table 5. Average compositions of amphibole in pegmatitic transitional (GR-II) and pegmatitic agpaitic (GR-III) dykes. Lithium determined by LA-ICP-MS.
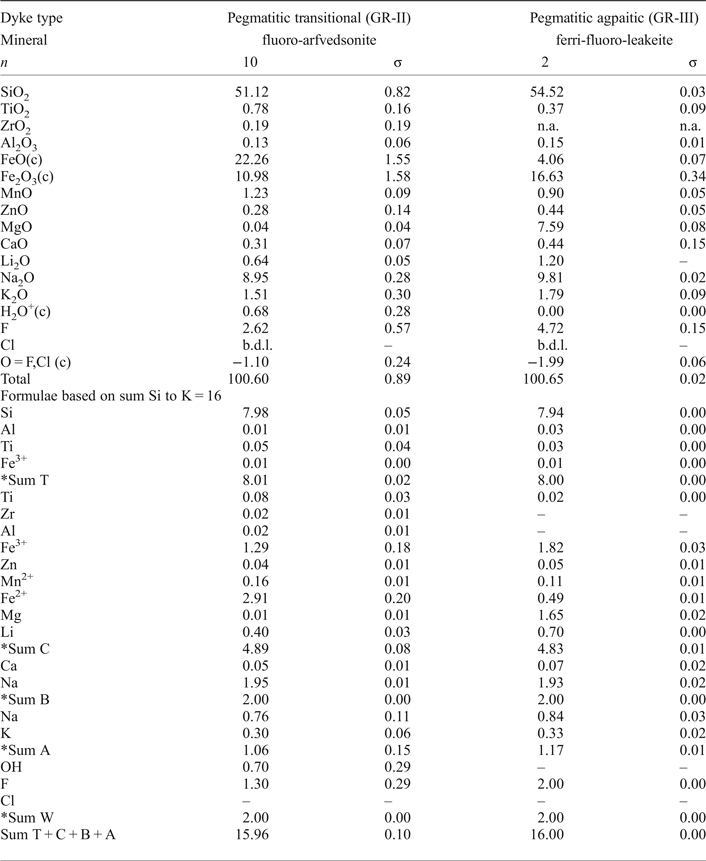
Abbreviations: b.d.l. = below detection limit, (c) = calculated, σ = standard deviation, n = number of analyses.
*Sum T is ideally 8 apfu; sum C ideally 5 apfu; sum B ideally 2 apfu; sum A from 0 to 1 apfu; sum W ideally 2 apfu.
Back-scatter electron images and X-ray element distribution mapping of a cpx-I phenocryst in GR-II illustrate the discontinuous zoning pattern between a homogeneous core and a zoned rim, as well as their compositional differences (Fig. 10). The qualitative X-ray Si map (Fig. 10b) shows the distribution of alkali feldspar (orange), clinopyroxene (green), quartz (red) and Zr-minerals (blue). The cpx-I shows a homogeneous light grey core enriched in Zr and Ca and depleted in Ti with respect to the external dark rim. This rim is characterized by porous, light and dark grey overgrowths containing inclusions of zircon, alkali feldspar and light grey patches (Fig. 10c,d,e,f). These features are restricted to the external rim and do not affect the light core. The light grey patches, porosity and zircon inclusions are mostly restricted to the light grey bands. The composition of zircon and light grey patches has been analysed qualitatively by energy-dispersive spectroscopy. Zircon contains significant concentrations of Ce, Ca, Fe and Al, whereas the light grey patches mainly contain Si, Zr and Na.
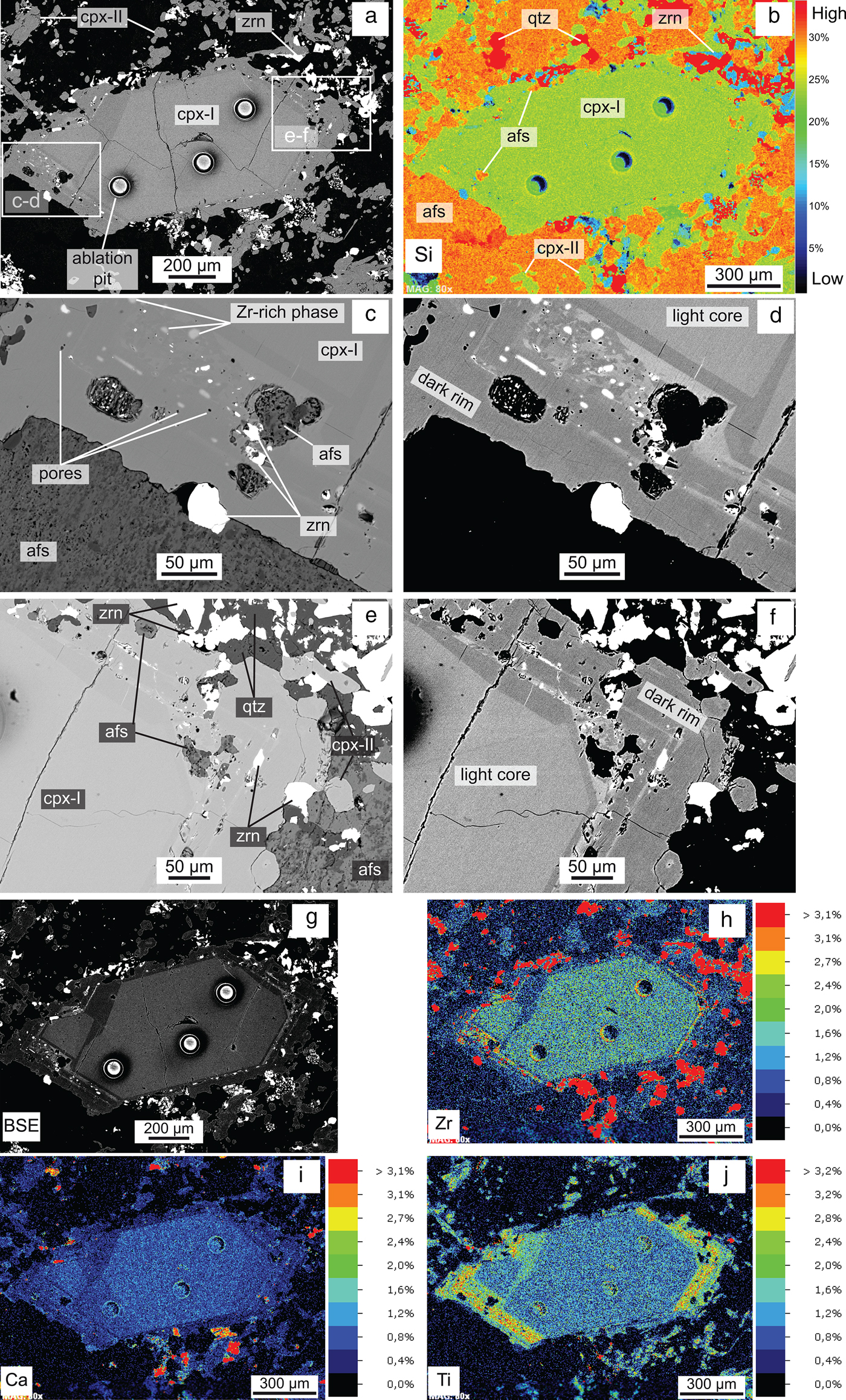
Fig. 10. (a) BSE image of zoned clinopyroxene phenocryst (cpx-I) surrounded by small clinopyroxene (cpx-II), zircon, alkali feldspar and quartz; (b) false-coloured X-ray map for Si of (a); (c–d) the left; and (e–f) right tips of the clinopyroxene phenocryst in (a) with different grey contrast. Band overgrowths contain pores, zircon and alkali feldspar inclusions, and Zr-rich light grey patches. (g–j) False-coloured X-ray maps showing the distribution of zirconium (Zr), calcium (Ca) and titanium (Ti). Abbreviations: afs: alkali feldspars; cpx: clinopyroxene, qtz: quartz; zrn: zircon.
Amphiboles in both dyke types are Na-rich, classified using the spreadsheet of Locock (Reference Locock2014) as an arfvedsonite in GR-II and as a ferri-fluoro-leakeite in GR-III. Lithium and fluorine contents in amphibole from GR-III are double those in GR-II. The most significant difference is the very high Mg content (7.6 wt.%) in amphibole from GR-III, which is very low (< 0.1 wt.%) in amphibole from GR-II. Their REE distribution patterns have a similar appearance in the LREE portion, with a higher content of LREE in GR-III than in GR-II, and a HREE portion with a steep positive slope in GR-III and a slightly flat curved shape in GR-II (Fig. 6e). Their trace-element distribution patterns have minor variations except for U, which is three orders of magnitude more concentrated in GR-III than in GR-II, and the geochemical twins Zr-Hf, which are one order of magnitude more concentrated in GR-II than in GR-III (Fig. 6e).
Discussion
Unusual occurrence of EGM in SiO2-oversaturated rocks
Chlorine solubility in the melt as the main limiting factor for EGM crystallization
The EGM described above are new examples occurring in SiO2-oversaturated rocks, and add to the list of other complex Na-K-Ca-Zr-silicates occurring in peralkaline granites (Table 1). It is now accepted that the term agpaitic, originally used to designate SiO2-undersaturated rocks that contain complex Na-K-Ca-Zr-silicates (Sørensen, Reference Sørensen1997; Le Maitre et al., Reference Le Maitre, Streckeisen, Zanettin, Le Bas, Bonin, Bateman, Bellieni, Dudek, Efremova, Keller, Lameyre, Sabine, Schmid, Sørensen and Woolley2002), can be extended to SiO2-oversaturated rocks (Marks et al., Reference Marks, Hettmann, Schilling, Frost and Markl2011). Marks et al. (Reference Marks, Hettmann, Schilling, Frost and Markl2011) also point out that most Zr minerals occurring in peralkaline granite (e.g. elpidite and dalyite) have a higher molar amount of SiO2 relative to alkalis and ZrO2 than Zr minerals in nepheline syenite (e.g. catapleiite and wadeite), due to the higher SiO2 activity in this type of melt. In addition, Zr minerals in peralkaline granite are either volatile free (e.g. dalyite and vlasovite) or H2O rich (e.g. elpidite). The EGM, unlike other agpaitic minerals found in peralkaline granites and pegmatites, are rich in Cl. Therefore, in addition to strongly peralkaline conditions and high Zr melt contents, the Cl content of the melt appears to be a critical parameter for crystallization of EGM in peralkaline granites (Sørensen, Reference Sørensen1997; Giehl et al., Reference Giehl, Marks and Nowak2014).
Chlorine is considered to be an incompatible element and is thus enriched in most evolved melts, although small amounts can be incorporated in amphibole, mica and apatite (Aiuppa et al., Reference Aiuppa, Baker and Webster2009). It has been shown that the fundamental controls on the solubility of Cl in silicate melts include the composition and structure of the melt, pressure and temperature (Signorelli and Carroll, Reference Signorelli and Carroll2002; Oppenheimer et al., Reference Oppenheimer, Fischer, Scaillet, Holland and Turekian2014). Chlorine solubility in silicic melts increases with decreasing pressure and increasing temperature (Webster, Reference Webster1992a). Chlorine is also known to have a high liquid/melt partition coefficient, leading to depletion of melts in Cl when a fluid phase is exsolved (Webster, Reference Webster1992b). The high capacity of peralkaline melts, and more particularly of agpaitic melts, to retain volatiles and rare elements has been discussed by several authors (e.g. Kogarko, Reference Kogarko and Sørensen1974; Metrich and Rutherford, Reference Metrich and Rutherford1992). Several experimental studies have shown that the solubility of Cl in peralkaline SiO2-oversaturated (pantellerititic) and undersaturated (phonolitic) melts is similar, in agreement with the concentration of Cl measured in natural samples (Metrich and Rutherford, Reference Metrich and Rutherford1992; Signorelli and Carroll, Reference Signorelli and Carroll2000, Reference Signorelli and Carroll2002). However, in granites and nepheline syenites, their intrusive equivalents, the retention of Cl is controlled by the composition of the crystallizing phases and by the eventuality of fluid exsolution. In SiO2-undersaturated rocks such as nepheline syenite, Cl can be incorporated into the Cl-rich rock-forming mineral sodalite, but in peralkaline granite, there are no major Cl-rich minerals. Furthermore, exsolution of a NaCl-rich fluid phase is common in peralkaline granites and is usually evoked to explain the scarcity of Cl in these rocks (Webster, Reference Webster1997). Thus, the window for EGM crystallization in SiO2-oversaturated rocks is rather restricted, requiring a highly evolved peralkaline melt composition and no, or very late, exsolution of a fluid phase. We thus suggest that the scarcity of EGM in peralkaline granites is related to the unavailability of Cl in the melt due to exsolution of a fluid phase at a late stage of crystallization. In the Ambohimirahavavy complex, we have shown in a previous paper that an orthomagmatic fluid exsolved from the melt at a late stage of crystallization of the peralkaline dykes (Estrade et al., Reference Estrade, Salvi, Béziat and Williams-Jones2015). The interstitial texture of EGM indicates that favourable conditions for its crystallization, i.e. a sufficient amount of Cl and Zr in the melt, were only achieved at the very late magmatic stage. However, these conditions evolved rapidly towards exsolution of an orthomagmatic fluid that partly altered the EGM.
The very unusual association of early magmatic EGM and quartz in the GR-III peralkaline pegmatite
The agpaitic GR-III granite represents the most differentiated lithology in the complex. This is shown by its agpaitic mineralogy and the coexistence of separate grains of albite and K-feldspar, indicating that the temperature of crystallization had decreased to below the feldspar solvus, and contrasts with crystallization of perthitic alkali feldspar in GR-II. These characteristics are attributed to the extremely evolved composition of the melt from which GR-III crystallized, and its extreme enrichment in HFSE, Na and volatiles (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a). To our knowledge, the association of early magmatic EGM and quartz in GR-III is the only known example of EGM as an early liquidus phase in granite. However, unlike the other main minerals, quartz is not present as inclusions in EGM, suggesting that quartz probably crystallized late, as is also indicated by its interstitial texture. This raises the question of whether quartz could have crystallized at a post-magmatic stage, i.e. from a hydrothermal fluid. However, the lack of reaction textures between these two minerals, or with any other agpaitic mineral, suggest a magmatic origin for quartz. Indeed, circulation of a hydrothermal fluid among these minerals would have severally affected the very unstable agpaitic minerals. The unusual composition of the GR-III melt compared to GR-II is further shown by the high Mg content of amphibole (Table 5, 8.0 wt.% MgO) and by the high Ca content of all the agpaitic minerals (Tables 2 and 3, 7.7 wt.% in EGM, 19.5 wt.% in nacareniobsite-(Ce) and 9.0 wt.% in turkestanite). High Mg contents, similar to those measured in GR-III, have been reported in ferri-fluoro-leakeite in nepheline syenite pegmatite from the Larvik Plutonic complex in Norway (Oberti et al., Reference Oberti, Boiocchi, Hawthorne and Kristiansen2014) and in the Verkhnee Espe deposit in Kazakhstan (Cámara et al., Reference Cámara, Hawthorne, Ball, Bekenova, Stepanov and Kotel'nikov2010). However, in the former occurrence, the Mg enrichment is not discussed, whereas in the latter, it is unclear whether the amphibole is magmatic or crystallized at a hydrothermal stage. The high Mg and Ca contents of these minerals contrasts with the composition of agpaitic melts, which are typically highly depleted in Mg – a highly compatible and early fractionating element – and moderately depleted in Ca (Sørensen, Reference Sørensen1997). To this effect, Marks et al. (Reference Marks, Hettmann, Schilling, Frost and Markl2011) have shown that the Ca content of evolved agpaitic melts varies depending on the nature of the parental melt and whether or not plagioclase had fractionated from the melt. The considerably negative Eu anomaly of the whole-rock REE patterns for both GR-II and GR-III dyke types (Fig. 6a) indicates significant fractionation of plagioclase from their parental melt; thus, the melt from which both dykes crystallized was depleted in Ca, following an evolution similar to the Ca-depleted trend of the Ilímaussaq complex (Marks et al., Reference Marks, Hettmann, Schilling, Frost and Markl2011). And, as far as we know, Mg enrichment has never been documented in alkaline complexes.
A possible explanation for the high Mg and Ca contents in the GR-III melt is contamination of the dykes by the host sediments (marls, mudstone and limestone) during emplacement. It is also possible that some of the Ca could have been introduced during fluid-rock interaction at a post-magmatic stage, such as described at Strange Lake during Ca-metasomatism (Salvi and Williams-Jones, Reference Salvi and Williams-Jones1996; Gysi et al., Reference Gysi, Williams-Jones and Collins2016; Vasyukova et al., Reference Vasyukova, Williams-Jones and Blamey2016). However, textural evidence clearly rules out a hydrothermal overprint of the primary Ca-bearing agpaitic minerals and Mg-bearing amphibole at Ambohimirahavavy.
The addition of Ca to the GR-III melt by contamination would have had a substantial effect on the solubility of Cl. Indeed, Chevychelov (Reference Chevychelov1999) has shown that the solubility of Cl in a melt correlates positively with its CaO content and with the CaO/SiO2 molar ratio. Using whole-rock compositional data from our previous studies on pegmatitic dykes, the CaO/SiO2 ratios are one order of magnitude higher in GR-III (0.053, sample EU01; Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a) compared with GR-II (≤0.007, samples AM30, AM50 and AM113A; Estrade et al., Reference Estrade, Béziat, Salvi, Tiepolo, Paquette and Rakotovao2014b). Therefore, we propose that the contamination of the highly evolved GR-III melt by Ca-rich material allowed for crystallization of early-magmatic eudialyte by increasing the concentration level of Cl in the melt because fluid saturation was suppressed. In GR-II, the low Mg content of amphibole and other minerals and the relatively low Ca content in cpx-I and EGM (Tables 2, 4 and 5) may indicate a lower contamination level than for GR-III.
REE and trace elements in EGM
High REE content of EGM
Trace-element patterns of EGM for SiO2-oversaturated (Ambohimirahavavy, Ascension Island and Straumsvola) and undersaturated (Ilímaussaq and Mont Saint Hilaire) complexes is displayed in Fig. 6a. In SiO2-oversaturated rocks, EGM generally have higher REE contents than in SiO2-undersaturated counterparts. In addition, most of the EGM from SiO2-undersaturated rocks that have similarly high REE contents are reported to be post-magmatic (hatched pattern in Fig. 7), and so probably crystallized from a REE-rich fluid (Figs 5b and 7). The REE enrichment in EGM in GR-II can be explained by the fact that EGM are the only REE-bearing minerals in GR-II, and thus scavenged most of the REE in the melt. By contrast, in GR-III, nacareniobsite-(Ce) is also a major REE-bearing mineral and competed with EGM for the uptake of REE. Harris et al. (Reference Harris, Cressey, Bell, Atkins and Beswetherick1982; Reference Harris and Rickard1987) considered the Ca-poor composition of peralkaline granites from Ascension Island and Straumsvola to explain the REE enrichment in EGM. They suggested that, because REE are incorporated in the same structural site as Ca in EGM, high amounts of REE entered the Ca site to compensate for its paucity in the melt from which the mineral crystallized. The negative correlation between La + Ce + Nd + Y and Ca clearly indicates that these elements compete for the same structural site (Fig. 5b). However, although whole-rock Ca contents in GR-II, Ascension Island and Straumsvola are similarly low (0.2 to 0.5 w. %), EGM from Straumsvola are depleted in HREE compared to those in the other two localities. Thus, another mechanism must be evoked to explain the difference in HREE content.
HREE enrichment in EGM: the role of melt composition
The HREE content of the melt can be estimated qualitatively by examining the REE distribution patterns of the major minerals present in the rocks (Fig. 6). In GR-II, the major minerals aegirine, amphibole and EGM all show HREE enrichments, suggesting that the GR-II melt was enriched in HREE. In GR-III, however, all minerals have lower HREE concentrations, surely reflecting an overall lower concentration of HREE in the GR-III melt. This difference is also evident from the whole-rock REE distribution patterns; in GR-II the HREE show a flat profile (Gd/Lu = 0.97), whereas it decreases slightly in GR-III (Gd/Lu = 1.73). We have pointed out above that the GR-III dykes are the most evolved lithology in the complex, and that they represent a melt produced by extensive fractionation. We suggest that at a late stage of evolution the HREE became compatible due to the abundant crystallization of phases that could incorporate them, such as aegirine and arfvedsonite, and were thus fractionated. The resulting melt, albeit very evolved, was thus relatively depleted in HREE.
U and Pb in EGM
Among all trace elements analysed in EGM from both dykes, U and Pb show the most markedly different behaviour (Fig. 6a). Uranium concentration in EGM, as for aegirine and amphibole, is higher in GR-III than in GR-II. As proposed for HREE above, the opposing behaviour of U in EGM might be attributed to its difference in concentration in the GR-II and GR-III melts. A much higher U concentration in the melt from which GR-III crystallized would be consistent with the presence of turkestanite in this granite, as U is a major constituent of this rare mineral, with up to 2 wt.% UO2. The higher U concentration in the GR-III melt thus reflects its highly incompatible behaviour, leading to an enrichment in the most evolved melts that were formed by significant fractional crystallization. The opposite observation can be made for Pb, which shows a positive and negative anomaly compared to its neighbouring elements in EGM from GR-II and GR-III, respectively (Fig. 6a). This opposite behaviour contrasts with the bulk Pb content, which is similar in both dykes. In GR-II, EGM and K-feldspar are the only minerals that can accommodate significant amounts of Pb, as aegirine and amphibole have very low Pb contents (few ppm to below ppm, see Supplementary material). In GR-III, in addition to EGM and K-feldspar, Pb is easily incorporated into turkestanite (~400 ppm, see Supplementary material). As feldspars are present in similar modal amounts in GR-II and GR-III, we think that they had a similar effect on bulk Pb content of the two melts. Therefore, we attribute the relative low Pb content in EGM from GR-III to its concomitant uptakes by turkestanite.
Destabilization of EGM and post-magmatic assemblage
Alteration of GR-II
The destabilization of EGM and concomitant sudden change in cpx-I composition in GR-II (Figs 8 and 10), together with the local destabilization of the three agpaitic minerals (EGM, nacareniobsite-(Ce) and turkestanite) in GR-III (Fig. 9), indicate that both granites experienced late- to post-magmatic alteration. The occurrence of alteration is a very common feature in peralkaline granites (Salvi and Williams-Jones, Reference Salvi and Williams-Jones2006; Kynicky et al., Reference Kynicky, Chakhmouradian, Xu, Krmicek and Galiova2011; Kempe et al., Reference Kempe, Möckel, Graupner, Kynicky and Dombon2015; Gysi et al., Reference Gysi, Williams-Jones and Collins2016; Vasyukova et al., Reference Vasyukova, Williams-Jones and Blamey2016) and in most cases the primary agpaitic minerals in these rocks undergo complete replacement, to the point that the precursor is not always easy to recognize. Examples include the Strange Lake complex in Canada, where most of the subsolvus granitic facies contain pseudomorphs of gittinsite after a euhedral precursor phase (Birkett et al., Reference Birkett, Miller, Roberts and Mariano1992; Mariano and Mariano, Reference Mariano and Mariano2014). Rare occurrences of partial replacement from drill core samples allowed identification of the primary mineral as elpidite, which was transformed to gittinsite via an intermediate step involving armstrongite (Salvi and Williams-Jones, Reference Salvi and Williams-Jones1995). Other instances of complete pseudomorphism include the Khan Bogd complex (Kynicky et al., Reference Kynicky, Chakhmouradian, Xu, Krmicek and Galiova2011) and Khaldzan Buregtey complex (Kempe et al., Reference Kempe, Möckel, Graupner, Kynicky and Dombon2015). Detailed mineralogical studies in some of these localities have shown that destabilization of these agpaitic minerals is a subsolidus process, related either to interaction with external fluids or to the exsolution of a fluid from the melt at a very late stage of crystallization. In some cases, fluid inclusion studies have documented the input of external fluids which may cause formation of relatively rare minerals, as was the case at Strange Lake, where Ca to form gittinsite was derived from a meteoric fluid (Salvi and Williams-Jones, Reference Salvi and Williams-Jones1990, Reference Salvi and Williams-Jones1996).
The above discussion indicates that interaction with a fluid, either of orthomagmatic or external origin, at late- to post-magmatic stages is a recurrent phenomenon in agpaitic peralkaline granite. This seems also to be the case in the Ambohimirahavavy complex, as shown in GR-II where secondary assemblages are characterized mainly by the occurrence of pseudomorphs of zircon and quartz after EGM plus an unidentified Ca-zirconosilicate (Fig. 8). Interaction with a fluid is also evidenced by the rims of cpx-I, which show a porous texture associated with zircon inclusions (Fig. 10). A similar texture has been described previously by the authors in zircon in a peralkaline granitic dyke of this complex (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014a). The association of Zr-rich patches, zircon inclusions and pores in the rim of the cpx-I can be interpreted in terms of coupled dissolution-reprecipitation which occurred at the fluid-cpx-I interface (Putnis, Reference Putnis, Oelkers and Schott2009). The presence of Al, Fe and Ca in zircon inclusions, elements that are not compatible in its structure, is further evidence of their hydrothermal origin (Geisler et al., Reference Geisler, Schaltegger and Tomaschek2007). The fact that the alteration is restricted to the light grey bands in the zoned rims of these crystals is intriguing (Fig. 10d, f). We suggest that this may be due to their higher content in Zr, thus making them more prone to alteration. The outermost dark rims are poor in Zr and probably precipitated during fluid-rock interaction concomitantly with the small cpx-II grains and late hydrothermal zircon (rich in Ca, Fe and Al) in the groundmass.
We propose that, during the interaction with a fluid, Cl and H2O were liberated to the fluid phase, causing the destabilization of previously formed EGM and precipitation of zircon and quartz. The elements initially present in EGM were redistributed in the rocks according to their respective mobility in the fluid phase. The precipitation of zircon within the edges of precursor EGM indicates that Zr could not be transported over a long distance. Moreover, Ti could no longer be incorporated in EGM and was therefore incorporated in the crystallizing rims of cpx-I and in the secondary cpx-II crystals (Fig. 10, Table 4). The similar Na2O content of cpx-I cores and rims indicate that Na activity did not change significantly between magmatic and late- to post-magmatic conditions. The amount of Ca incorporated in secondary phases (mainly in secondary Zr-minerals) could be derived partly from the Ca released in the fluid during the replacement of EGM and cpx-I. However, it is possible that some Ca was added to the system by an external fluid.
Alteration in GR-III is local and Ca-rich
Unlike GR-II, where most of the EGM have been completely replaced, alteration of EGM in GR-III is only a local phenomenon, and most of the EGM are pristine. In the altered parts, the three main agpaitic minerals (EGM, nacareniobsite-(Ce) and turkestanite) are destabilized and replaced, but aegirine and amphibole are mostly preserved. The most significant effect of alteration is the systematic removing of alkalis from the three agpaitic minerals and the precipitation of Ca-rich phases such as calcite and titanite in secondary assemblages (Fig. 9 and Tables 2 and 3). These observations suggest that, similarly to GR-II, a Ca-rich fluid interacted with GR-III at a late- to post-magmatic stage. The origin of the fluid could be both magmatic and external (Estrade et al., Reference Estrade, Salvi, Béziat and Williams-Jones2015).
Conclusions
We document two new occurrences of EGM in two types of peralkaline granitic dykes from the Ambohimirahavavy complex in northwest Madagascar. We propose that Cl availability is the main limiting factor for the crystallization of EGM in SiO2-oversaturated rocks. The transitional GR-II granite was produced from a melt that only achieved a sufficient Cl content to crystallize EGM at very late stages, resulting in formation of interstitial EGM. The melt rapidly evolved towards fluid exsolution, losing its Cl and thus hampering EGM crystallization. The resulting orthomagmatic fluid altered the EGM, although only to a limited extent. The agpaitic GR-III granite contains early-formed, abundant EGM. Contamination of the highly evolved GR-III melt by Ca-rich material increased Cl solubility, inducing early EGM crystallization. EGM in these granites have higher REE contents than most occurrences in undersaturated complexes. This is explained by the low Ca content of peralkaline melts, which favours REE intake in EGM as substitution for Ca. The enrichment of the heavy REE in GR-II with respect to EGM in GR-III is a feature inherited from the respective melt compositions, related to fractionation of the more compatible HREE in late-forming minerals at late stages of GR-III melt differentiation. In GR-II, exsolution of a NaCl-rich fluid caused destabilisation of EGM and precipitation of zircon and quartz. In GR-III, the exsolving fluid was rich in Ca and produced a calcic alteration assemblage.
Acknowledgements
Financial support for this research was provided by a CESSUR grant from the French Institut National des Sciences de l'Univers to Stefano Salvi, and by the company Tantalus Rare Earth A.G., who also kindly provided access to the Ambohimirahavavy complex and logistical support during fieldwork. We thank Wolfgang Hampel for his invaluable help in the field, Olivier Bruguier for laboratory assistance with the LA-ICP-MS and Sophie Gouy, Philippe De Parseval and Thierry Aigouy for assistance with EPMA and SEM analyses. We are grateful to Roger H. Mitchell and Iain Samson for their constructive and thorough reviews, which helped improve the quality of this manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.053