INTRODUCTION
Toxoplasma gondii is an obligate intracellular apicomplexan parasite that infects nucleated cells of birds and mammals (reviewed by Tenter et al. Reference Tenter, Heckeroth and Weiss2000). Since T. gondii is commonly acquired through oral ingestion of tissue cysts or oocysts, the intestinal epithelial cells constitute the first line of defence against the parasite. However, despite the local mechanisms to control the infection, some parasites are able to migrate through the epithelium and spread to other tissues (Buzoni-Gatel et al. Reference Buzoni-Gatel, Schulthess, Menard and Kasper2006). The migration of cells of the immune system from the blood into the site of infection in peripheral tissues is mediated by chemotactic factors and cell surface adhesion molecules, resulting in an acute inflammatory cellular infiltrate characterized by dendritic cells, granulocytes, NK and NKT cells (Iwasaki and Medzhitov, Reference Iwasaki and Medzhitov2004).
Among granulocytes, neutrophils are the earliest phagocytic cells recruited to the site of infection and play a key role in the clearance and killing of invading pathogens by phagocytosis, the release of antimicrobial compounds from their granules, and the production of reactive oxygen species (ROS) and nitric oxide (NO) (van Gisbergen et al. Reference van Gisbergen, Geijtenbeek and van Kooyk2005). Previous studies showed that depletion of neutrophils in early infection results in increased susceptibility to T. gondii (Bliss et al. Reference Bliss, Gavrilescu, Alcaraz and Denkers2001). However, there is no effect if neutrophils are removed at later stages of infection, indicating a major role for these cells in the initial response to T. gondii (Bliss et al. Reference Bliss, Gavrilescu, Alcaraz and Denkers2001).
A recent study showed that glycosylphosphatidylinositol (GPI)-anchored proteins on the surface of T. gondii tachyzoites bind human galectin-3 (Gal-3) with strong affinity, and in a dose-dependent manner (Debierre-Grockiego et al. Reference Debierre-Grockiego, Niehus, Coddeville, Elass, Poirier, Weingart, Schmidt, Mazurier, Guérardel and Schwarz2010). Gal-3 belongs to a family of lectins that bind β-galactoside-containing glycoconjugates (Barondes et al. Reference Barondes, Cooper, Gitt and Leffler1994). This lectin is expressed in different tissues by various non-immune and immune cell types (Kim et al. Reference Kim, Lee, Hyun, Park, Joo and Shin2007) and is markedly expressed by innate cells, including mast cells, neutrophils and eosinophils (Liu, Reference Liu2005).
The recruitment of effector cells dependent on Gal-3 may be an important mechanism of resistance to parasite infection (Debierre-Grockiego et al. Reference Debierre-Grockiego, Niehus, Coddeville, Elass, Poirier, Weingart, Schmidt, Mazurier, Guérardel and Schwarz2010). For example, high levels of Gal-3 were found in the granulomas surrounding eggs and worms during Schistosoma mansoni infection (van den Berg et al. Reference van den Berg, Honing, Franke, van Remoortere, Schiphorst, Liu, Deelder, Cummings, Hokke and van Die2004), whereas the size of the granulomas was significantly decreased in Gal-3 deficient mice (Gal-3−/−) compared with their wild type counterparts (Breuilh et al. Reference Breuilh, Vanhoutte, Fontaine, van Stijn, Tillie-Leblond, Capron, Faveeuw, Jouault, van Die, Gosset and Trottein2007). Currently, there is a single study on the importance of Gal-3 in the innate and adaptive immunity during in vivo T. gondii infection with a low-virulent strain, ME-49, in the mouse model (Bernardes et al. Reference Bernardes, Silva, Ruas, Mineo, Loyola, Hsu, Liu, Chammas and Roque-Barreira2006). The authors showed an upregulation of Gal-3 expression in various tissues in wild type mice, while Gal-3−/− mice developed lower inflammatory responses (Bernardes et al. Reference Bernardes, Silva, Ruas, Mineo, Loyola, Hsu, Liu, Chammas and Roque-Barreira2006). Recently, we have reported, in an in vitro T. gondii infection model, that Gal-3 plays an important modulatory role by interfering in neutrophil life span and activation during early stages of the infection (Alves et al. Reference Alves, Silva, Azzolini, Marzocchi-Machado, Carvalho, Pajuaba, Lucisano-Valim, Chammas, Liu, Roque-Barreira and Mineo2010).
Considering that T. gondii GPIs are ligands for Gal-3 and recognizing the importance of neutrophils during the early stages of this parasitic infection, in the present study we investigated the role of Gal-3 in the recruitment of peritoneal inflammatory leukocytes and activation of neutrophils from C57BL/6 mice after intraperitoneal infection with a virulent T. gondii RH strain.
MATERIALS AND METHODS
Mice and parasites
All experiments were carried out with 6 to 8-week-old C57BL/6 Gal-3 deficient mice (Gal-3−/−) and wild type (Gal-3+/+) mice that were maintained under standard conditions in the Animal Experimental Center, Universidade Federal de Uberlândia, Brazil. Gal-3−/− mice were obtained from the Animal Experimental Center of the Faculty of Medicine of Ribeirão Preto, Universidade de São Paulo, Brazil. All procedures were conducted according to institutional guidelines for animal ethics.
Toxoplasma gondii RH strain tachyzoites were maintained by serial passage in Swiss mice for 48 to 72 h (Mineo et al. Reference Mineo, Camargo and Ferreira1980). Peritoneal exudates were harvested and washed in phosphate-buffered saline (PBS, pH 7·2). Parasite suspensions were counted in a haemocytometer and re-suspended in RPMI medium.
Induction of peritoneal inflammation
Mice were injected intraperitoneally (i.p.) with 1 ml of sterile 3% thioglycollate or PBS as a control (Baron and Proctor, Reference Baron and Proctor1982). Peritoneal cells were harvested by gentle lavage with PBS after 3, 6 and 9 h of injection. Cells were washed once in PBS and counted in a haemocytometer for quantitation of total leukocytes. The proportion of leukocyte subpopulations was determined on the basis of standard morphological criteria in Giemsa-stained smears. Cell subpopulation counts were obtained in triplicate for each sample from 100–200 cells using a 100X oil immersion objective and expressed as mean absolute values in relation to total leukocyte number.
To evaluate the T. gondii-induced leukocyte influx to the peritoneal cavity, mice were inoculated (200 μl) i.p. with 1 × 102 tachyzoites of RH strain or peritoneal exudates from non-infected Swiss mice as the control. Peritoneal cells were harvested and analysed as described above.
T. gondii infection in vivo
Gal-3−/− and wild type mice (n = 10 animals per lineage) were infected i.p. (200 μl) with 1 × 102 tachyzoites of T. gondii RH strain. As the control, 4 mice of each lineage were inoculated with PBS alone. Animals were observed daily for clinical signs and mortality, by using morbidity scores according to the previously proposed protocol (Bartley et al. Reference Bartley, Wright, Sales, Chianini, Buxton and Innes2006) as follows: (0) sleek/glossy coat, bright and active, weight maintained at pre-infection level; (1) ruffled coat, hunched, tottering gait, reluctance to move, 10% weight loss; (2) starry stiff coat, 20% weight loss.
Isolation of inflammatory peritoneal neutrophils from T. gondii-infected mice
After 1, 3 and 5 days of T. gondii infection, neutrophils were obtained from the peritoneal cavity of mice by injecting i.p. 1 ml of 3% thioglycollate 6 h prior to the end of each time of infection. Cells were harvested from the peritoneal cavity by gentle lavage with PBS and layered on Ficoll-Hypaque solution (d = 1·077 g/ml; Sigma Chemical Co., St Louis, MO, USA) as previously described (Wilkie et al. Reference Wilkie, Vissers, Dragunow and Hampton2007). After centrifugation at 1000 g for 20 min at 20 °C, the supernatant containing the mononuclear cells was removed by aspiration and the neutrophil pellet was washed in RPMI medium. Cells were treated with erythrocyte lysis buffer (0·16 M NH4Cl, 0·17 M Tris-HCl, pH 7·5) for 5 min at 37 °C, when appropriate. After new washing, cells were suspended in complete RPMI medium (supplemented with 10% heat-inactivated fetal calf serum, 2 mm glutamine, 100 U penicillin/ml and 100 μg streptomycin/ml) and viable cells were counted in a haemocytometer by trypan blue exclusion. Neutrophils obtained in this manner were routinely >95% viable and 80–90% pure.
Reactive oxygen species measurement
Chemiluminescence (CL) assays were performed according to the previously described protocol (Cheung et al. Reference Cheung, Archibald and Robinson1983; Alves et al. Reference Alves, Marzocchi-Machado, Carvalho and Lucisano-Valim2003). Inflammatory peritoneal neutrophils (5 × 105 cells/500 μl) obtained from mice infected or not with T. gondii after 1, 3 or 5 days of infection were mixed with 5 μl of 280 μM luminol in Hanks' balanced saline solution (HBSS) at 37 °C for 2 min. Cells were then stimulated with 50 μl of 10−7 M phorbol myristate acetate (PMA) and cells incubated with luminol in the absence of PMA were used as control of reactive oxygen species (ROS) spontaneous release. CL measurements were performed at 37 °C for 20 min in an Auto Lumat luminometer (Berthold, Bad Wildbad, Germany). Results were expressed as the integrated area under the curve of CL profiles by subtracting the spontaneous release.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). The Student's t-test was used for comparison of data obtained between Gal-3+/+ and Gal-3−/− mice as well as T. gondii infection in relation to non-infected controls. Differences between times after inoculation for neutrophil influx as well as differences between peaks of ROS production after T. gondii infection were analysed using the ANOVA and Tukey multiple comparison post-test. The Kaplan-Meier method was applied to estimate the percentage of mice surviving at each time-point after infection and survival curves were compared using the log-rank test. A value of P < 0·05 was considered statistically significant.
RESULTS
Galectin-3 upregulates mouse peritoneal inflammation, with enhanced recruitment of neutrophils and lymphocytes after stimulation with thioglycollate
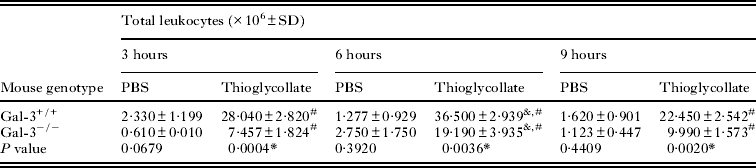
To investigate the effect of Gal-3 in the recruitment of inflammatory leukocytes into the mouse peritoneal cavity, we evaluated the influx of leukocytes after different times of peritoneal inflammation induced by thioglycollate in Gal-3+/+ and Gal-3−/− mice comparatively to PBS (Tables 1 and 2). Although both mouse lineages exhibited peritoneal inflammation with significant recruitment of leukocytes after stimulation with thioglycollate at all times analysed, Gal-3+/+ mice showed increased inflammation with higher total leukocyte influx compared to Gal-3−/− mice (P < 0·05). Also, a peak influx of peritoneal leukocytes was noted after 6 h of stimulation in both mouse lineages. After PBS inoculation, there were no significant differences in the total leukocyte population into the peritoneum between both mouse genotypes (Table 1).
Table 1. Kinetics of inflammatory peritoneal total leukocyte recoveries from C57BL/6 wild type (Gal-3+/+) and galectin-3 deficient (Gal-3−/−) mice treated with thioglycollate
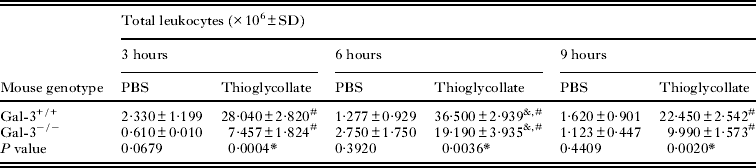
Table 2. Kinetics of inflammatory peritoneal leukocyte subpopulation recoveries from C57BL/6 wild type (Gal-3+/+) and galectin-3 deficient (Gal-3−/−) mice treated with thioglycollate

a, b, c Different letters indicate statistically significant differences between leukocyte subpopulations in each genotype and time of stimulus analysed.
# Statistical significance for neutrophil influx peak among different times after stimulation (ANOVA followed by Tukey's multiple comparison post-test, P < 0·05).
ND, not done.
When different populations of leukocytes were analysed after thioglycollate stimulation, there was a predominance of neutrophils and lymphocytes at all times analysed in both Gal-3+/+ and Gal-3−/− mice. However, Gal-3+/+ mice exhibited a significantly higher number of these cells compared to Gal-3−/− in all time-points (P < 0·05). Also, a peak in the influx of neutrophils was observed in both mouse lineages after 6 h of stimulation, with a higher number of neutrophils than lymphocytes in Gal-3+/+ mice, but not in Gal-3−/− mice at this time-point. The numbers of monocytes and eosinophils were comparable between both groups of mice in all conditions analysed, except for monocytes after 3 h of thioglycollate stimulation. After PBS inoculation, no significant difference was found between mouse genotypes for all populations of leukocytes analysed (Table 2). Taken together, these results indicate that Gal-3 enhances the peritoneal inflammation in C57BL/6 mice stimulated intraperitoneally with thioglycollate, with a significant increase in the recruitment of neutrophils and lymphocytes, but it had no effect on the total leukocyte populations in the peritoneum after PBS inoculation.
Galectin-3 does not influence the enhanced recruitment of neutrophils in early stages of T. gondii infection
In order to determine whether Gal-3 could be involved in regulating the migration of leukocytes, especially neutrophils, into the peritoneal cavity of C57BL/6 mice after i.p. infection with the RH strain of T. gondii, we used the same model as above described. In contrast with the animals that were stimulated with thioglycollate, no significant difference was observed in the recruitment of total leukocytes after T. gondii infection in Gal-3+/+ mice. However, Gal-3−/− mice exhibited increased leukocyte influx after the first 3 h of infection as compared to the non-infected controls. On the other hand, after 3 h of inoculation with non-infected peritoneal exudate (control), Gal-3−/− mice showed decreased leukocyte influx in relation to Gal-3+/+ mice (Fig. 1A). Thus, the differential count of leukocytes in the peritoneal exudate of mice was performed only at this time-point. As shown in Fig. 1B, there was a significant increase in lymphocyte populations, and to a lesser extent, in the number of neutrophils, in infected Gal-3−/− mice compared to the non-infected controls. In contrast, infected Gal-3+/+ mice showed a significant increase only in the neutrophil population compared to non-infected controls (Fig. 1B), but this increase did not change the total number of leukocytes as seen in Fig. 1A. At this time-point, in the absence of infection, Gal-3−/− mice showed decreased lymphocyte influx in relation to Gal-3+/+ mice (Fig. 1B).

Fig. 1. Influx of leukocytes from C57BL/6 wild type (Gal-3+/+) and galectin-3 deficient (Gal-3−/−) mice. Mice were injected i.p. with 1 × 102 tachyzoites of Toxoplasma gondii RH strain or peritoneal exudate from non-infected mice (control). (A) Kinetics of total leukocyte influx was analysed after 3, 6 and 9 h of infection. (B) Leukocyte subpopulation influx was analysed after 3 h of infection. (C) Kinetics of influx of neutrophils. (D) Representative photomicrographs of leukocyte population in Giemsa-stained smears: a. monocyte; b. lymphocytes; c. neutrophils. Scale Bar = 10 μm. Data are expressed as mean ± S.D. from 2 independent experiments. *Comparison between infected mice and control for each condition (Student's t-test; P < 0·05). #Comparison between Gal-3+/+ and Gal-3−/− controls for each condition (Student's t-test; P < 0·05); &Predominance of lymphocytes in relation to other leukocytes (ANOVA and Tukey multiple comparison post-test; P < 0·05).
To confirm these findings, the neutrophil population was analysed in both mouse lineages in all time-points, and an increased number of these cells was observed only after 3 h of infection with T. gondii as compared to non-infected controls (Fig. 1C). The morphological aspects of the leukocyte populations are shown in Fig. 1D. Taken together, these results suggest that Gal-3 does not change the influx of total inflammatory leukocytes in early stages of T. gondii infection, and the recruitment of neutrophils is enhanced regardless of galectin-3. In the absence of infection, however, Gal-3 upregulates the influx of total leukocytes especially the lymphocytes.
Galectin-3 upregulates PMA-induced ROS generation by inflammatory peritoneal neutrophils from mice infected with T. gondii RH strain
Once the recruitment of neutrophils into the peritoneal cavity increased after early infection with T. gondii in both mouse genotypes, we verified whether Gal-3 could interfere with the generation of ROS by inflammatory peritoneal neutrophils from mice infected with virulent T. gondii RH strain. After 1, 3 and 5 days of infection, the PMA-induced ROS production was evaluated by luminol-dependent CL responses (Fig. 2). After 1 day of infection (Fig. 2A), the ROS production increased in Gal-3+/+ neutrophils and decreased in Gal-3−/− neutrophils as compared to non-infected controls (P < 0·05). In addition, ROS production was higher in infected Gal-3+/+ than Gal-3−/− neutrophils at this time point (P < 0·05). After 3 days of infection (Fig. 2B), the ROS production profile was similar to the first day after infection, with increased ROS production in Gal-3+/+ and decreased in Gal-3−/− neutrophils as compared with the respective non-infected controls (P < 0·05). In addition, ROS production was not significantly different between both genotypes at this time-point of infection. On the other hand, after 5 days of infection (Fig. 2C), both Gal-3+/+ and Gal-3−/− neutrophils exhibited decreased ROS production in relation to non-infected controls, although Gal-3+/+ neutrophils had higher ROS production than Gal-3−/− neutrophils (P < 0·05). In non-infected control groups, ROS production in Gal-3−/− neutrophils was higher than Gal-3+/+ neutrophils at all time-points analysed (Fig. 2A–C) (P < 0·05).

Fig. 2. PMA-induced ROS production measured by chemiluminescence (CL) in inflammatory peritoneal neutrophils from C57BL/6 wild type (Gal-3+/+) and galectin-3 deficient (Gal-3−/−) mice after 1 (A), 3 (B) and 5 (C) days of infection with 1 × 102 tachyzoites of Toxoplasma gondii RH strain compared to non-infected controls. Neutrophils were stimulated with PMA and ROS release was measured in the presence of luminol. (D) CL profile in neutrophils from Gal-3+/+ and Gal-3−/− mice after different times of infection or PBS injection. Data are expressed as mean ± S.D. of areas under the curves of CL profiles from 3 independent experiments performed in triplicate. *Comparison between non-infected and infected neutrophils in each mouse genotype; #Significant differences in relation to Gal-3+/+ in each condition (Student's t-test; P < 0·05); &Statistical significance among different times after infection or PBS injection (ANOVA and Tukey multiple comparison post-test; P < 0·05).
When the kinetics of ROS production by Gal-3+/+ and Gal-3−/− neutrophils was analysed in parallel (Fig. 2D), ROS production increased after 1 day of infection and decreased progressively on days 3 and 5 after infection in Gal-3+/+ neutrophils (P < 0·05). In Gal-3−/− neutrophils, however, the peak in the ROS generation occurred after 3 days of infection and significantly decreased on day 5 after infection (P < 0·05). In non-infected groups, the kinetics of ROS production was not significantly changed in both mouse genotypes (Fig. 2D).
It is worth mentioning that T. gondii infection did not change the spontaneous release of ROS in relation to controls in both mouse lineages and at all times analysed (data not shown). These results show that galectin-3 upregulates ROS generation in inflammatory peritoneal neutrophils from C57BL/6 mice infected with the RH strain of T. gondii, but downregulates ROS generation by neutrophils from non-infected mice.
Galectin-3 does not influence the survival of C57BL/6 mice after infection with the virulent T. gondii RH strain
To evaluate the role of Gal-3 in the T. gondii infection outcome, Gal-3+/+ and Gal-3−/− mice were infected i.p. with virulent RH strain tachyzoites and monitored for survival. As shown in Fig. 3A, Gal-3−/− mice showed a slightly lower survival percentage than Gal-3+/+ mice, but this difference was not significant (P > 0·05). However, survival curves of both mouse lineages inoculated with T. gondii were significantly lower in relation to the non-infected controls (P < 0·05). These findings demonstrate that Gal-3 has no apparent effect on the survival of C57BL/6 mice after infection with the virulent T. gondii RH strain.

Fig. 3. Survival curves (A) and morbidity scores (B) of C57BL/6 wild type (Gal-3+/+, n = 10) and galectin-3 deficient (Gal-3−/−, n = 10) mice after intraperitoneal infection with 1 × 102 tachyzoites of Toxoplasma gondii RH strain or PBS injection (control). Survival data are representative of 1 from 3 independent experiments. Data from morbidity scores are expressed as mean ± S.D. from 3 independent experiments. Survival curves of Gal-3+/+ and Gal-3−/− mice inoculated with T. gondii were significantly different from controls (Log rank test; P < 0·05).
The evaluation of the morbidity scores showed that both infected Gal-3+/+ and Gal-3−/− mice started to present clinical signs at 6 days after infection, reaching a morbidity peak on day 7 post-infection (Fig. 3B), which was coincident with the highest rate of mortality (70–80%) occurring at 7–8 days after infection. The remaining animals, as well the uninfected controls, showed no clinical signs.
DISCUSSION
In the present study we outline an experimental model of in vivo T. gondii infection with the virulent RH strain using C57BL/6 Gal-3+/+ and Gal-3−/− mice to assess the role of this lectin in acute inflammatory processes. First, we evaluated whether Gal-3 is involved in acute inflammation after 3, 6, and 9 h of thioglycollate stimulation compared to homeostasis condition (PBS). Both mouse lineages were able to display peritoneal inflammation in response to thioglycollate; however, this inflammation was decreased in the absence of Gal-3, as shown by reduced total leukocytes in Gal-3−/− mice. Furthermore, at all times analysed, neutrophils and lymphocytes were the predominant population in both mouse genotypes, although after 6 h of stimulation, the number of neutrophils was higher compared to the other leukocytes in Gal-3+/+, but not Gal-3−/− mice. In homeostasis condition, however, Gal-3 seems not to have an effect on the leukocyte populations in the peritoneum. Previous studies evaluating the kinetics of leukocyte recruitment into the peritoneal cavity of Gal-3−/− and wild type mice after 1, 2 and 4 days of i.p. injection of thioglycollate also showed that Gal-3−/− mice exhibited a reduced number of neutrophils (Colnot et al. Reference Colnot, Ripoche, Milon, Montagutelli, Crocker and Poirier1998) and macrophages (Hsu et al. Reference Hsu, Yang, Pan, Yu, Salomon, Fung-Leung and Liu2000) compared to wild type mice. Taken together, these results suggest that endogenous Gal-3 upregulates the acute peritoneal inflammation in mice stimulated with thioglycollate, mainly by increasing the recruitment of neutrophils and lymphocytes, but it does not influence the leukocyte populations under homeostasis condition.
Considering that T. gondii is able to elicit a strong inflammatory response developed by several cell types, such as granulocytes, macrophages, and lymphocytes (Munoz et al. Reference Munoz, Liesenfeld and Heimesaat2011), we investigated whether Gal-3 has a pro-inflammatory role in mice infected with the virulent T. gondii RH strain. In this infection condition, no significant difference was observed in the recruitment of total leukocytes in Gal-3+/+ mice, and only an increase occurred in the population of neutrophils within the first 3 h of infection. On the other hand, infected Gal-3−/− mice exhibited a significant inflammatory response with increased leukocyte influx associated with a marked increase in the neutrophils and mostly, the lymphocyte population, in the earlier time after infection. Previous studies have demonstrated a significant influx of neutrophils into the peritoneal cavity of C57BL/6 or BALB/c mice, after 4 h of infection with 2 × 106 tachyzoites of RH strain of T. gondii (Bliss et al. Reference Bliss, Butcher and Denkers2000; Del Rio et al. Reference Del Rio, Bennouna, Salinas and Denkers2001). It is noteworthy, however, that the parasite inoculum used in our model of infection (1 × 102 tachyzoites) consisted of a 20 000 times lower parasite load, and it was even able to induce significant recruitment of neutrophils in both Gal-3+/+ and Gal-3−/− mice. These results suggest that the parasite, but not Gal-3, upregulates the influx of inflammatory neutrophils in early stages of this infection model. In conditions without infection, however, Gal-3 was shown to be important for maintaining the influx of total leukocytes, especially the lymphocytes.
Although T. gondii infection did not cause an increase in the total inflammatory leukocytes, the increased inflammatory peritoneal neutrophils at the earlier time after infection in both mouse genotypes reflects the ability of these cells to rapidly respond to a potent inflammatory stimulus such as T. gondii. Studies have demonstrated the critical importance of neutrophils in the initial response to T. gondii (Sayles and Johnson, Reference Sayles and Johnson1996; Bliss et al. Reference Bliss, Gavrilescu, Alcaraz and Denkers2001). C57BL/6 mice depleted of neutrophils and infected with T. gondii ME49 strain succumb during the acute phase, showing lesions in multiple organs and a high parasite load (Bliss et al. Reference Bliss, Gavrilescu, Alcaraz and Denkers2001). The role of Gal-3 was investigated in infection with the low-virulent ME-49 strain of T. gondii and it was shown that Gal-3−/− mice infected i.p. exhibited lower survival rates associated with lower infiltration of neutrophils and monocytes/macrophages into the peritoneal cavity than Gal-3+/+ mice on day 4 after infection. In addition, when mice were orally infected, Gal-3−/− mice developed reduced inflammatory responses, exhibiting a higher parasite burden in the lungs and brain, although they produced a higher Th1-polarized response with higher levels of IL-12 and IFN-γ than the wild type counterparts (Bernardes et al. Reference Bernardes, Silva, Ruas, Mineo, Loyola, Hsu, Liu, Chammas and Roque-Barreira2006). Those authors concluded that Gal-3 showed a tendency to suppress Th1 responses. In another model of infection, Gal-3−/− mice were more susceptible to infection by Paracoccidioides brasiliensis and developed a Th2-polarized immune response compared to Gal-3+/+ mice (Ruas et al. Reference Ruas, Bernardes, Fermino, de Oliveira, Hsu, Liu, Chammas and Roque-Barreira2009). Considering these reports and the findings of the present study, it is remarkable that Gal-3 can display diverse roles in different models of infection, which include the nature of the pathogen, as well as its genotype and the route of infection.
As a significant recruitment of neutrophils into the peritoneal cavity after early T. gondii infection was observed in both mouse genotypes, we then investigated whether Gal-3 could affect ROS generation in these cells. We observed that after T. gondii infection Gal-3+/+ neutrophils showed an increase in PMA-induced ROS generation followed by a decline after 5 days of infection, in contrast to a decrease in ROS production by Gal-3−/− neutrophils compared to the non-infected groups. These findings suggest that Gal-3 is important in generating ROS by neutrophils from infected mice as an attempt to control parasite growth in the acute phase of infection, whereas in the absence of Gal-3 the parasite is able to decrease ROS production by neutrophils as a strategy to evade the immune response. Therefore, endogenous Gal-3 may exert upregulation of this neutrophil function against early infection by T. gondii. Moreover, the decline in ROS generation by neutrophils after 5 days of infection may be related to the clinical presentation of animals at this time, as demonstrated by the highest morbidity scores and mortality rates at this stage of infection. On the other hand, the lower ROS generation in Gal-3+/+ than Gal-3−/− neutrophils from non-infected mice at all time-points analysed can reflect a potential role of Gal-3 in maintaining baseline ROS production under homeostasis conditions.
Recently, in in vitro infection with T. gondii RH strain we have shown decreased PMA-induced ROS production by Gal-3+/+ neutrophils after 30 min of infection compared to non-infected controls, while in Gal-3−/− neutrophils the ROS generation was similar between infected and uninfected cells. On the other hand, a higher ROS production by Gal-3−/− compared with Gal-3+/+ neutrophils was observed regardless of infection (Alves et al. Reference Alves, Silva, Azzolini, Marzocchi-Machado, Carvalho, Pajuaba, Lucisano-Valim, Chammas, Liu, Roque-Barreira and Mineo2010). These findings, along with those herein described, suggest that Gal-3 downregulates ROS generation by neutrophils in the absence of T. gondii infection in both in vitro and in vivo models. After infection, however, the ROS generation is differentially modulated by Gal-3 when assessed in in vivo or in vitro models, probably due to multiple cell interactions that occur in the former condition.
Finally, we demonstrated that Gal-3 does not change the estimated survival and mean morbidity scores of mice after i.p. infection with this virulent strain of T. gondii. These findings corroborate previous results demonstrating that the mortality of Gal-3−/− mice was comparable with its wild-type counterparts when orally infected with the low-virulent ME-49 strain (Bernardes et al. Reference Bernardes, Silva, Ruas, Mineo, Loyola, Hsu, Liu, Chammas and Roque-Barreira2006). However, when mice were i.p. infected with this strain, the mortality rate was higher in Gal-3−/− than wild type mice (Bernardes et al. Reference Bernardes, Silva, Ruas, Mineo, Loyola, Hsu, Liu, Chammas and Roque-Barreira2006), reinforcing the idea that the degree of strain virulence as well as the route of inoculation are important issues to be considered in the outcome of infection.
Altogether, the results of the present study suggest that Gal-3 upregulates peritoneal inflammation in C57BL/6 mice, with enhanced recruitment of neutrophils and lymphocytes after stimulation with thioglycollate, but does not have any influence in the enhanced recruitment of neutrophils in early stages of virulent T. gondii RH strain infection. In addition, Gal-3 upregulates PMA-induced ROS production by inflammatory peritoneal neutrophils from mice infected with T. gondii RH strain, but downregulates its production by neutrophils from non-infected animals. Thus, during in vivo infection with virulent T. gondii strain, Gal-3 is essential for ROS generation by neutrophils and this mechanism can represent an attempt to control the parasite growth in the acute phase of infection. Further studies should be conducted to prove if Gal-3+/+ neutrophils are more able to kill parasites than Gal-3−/− neutrophils, and thus confirming the microbicidal potential of ROS generation by neutrophils in the initial acute phase of T. gondii infection.
ACKNOWLEDGEMENTS
We thank Dr Roger Chammas and Dr Fu-Tong Liu for provide us galectin-3 deficient mice.
FINANCIAL SUPPORT
This work was supported by the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), Brazil.