INTRODUCTION
Coral reefs worldwide have been in decline for decades under increasing anthropogenic disturbances, such as overfishing, pollution and coral bleaching resulting from global climate change (Pandolfi et al., Reference Pandolfi, Bradbury, Sala, Hughes, Bjorndal, Cooke, McArdle, McClenachan, Newman, Paredes, Warner and Jackson2000). It is crucial to gain more knowledge on the multitude of biotic and abiotic stressors that threaten coral reefs in order to successfully implement conservation plans for their protection.
The encrusting cyanobacteriosponge Terpios hoshinota Rützler & Muzik (Reference Rützler and Muzik1993) has come to the attention as another potential threat to Indo-Pacific coral reefs. Although sponges are an important component of coral reef biodiversity, and together with stony and soft corals form complex three-dimensional structures that provide shelter to numerous marine organisms, they are also very successful competitors for space on reefs, at the expense of corals (Aerts & van Soest, Reference Aerts and van Soest1997; de Voogd et al., Reference de Voogd, Becking, Noor, Hoeksema and van Soest2004; Wulff, Reference Wulff2012). Generally, sponges have a competitive advantage over corals because they can successfully resist coral defences and persevere in environments that are unfavourable to corals (Chadwick & Morrow, Reference Chadwick, Morrow, Dubinsky and Stambler2011). Additionally, the sponge T. hoshinota can actively kill and overgrow live coral tissue. This sponge occasionally exhibits outbreaks that can cover huge areas, resulting in the mass mortality of corals and other sessile organisms (Rützler & Muzik, Reference Rützler and Muzik1993; Liao et al., Reference Liao, Tang, Hsu, Wen, Wu, Chen and Chen2007). During outbreaks, T. hoshinota can rapidly decimate corals due to growth rates of approximately 1–2 mm per day, and its capacity to occupy reef substrata for up to several years (Plucer-Rosario, Reference Plucer-Rosario1987; Schils, Reference Schils2012). This may prevent the settlement of coral larvae, hereby hindering reef recovery (Bryan, Reference Bryan1973; Plucer-Rosario, Reference Plucer-Rosario1987). It is clear that this sponge is very persistent on coral reefs, and once it has invaded a new area, it appears to remain there permanently (Reimer et al., Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a, Reference Reimer, Nozawa and Hiroseb). However, the outbreaks do not always lead to irreversible reef degradation because coral recovery has also been observed with the near disappearance of T. hoshinota (Plucer-Rosario, Reference Plucer-Rosario1987; Reimer et al., Reference Reimer, Nozawa and Hirose2011b).
Terpios hoshinota has been described from the Ryukyu Archipelago, Japan and Guam (Rützler & Muzik, Reference Rützler and Muzik1993), but outbreaks of the sponge have been reported and studied earlier in Guam, the Caroline Islands, the Philippines, Taiwan and American Samoa (Bryan, Reference Bryan1973; Plucer-Rosario, Reference Plucer-Rosario1987). More recently, the sponge has been reported in the Great Barrier Reef (Fujii et al., Reference Fujii, Keshavmurthy, Zhou, Hirose, Chen and Reimer2011), Indonesia (de Voogd et al., Reference de Voogd, Cleary and Dekker2013), Malaysia (Hoeksema et al., Reference Hoeksema, Waheed and de Voogd2014b) and the Maldives (Montano et al., Reference Montano, Chou, Chen, Galli and Reimer2015). Most information about T. hoshinota is obtained through studies during and after outbreaks. However, pre-outbreak data on T. hoshinota are still lacking, and baseline data concerning its ecology, habitat preferences and outbreak triggers are therefore urgently needed.
Recently, small patches of T. hoshinota were observed on an offshore reef in the Spermonde Archipelago, Makassar Strait, Indonesia (de Voogd et al., Reference de Voogd, Cleary and Dekker2013). Its identity was confirmed by morphological analyses and cytochrome oxidase I mitochondrial DNA sequences. The reefs of the Spermonde Archipelago have been extensively studied during sponge surveys since 1997 (de Voogd et al., Reference de Voogd, Becking, Noor, Hoeksema and van Soest2004, Reference de Voogd, Haftka and Hoeksema2005, Reference de Voogd, Cleary, Hoeksema, Noor and van Soest2006; Cleary & de Voogd, Reference Cleary and de Voogd2007; Hoeksema et al., Reference Hoeksema, Dekker and de Voogd2014a, Reference Hoeksema, Waheed and de Voogdb), but T. hoshinota has not been recorded before 2012. Hence, this area is highly suitable for a baseline study. Future surveys in this area may reveal whether the occurrence of T. hoshinota is stable, or whether its presence will inevitably always lead to an outbreak event.
The objectives for the present baseline study were (1) to map the current distribution of T. hoshinota in the Spermonde Archipelago; (2) to study its distribution and abundance in relation to cross-shelf variation in benthic community structure; (3) to record its coral substrate preference under regular (non-outbreak) conditions; and (4) to analyse the genetic diversity within a T. hoshinota population by comparing sequences obtained by use of the Folmer partition and the I3-M11 partition of the mitochondrial CO1 and the D3–D5 region of the nuclear ribosomal 28S gene with sequences from the marine region of China available in GenBank.
MATERIALS AND METHODS
Study area
The Spermonde Archipelago in south-west Sulawesi is situated within the Coral Triangle, the centre of maximum marine benthic biodiversity (Hoeksema, Reference Hoeksema and Renema2007). The shelf ranges 32–56 km in width and approximately 128 km in length and is covered by over ~100 coral cay islands and submerged reefs (Figure 1). Its depth ranges towards a maximum depth of >50 m near the offshore shelf edge in the Makassar Strait. Several near-shore reefs are located close to the port of Makassar, which houses over a million people and exerts environmental stress on the nearby reefs by sewage seepage and industrial pollution. The mouth of the Jene Berang River, south of Makassar, discharges terrigenous sediments and inland-pollution into the Spermonde Archipelago. The near-shore reefs off Makassar, up to 4 km from the coastline, are directly influenced by this river through high sedimentation and freshwater discharge causing these reefs to have shallower euphotic zones, higher nutrient levels, higher silt and sand content and lower salinity (Edinger et al., Reference Edinger, Jompa and Limmon1998; Renema & Troelstra, Reference Renema and Troelstra2001; Renema et al., Reference Renema, Hoeksema and Van Hinte2001; Cleary et al., Reference Cleary, Becking, de Voogd, Renema, de Beer, van Soest and Hoeksema2005), but they are more sheltered from wave energy and cold-water upwelling (Hoeksema, Reference Hoeksema2012a). Most of the coral cay islands are inhabited by fishing communities, who may use destructive fishing methods such as blast fishing (Pet-Soede et al., Reference Pet-Soede, Cesar and Pet1999; Hoeksema, Reference Hoeksema and Visser2004). Furthermore, corals in this area are being collected for the aquarium trade (Knittweis & Wolff, Reference Knittweis and Wolff2010). Although the reefs around the most offshore island on the shelf, Kapoposang, have been designated a Marine Protected Area (MPA) category V by IUCN, there is little surveillance in the area.

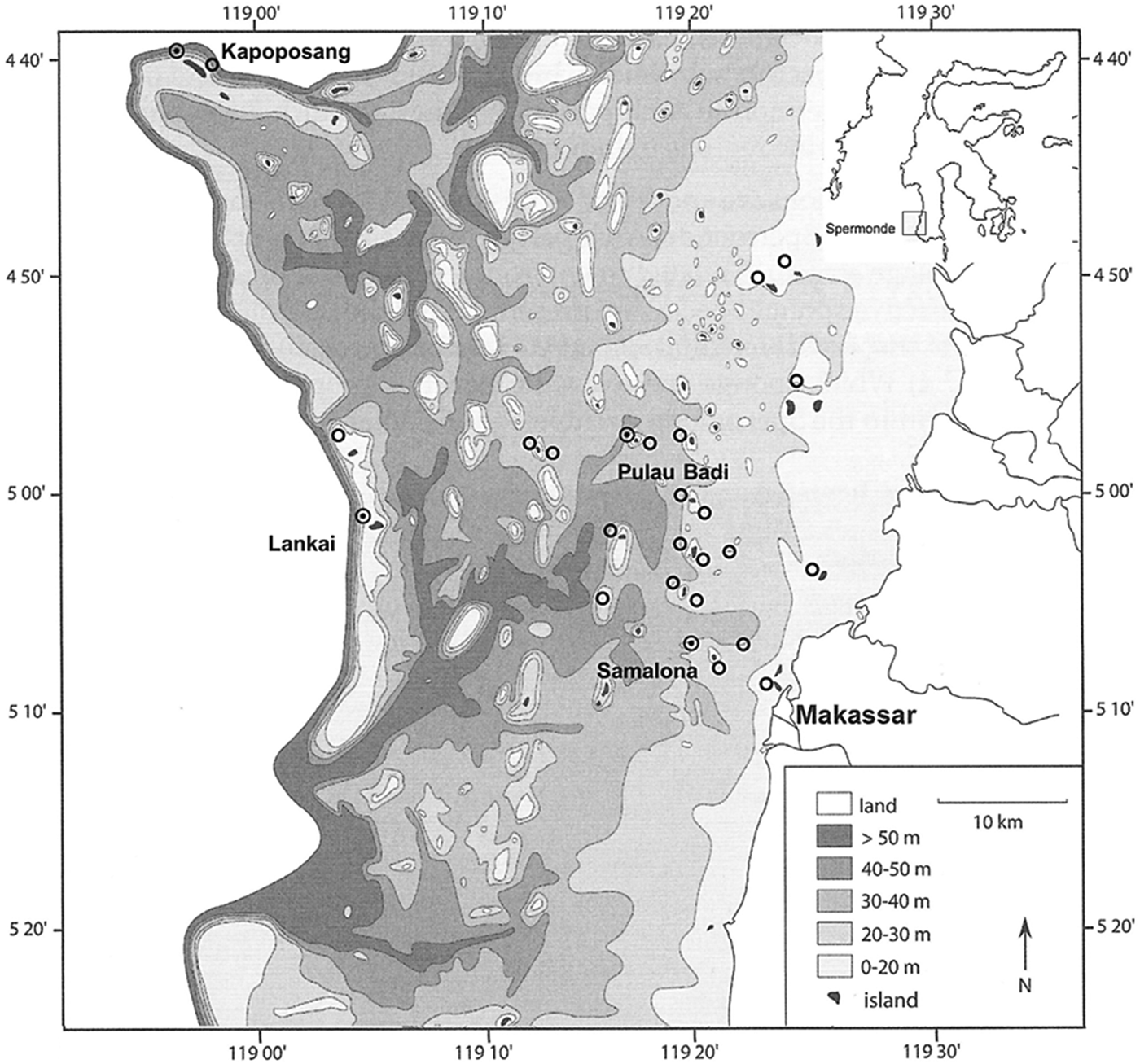
Fig. 1. Bathymetric map of the Spermonde Archipelago showing ![]() = surveyed sites. ⊙ T. hoshinota observation. Inset showing the location of the Spermonde Archipelago in relation to the island of Sulawesi.
= surveyed sites. ⊙ T. hoshinota observation. Inset showing the location of the Spermonde Archipelago in relation to the island of Sulawesi.
The Spermonde Archipelago thus presents a diverse array of environmental conditions and cross-shelf diversity, making it a very suitable area for research. It is one of the best-explored marine regions in Indonesia and has been well-documented in literature, as extensive marine biological and physical geographic studies have been conducted during the last 35 years (Moll, Reference Moll1983; Verheij & Erftemeijer, Reference Verheij and Erftemeijer1993; Verheij & Prud'homme van Reine, Reference Verheij and Prud'homme van Reine1993; Erftemeijer, Reference Erftemeijer1994; Sterrenburg et al., Reference Sterrenburg, Erftemeijer and Nienhuis1995; de Voogd et al., Reference de Voogd, Becking, Noor, Hoeksema and van Soest2004, Reference de Voogd, Haftka and Hoeksema2005, Reference de Voogd, Cleary, Hoeksema, Noor and van Soest2006; Cleary et al., Reference Cleary, Becking, de Voogd, Renema, de Beer, van Soest and Hoeksema2005; Becking et al., Reference Becking, Cleary, de Voogd, Renema, de Beer, van Soest and Hoeksema2006; Cornils et al., Reference Cornils, Schulz, Schmitt, Lanuru, Richter and Schnack-Schiel2011; Hoeksema & Crowther, Reference Hoeksema and Crowther2011; Sawall et al., Reference Sawall, Teichberg, Seemann, Litaay, Jompa and Richter2011; Hoeksema, Reference Hoeksema2012a, Reference Hoeksemab; Hoeksema & de Voogd, Reference Hoeksema and de Voogd2012).
Benthic community of reef sites
A total of 27 reef sites, spread over 20 islands in the Spermonde Archipelago, were thoroughly surveyed by scuba diving for the presence of T. hoshinota from 15 August to 2 September 2013. The sessile benthic community of the surveyed reefs was assessed by carrying out line intercept transects (LIT). Rather than determining the benthic community at species level, the LIT method is based on classification of sessile life forms in broader, environmentally significant categories, in order to give an impression of the general state of coral reefs in the Spermonde Archipelago (English et al., Reference English, Wilkinson and Baker1997). At each site two 30-m parallel transects were laid down, at 3 and 6 m. The transects were recorded using a digital underwater camera. The LIT recordings were analysed and percentages in which the different benthic life form categories occurred over the transect line were calculated, which were categorized as ‘live coral’, ‘dead coral (with algae)’, ‘algae’, ‘sponges’, ‘rubble’, ‘rock’, ‘sand’ and ‘others’. The category ‘live coral’ was comprised of the categories ‘branching’, ‘encrusting’, ‘foliose’, ‘massive’ and ‘submassive’ corals. The reef areas surrounding the transects were surveyed for patches of T. hoshinota by using the Roving Diver Technique (RDT) (Munro, Reference Munro, Eleftheriou and McIntyre2005; Hoeksema & Koh, Reference Hoeksema and Koh2009).
Sampling
Sponge identification in situ was based on the morphological characteristics given by Rützler & Muzik (Reference Rützler and Muzik1993) and by comparison of sponge specimens with those presented in pictures of T. hoshinota in other localities (Reimer et al., Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a, Reference Reimer, Nozawa and Hiroseb; de Voogd et al., Reference de Voogd, Cleary and Dekker2013). All sponges were photographed using a digital camera, along with a ruler for scale. Additionally, the surrounding coral substrates were photographed for coral host identification. Fragments of approximately 2 cm2 of sponge tissue were collected for DNA and morphological analyses. Fragments were immediately stored in ethanol (96%) and kept at −20°C. Vouchers are housed in the sponge collection of Naturalis Biodiversity Center, Leiden, the Netherlands.
Molecular analyses
DNA was extracted from the sponge tissue samples using the DNeasy Blood & Tissue kit (Qiagen) following the instructions of the manufacturer. Partitions of a mitochondrial and nuclear marker were amplified: two regions of the cytochrome oxidase subunit 1 (CO1), the standard DNA-barcoding partition called the ‘Folmer partition’, as well as the I3-M11 partition, and the D3–D5 region of the nuclear ribosomal 28S gene (28S).
The Folmer partition of CO1 was amplified by using a specific forward primer designed for the family Suberitidae, SUB-CO1F (5′-GGAATGATCGGGACAGCTTTTAGCATG-3′) designed by Becking et al. (Reference Becking, Erpenbeck, Peijnenburg and de Voogd2013) and the degenerated reverse primer from Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) designed by Meyer et al. (Reference Meyer, Geller and Paulay2005), dgHCO2198 (5′-TAAACTTCAGGGTGACCAAARAAYCA-3′). These primers amplified a fragment of 600 bp. Amplifications were carried out in 25 μL reaction volumes containing 15.75 μL of MilliQ water, 2.5 μL 10× reaction buffer, 1 μL MgCl2 (25 mM), 2 μL dNTPs (2.5 mM), 1 μL of each primer (10 μM), 0.25 μL Taq polymerase, and 1.5 μL of DNA. The PCR profile was: initial denaturing step of 94°C for 4 min, 38 cycles of denaturing at 94°C for 30 s, annealing at 54°C for 30 s, extension at 72°C for 40 s, and a final extension step at 72°C for 4 min. The I3-M11 partition of CO1 was amplified by a nested approach using primers designed by Misof et al. (Reference Misof, Erpenbeck and Sauer2000) and Erpenbeck et al. (Reference Erpenbeck, Knowlton, Talbot, Highsmith and Van Soest2004). The first primers used were C1J2165 (5′-GAAGTTTATATTTTAATTTTACCDGG-3′) and C1Npor2760 (5′-TCTAGGTAATCCAGCTAAACC-3′), which amplified a fragment of 544 bp. Amplifications were carried out in 25 μL reaction volumes containing 15.75 μL of MilliQ water, 2.5 μL 10× reaction buffer, 1 μL MgCl2 (25 mM), 2 μL dNTPs (2.5 mM), 1 μL of each primer (10 μM), 0.25 μL Taq polymerase and 1.5 μL of DNA. The PCR profile was: initial denaturing step of 94°C for 4 min, 38 cycles of denaturing at 94°C for 30 s, annealing at 50°C for 30 s, extension at 72°C for 45 s, and a final extension step at 72°C for 4 min. Then, 1 μL of the obtained PCR product was used as a DNA template in a consecutive amplification using primers CO1porF1 (5′-CCNCANTTNKCNGMNAAAAAACA-3′) and CO1porR1 (5′-AANTGNTGNGGRAARAANG-3′), which amplified a fragment of 473 bp. Amplifications were carried out in 25 μL reaction volumes containing 15.75 μL of MilliQ water, 2.5 μL 10× reaction buffer, 1 μL MgCl2 (25 mM), 2 μL dNTPs (2.5 mM), 1 μL of each primer (10 μM), 0.25 μL Taq polymerase and 1.5 μL of DNA. The PCR profile was: initial denaturing step of 94°C for 4 min, 35 cycles of denaturing at 94°C for 30 s, annealing at 45°C for 30 s, extension at 72°C for 45 s and a final extension step at 72°C for 4 min. The D3–D5 region of 28S was amplified using primers from McCormack & Kelly (Reference McCormack and Kelly2002), RD3A (5′-GACCCGTCTTGAAACACGA-3′) and RD5B2 (5′-ACACACTCCTTAGCGGA-3′), which amplified a fragment of 596 bp. Amplifications were carried out in 25 μL reaction volumes containing 8.9 μL of MilliQ water 5 μL 5× Phire® reaction buffer, 5 μL Q-solution, 2 μL dNTPs (2.5 mM), 1.3 μL of each primer (10 μM), 0.5 μL Phire® Hotstart II Taq polymerase and 1 μL DNA. The PCR profile was: initial denaturing step of 98°C for 30 s, 35 cycles of denaturing at 98°C for 5 s, annealing at 50°C for 5 s, extension at 72°C for 15 s and a final extension step at 72°C for 5 min.
Following PCR, amplification success was checked on a 1.5% agarose gel. PCR products were then sent to Macrogen Europe for sequencing. The obtained sequences were checked, trimmed and aligned using Geneious version 4.8.3. The poriferan origin of the sequences was confirmed through BLAST searches in GenBank, which revealed best matches of the CO1 and 28S data with sequences of T. hoshinota (KJ008098; Yongxing Island, South China Sea) and other species of the family Suberitidae, respectively. Haplotype and nucleotide diversities were calculated for both the Folmer partition as I3-M11 partition using DnaSP (version 5.0, Librado & Rozas, Reference Librado and Rozas2009).
Additionally, one sequence of the Folmer partition of CO1 of a T. hoshinota specimen that was sampled during a previous survey in the Spermonde Archipelago (2012) was used for comparison, as well as one sequence of sponge tissue sampled during fieldwork in the Thousand Islands, Java (de Voogd et al., Reference de Voogd, Cleary and Dekker2013).
RESULTS
Presence of Terpios hoshinota in the Spermonde Archipelago
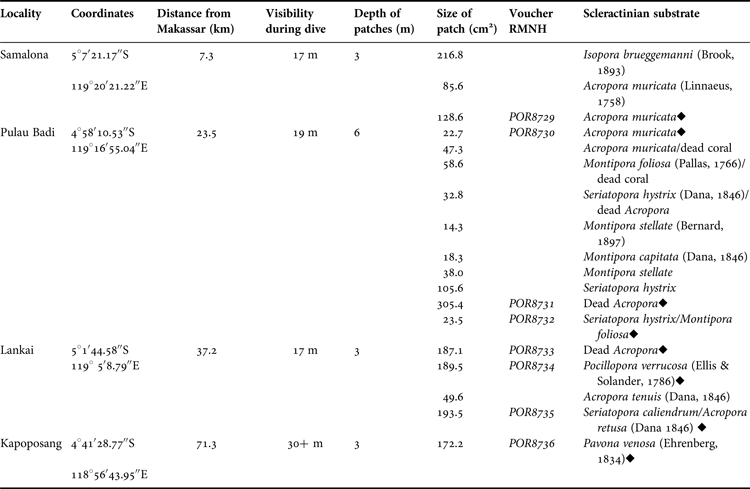
A total of 18 patches of T. hoshinota were found at four of the 27 surveyed sites (Figures 1 & 2; Table 1). Three patches of T. hoshinota were found at Samalona Reef (~7.5 km from Makassar), varying in size between 86 and 217 cm2 and a total cover of ~431 cm2. Terpios hoshinota was most abundant on the reef of Pulau Badi (distance from Makassar ~23.5 km) with 10 individuals. Patch size varied between 14 and 305 cm2 and the total covered reef area was ~667 cm2. Small patches of T. hoshinota were also observed at the outer reef of Lankai (~37 km from Makassar), where it was first recorded during the survey in 2012. These patches varied between 50 and 194 cm2 in size, covering a total reef area of 620 cm2. Finally a single patch of ~172 cm2 was found within the MPA of Kapoposang (~71 km from Makassar). The sponge was not encountered on surveyed reefs that were most near shore, i.e. Lae-Lae, Bone Lola and Bone Baku (Figure 1).

Fig. 2. (A) Patch of Terpios hoshinota with directional growth to the left, toward a live coral colony of Acropora retusa. A colony of Seriatopora caliendrum is already overgrown (although a few living branches can still be seen). Filamentous algae start to cover the dead coral substrate. (B) A thick tissue thread growing away from the substrate (encircled). The encircled tissue was observed to grow only on the site of the coral that was exposed to sunlight, and not underneath it. (C) The sponge shows discolouration at the growth front with the coral Acropora muricata. (D) Hairy tips can be seen at the growth front in an interaction with the coral.
Table 1. Specifications of locations where Terpios hoshinota was found in the Spermonde Archipelago, Indonesia, including the coral substrates that the individual patches were covering. (♦) Indicates the patches that were sampled for DNA analyses.

Distribution in relation to the benthic community
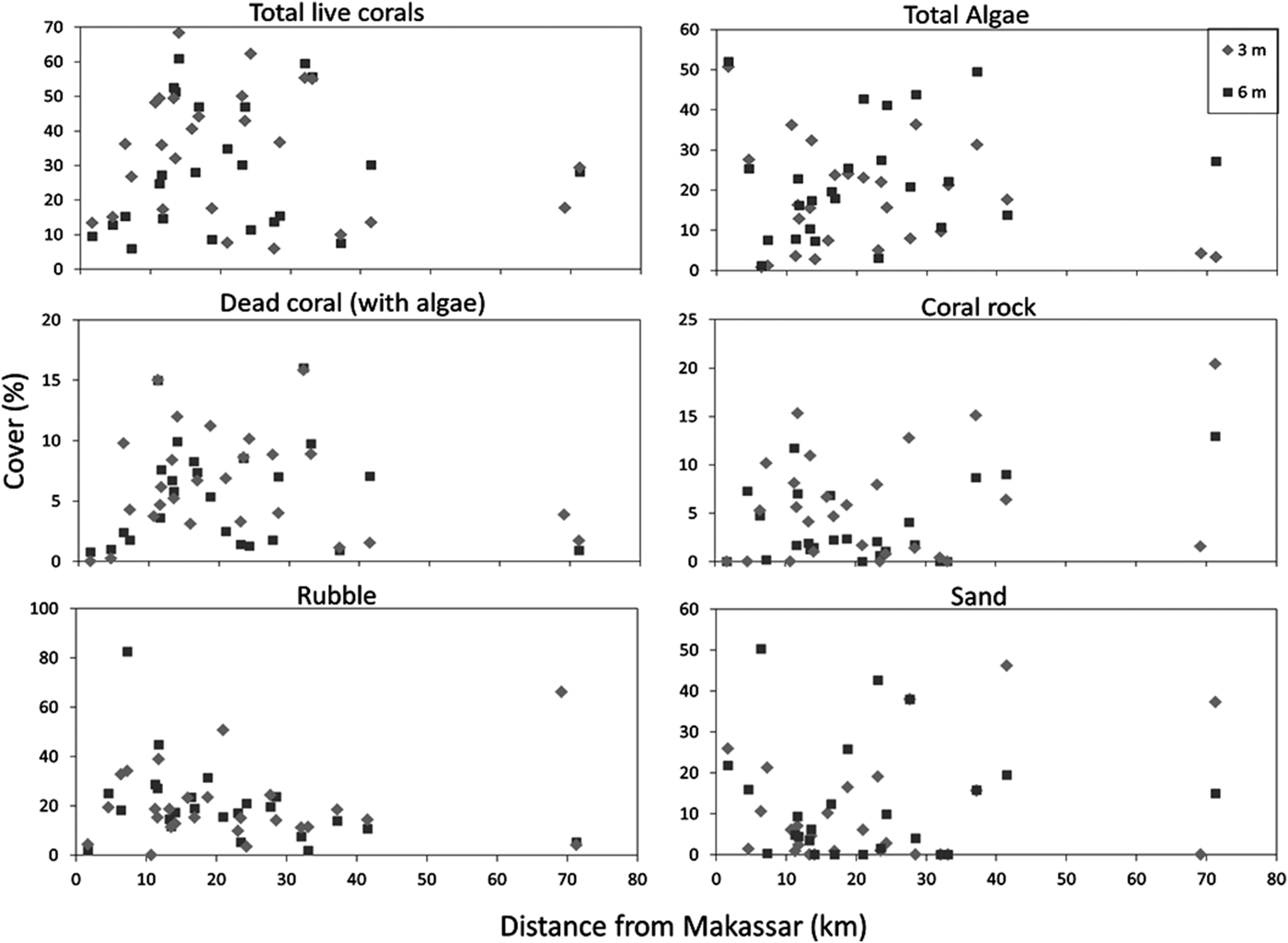
Some variables of the benthic community composition showed a distinct distribution with respect to the distance to Makassar (Figure 3). The total live coral cover at the inner-shelf reefs, ≤10 km away from Makassar, was low (ranging from 9 to 15%). Over the mid-shelf it varied greatly, ranging between 6% at Lumulumu (3 m depth) and 68% at Barrang Lompo (3 m depth). Although Barrang Lompo is one of the largest and most densely populated islands in the Spermonde Archipelago, the reef was largely covered by large foliose corals (35%; Figure 3), spanning the entire northern reef area. The outer-shelf reefs had a lower live coral cover (7.5–30%). The total live coral cover encompassed all major coral growth forms (Figure 4). Branching and massive corals were the dominant coral growth forms, although most were small (<20 cm). Furthermore, the total algae cover showed high variation, in the range of 1–52%, although it was generally high throughout the Spermonde Archipelago. The dead coral rock cover (also bare hard substrate) varied throughout the archipelago (range 0–20%). The benthic community composition was not significantly different between the two depths for any category except massive coral cover, which showed a higher cover at 3 m depth (two-tailed t-test; P = 0.02). The benthic environment of the four sites where the sponge T. hoshinota was found is also depicted (Figures 3 & 4).

Fig. 3. Variation in the cover of live coral, total algae, dead coral (with algae), coral rock, rubble and sand, as a function of distance from Makassar.

Fig. 4. Variation in the cover of the individual growth forms of live corals, as a function of distance from Makassar.
Substrate preferences
The coral substrate of each observed T. hoshinota sponge was identified (Table 1) following Veron (Reference Veron2000). All T. hoshinota patches were overgrowing live corals, most of which belonged to species of the genera Acropora, Isopora and Montipora (Acroporidae), Seriatopora and Pocillopora (Pocilloporidae) and the species Pavona venosa (Agariciidae) (Figure 2).
Genetic diversity
All sequences were submitted to NCBI GenBank under accession numbers KP764919 (28S), KP764915-KP764916 (Folmer partition of CO1) and KP764917-KP764918 (I3-M11 partition of CO1).
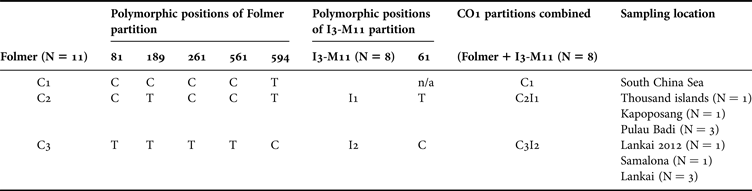
Final alignments for the sponge T. hoshinota were obtained for the Folmer partition of CO1 of 600 bp (Tables 2 & 3). These alignments revealed five variable sites, which all exhibited C-T transitions and all resulting in silent mutations. This sequence variation revealed two haplotypes in the sequences obtained from samples from the Spermonde Archipelago, separating haplotype C2, comprising three specimens from the mid-shelf island of Pulau Badi and one specimen from the outer-shelf island of Kapoposang, from haplotype C3, comprising one specimen from the inner-shelf island of Samalona and three specimens from the outer-shelf island of Lankai. The sequence obtained from Lankai during the 2012 survey, was identical to haplotype C3. The sequence from the Thousand Islands, Java, however was similar to haplotype C2. The sequence from GenBank of the Folmer partition of CO1 of T. hoshinota (KJ008098) is 1 bp different from C2 and 5 bp different from C3, separating it in another haplotype, C1. Mitochondrial diversity based on the sequences were 0.636 (SD = 0.089) (haplotype diversity) and 0.00394 (SD = 0.00052) (nucleotide diversity).
Table 2. Polymorphic positions of Terpios hoshinota for two regions of the mitochondrial marker COI, the standard DNA-barcoding partition (Folmer partition) and the I3-M11 partition. Three haplotypes (C1–3) are found for the Folmer partition with a total of five variable sites. Two haplotypes (I1–I2) are found for the I3M11 partition, with one variable site. When these two regions of the mitochondrial marker CO1 are combined, three haplotypes are found (C1, C2I1, C3I2).

Table 3. Genetic composition and mitochondrial diversity (based on the Folmer partition and I3-M11 partition of the mitochondrial marker CO1) of Terpios hoshinota. N = number of samples, nH = number of haplotypes, H = haplotype (gene) diversity, π = nucleotide diversity (SD in parentheses). C1–C3 and I1–I2 refer to the relative frequencies of haplotypes.

Final alignments for the I3-M11 partition of CO1 of 473 bp were obtained from all eight samples from the Spermonde Archipelago (Tables 2 & 3). This region exhibited almost no sequence variation; only one variable site was observed, resulting in two haplotypes. These two haplotypes, I1 and I2, resulted in the same separation of specimens as haplotype C2 and C3, respectively. The two haplotypes resulted from a C-T transition at position 61 of the fragment. This missense mutation causes the encoding of different amino acids, creating cysteine in I1, and arginine in I2. Mitochondrial diversity based on the sequences were 0.571 (SD = 0.094) (haplotype diversity) and 0.00121 (SD = 0.00020) (nucleotide diversity).
The sequences of the D3–D5 region of 28S showed no nuclear variation within the specimens from the Spermonde Archipelago.
Since the Folmer partition and the I3-M11 partition are both located on the same mitochondrial marker (CO1), the haplotypes found for these partitions could be combined, creating two haplotypes in total, separating haplotype C2I1, comprising specimens from the mid-shelf island of Pulau Badi and the outer-shelf island of Kapoposang, from haplotype C3I2, comprising specimens from the inner-shelf island of Samalona and the outer-shelf island of Lankai.
DISCUSSION
Since its first sighting in 2012 in the Spermonde Archipelago on the reef of Lankai (de Voogd et al., Reference de Voogd, Cleary and Dekker2013), T. hoshinota has been advancing throughout the archipelago. During the survey in 2012 it was found at only one out of 14 surveyed reefs in the Spermonde Archipelago (~7%), on the off-shore reef of Lankai. The present study confirms the occurrence of T. hoshinota in the Spermonde Archipelago at four of the 27 surveyed reef sites (~15%).
The sites where T. hoshinota has been observed during the present survey are all environmentally and spatially very different from each other (Edinger et al., Reference Edinger, Jompa and Limmon1998; Renema et al., Reference Renema, Hoeksema and Van Hinte2001; Cleary et al., Reference Cleary, Becking, de Voogd, Renema, de Beer, van Soest and Hoeksema2005; Cleary & de Voogd, Reference Cleary and de Voogd2007; Hoeksema, Reference Hoeksema2012a). The sponge was found on the mid-shelf reef at Samalona and Pulau Badi, as well as at the outer edge of the Spermonde Archipelago, at Lankai and Kapoposang, indicating that it can also occur in relatively pristine reefs. These findings are supported by a survey of the Ryukyu Archipelago by Reimer et al. (Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a), where T. hoshinota was also found in more pristine reefs, as well as reefs that suffered under anthropogenic stressors. Schils (Reference Schils2012) recorded an outbreak after volcanic eruptions, indicating that T. hoshinota and its symbiotic cyanobacteria benefit from the induced nutrient enrichment by volcanic ash. A massive T. hoshinota outbreak in an area with high turbidity and ‘murky’ water was also recorded by Rützler & Muzik (Reference Rützler and Muzik1993). However, the present study showed no large variation in patch size between the four sites where T. hoshinota was observed, indicating that no clear relation can be found between the size of the sponges and the distance offshore.
In the present study, the sponge was observed across the whole shelf width except for the inner-shelf reefs, near the city of Makassar, where the photic zone was much shallower and visibility during dives was less than 5 m. A previous study in the Spermonde Archipelago showed that water velocity and salinity and the variation in water velocity and temperature increased from the inner- to the outer-shelf due to the decreasing influence of the Jene Berang river and to increasing oceanic influences, which had a distinct influence on the cross-shelf sponge community (Cleary & de Voogd, Reference Cleary and de Voogd2007). While T. hoshinota in the present study was found at various locations across the shelf, indicating that it can survive under different abiotic conditions, it is not clear whether these abiotic variables constrain or promote the expansion of the sponge.
The benthic community did not vary between the two surveyed depths with exception of the cover of massive corals. Terpios hoshinota was found both at shallow depth (3 m) and deeper on the reef slope (6 m). Reimer et al. (Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a) also recorded T. hoshinota mostly on shallow reefs (<5 m; 87%) although it was also recorded as deep as 17 m. The largest patches in the present study were observed at Pulau Badi at 6 m depth, which is arguably still relatively shallow and confirms T. hoshinota as a shallow reef sponge. Its symbiotic relationship with intercellular bacteria (5 × 105 cells cm−2), mainly cyanobacteria (61–98%) also makes it essential for T. hoshinota to grow in the light (Rützler & Muzik, Reference Rützler and Muzik1993; Tang et al., Reference Tang, Hong, Liao, Jane, Chiang, Chen and Chen2011; Hoeksema et al., Reference Hoeksema, Waheed and de Voogd2014b). Terpios hoshinota was not observed beyond a depth of 6 m in the present study.
The benthic community shows much cross-shelf variation, although both live coral, algae and rubble were dominant on all reefs. The cover of rubble, caused by storms, anchor damage and blast fishing, was generally high, up to 83%, throughout the Spermonde Archipelago. Reefs on the inner-shelf, less than 10 km away from Makassar, showed a very low cover of live coral, while over the mid-shelf area the cover varied extremely between sites and between all benthic variables, and the most off-shore reef had a relatively low coral cover again. A similar unimodal pattern was observed by Cleary et al. (Reference Cleary, De Vantier, Vail, Manto, de Voogd, Rachello-Dolmen and Hoeksema2008) in the Thousand Islands, Jakarta, where T. hoshinota has also been recorded. It is clear that the Spermonde Archipelago suffers from human impact. Although branching and massive corals were the dominant coral growth forms, most branching corals that occurred mid-shelf were very small colonies (<20 cm), growing on top of large piles of coral rubble. Thus the high cover of branching corals does not imply that healthy reefs were present, which could sustain large fish populations, but rather that reefs in the Spermonde Archipelago are in the process of recovery.
The four sites where patches of T. hoshinota were discovered also varied distinctly from each other in benthic community structure; the sponges were found at sites with both high, intermediate and low cover of live corals, algae, rubble and sand. This shows that the sponge can survive within different benthic community structures, at least prior to an outbreak, but it does not explain why it has not expanded to other reefs in the Spermonde. Since an outbreak has not occurred in either of these structurally variable sites, Terpios hoshinota outbreaks are probably not related to (variation in) benthic community structure. It is noteworthy that at all four sites, T. hoshinota was almost exclusively found on branching corals, yet the benthic community of the reefs where they were found had a generally low cover of branching corals; with the exception of Pulau Badi (16%), all sponges were found at sites with a branching coral cover of less than 2%. During massive outbreaks T. hoshinota is known to overgrow any coral form and other benthic structures (Rützler & Muzik, Reference Rützler and Muzik1993; Reimer et al., Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a).
Terpios hoshinota was mostly covering and interacting with living corals. Bryan (Reference Bryan1973) suggested that the killing of live coral tissue may provide the sponge with additional nutrients, which would explain why the sponge prefers expanding over on live corals. However, Plucer-Rosario (Reference Plucer-Rosario1987) demonstrated that cleaned coral substrate resulted in even higher growth rates (1–2 mm/day) and concluded that T. hoshinota may be killing corals in competition for space on a reef.
In the present study all live coral substrates were identified to species level. Sponge patches were mostly found on corals of the family Acroporidae, and also to a lesser extent on corals of the family Pocilloporidae. This study is the first to record such a preference in regular conditions outside the context of an outbreak, and although T. hoshinota is also known to grow over any benthic structure during massive outbreaks (Rützler & Muzik, Reference Rützler and Muzik1993; Reimer et al., Reference Reimer, Mizuyama, Nakano, Fujii and Hirose2011a), this information can be crucial to understanding colonization preference before an outbreak of T. hoshinota. In the Thousand Islands, Jakarta, where no outbreak event had occurred yet, the sampled T. hoshinota patches were also overgrowing corals of the family Acroporidae, specifically the foliose coral Montipora sp. (de Voogd et al., Reference de Voogd, Cleary and Dekker2013).
When expanding over live coral tissue, T. hoshinota shows directional growth and has an active growth front, which features structures like thin tissue threads, hairy tips, compact edges and disintegrated tissues. The hairy tips tissue threads contain cyanobacteria, sponge tissue and spicules, and play an active role in killing adjacent corals (Soong et al., Reference Soong, Yang and Chen2009; Wang et al., Reference Wang, Chen, Meng, Sune, Hsu, Wei and Chen2012). In a patch more cryptically hidden in the reef, tissue threads were observed to grow towards the light. This growth strategy further highlights the potential of T. hoshinota to rapidly invade reefs.
Finally a study was performed on the genetic diversity in the sponge T. hoshinota. The D3–D5 region of the nuclear ribosomal 28S gene has been used to distinguish genera and species of a wide range of demosponge taxa, but it has also been found to be too conserved to discriminate between closely related species (Reveillaud et al., Reference Reveillaud, Remerie, van Soest, Erpenbeck, Cárdenas, Derycke and Vanreusel2010; Becking et al., Reference Becking, Erpenbeck, Peijnenburg and de Voogd2013). Indeed, no genetic variation in sequences was observed in the D3–D5 region of the 28S gene. However, two distinct haplotypes of T. hoshinota could be distinguished by sequencing the Folmer partition and the I3-M11 partition of the mitochondrial CO1 in the present samples. These two haplotypes both occur in the Spermonde in the same frequency; haplotype C2I1 represents specimens from the mid-shelf island of Pulau Badi and the outer-shelf island of Kapoposang, and also includes the specimen from the Thousand islands, Jakarta, and haplotype C3I2 represents specimens from the inner-shelf island of Samalona and the outer-shelf island of Lankai. The CO1 sequence of T. hoshinota obtained from GenBank, originates from Yongxing Island, South China Sea, where the sponge has had outbreak events in 2008 and 2010 (Shi et al., Reference Shi, Liu, Yan and Zhang2012). This sequence varied slightly from those found in the Spermonde Archipelago, and was separated in another haplotype, C1.
The sponge T. hoshinota is recognized as an aggressive coral-killing sponge and has drawn the attention for extensive studies. There are a multitude of encrusting sponge species that are able to kill corals, though usually by simply overgrowing and smothering them to death (Aerts & van Soest, Reference Aerts and van Soest1997). Some of the most aggressive coral-killing sponges belonging to the family Clionaidae are encrusting and excavating sponges that are overgrowing and killing already stressed corals in the Caribbean at a disturbingly fast rate (Rützler, Reference Rützler2002; Chaves-Fonnegra & Zea, Reference Chaves-Fonnegra and Zea2011). Another coral-killing cyanobacteriosponge, Chalinula milnei, exhibits similar aggressive growth strategies as T. hoshinota (Hoeksema et al., Reference Hoeksema, Dekker and de Voogd2014a) and was more abundantly observed than T. hoshinota throughout the entire Spermonde Archipelago during surveys in 2012 and 2013 (F. Dekker, unpublished data). However, it has received much less attention than T. hoshinota, while it may also impose a threat to coral reefs.
Recently, T. hoshinota was not observed at Yonama, Southern Japan where a massive outbreak of the ‘black disease’ occurred a quarter of a century ago (Reimer et al., Reference Reimer, Nozawa and Hirose2011b). In addition, this outbreak did not cause irreversible damage to the reefs. However, in the present study we observed dead coral next to T. hoshinota covered by filamentous algae. It is known that a phase-shift from a coral to more algae-dominated reef may in fact be irreversible (Bellwood et al., Reference Bellwood, Hughes, Folke and Nyström2004), and this way an outbreak of T. hoshinota can indirectly cause irreversible degradation of coral reefs.
Currently, there has been no major outbreak event in the Spermonde Archipelago yet, making this site and the T. hoshinota patches recorded during this study of particular interest for further research. The present results suggest that the sponge tolerates large variations in benthic community structure and other environmental and spatial conditions, indicating there are still other unknown mechanisms or stressors, either biotic or abiotic, that may play a role in the onset of T. hoshinota outbreaks. Liao et al. (Reference Liao, Tang, Hsu, Wen, Wu, Chen and Chen2007) recorded the sponge in Taiwan, suggesting that T. hoshinota is widening its distribution in the north-western Pacific. Indeed, T. hoshinota has now been recorded as far west as the Maldives in the Indian Ocean (Montano et al., Reference Montano, Chou, Chen, Galli and Reimer2015) and as far south-east as the Great Barrier Reef (Fujii et al., Reference Fujii, Keshavmurthy, Zhou, Hirose, Chen and Reimer2011). It is unclear whether additional records are a consequence of range expansion, more outbreaks, or easier recognition as the species is more widely known than before.
ACKNOWLEDGEMENTS
Research permits were issued by the Indonesian State Ministry for Research and Technology (RISTEK) and the Indonesian Institute of Sciences (PPO-LIPI). Hasanuddin University at Makassar provided local support. We thank F. Dekker and S. Sradaputta for their help during fieldwork. We thank K. Beentjes, M. Eurlings, B. Reijnen and C. de Leeuw for advice and help with lab techniques and molecular analyses.
FINANCIAL SUPPORT
This research was funded by the Treub-Maatschappij, Stichting Jan Joost ter Pelkwijk Fonds and Mej. A.M. Buitendijk Fonds.