The 2014 Ebola outbreak in West Africa was arguably the most terrifying epidemic in recent decades. While both its onset and the declaration of closure occurred relatively quickly, the spread of the disease occupied a prominent place in media coverage for most of a year. The gruesome symptoms of infected patients and the high incidence of mortality seemed to overwhelm underprepared medical facilities in countries where infection rates were high. After health authorities failed to recognize the severity of the epidemic, a massive international effort was established to stop the spread of Ebola. One important feature of this effort was the rapid testing of potential vaccines.
Ghana, a West African country without a single case of Ebola, was chosen as one of nine sites for Ebola vaccine trials (EVTs).Footnote 1 The announcement generated controversy in the Ghanaian news media, leading to public debates and protests on radio, television, and social media. The Parliament of Ghana eventually suspended the planned trials, which were still under review at the time by the Ghana Food and Drugs Authority (FDA). After five months of investigation of the safety of the planned EVTs, the Ghanaian Parliament did approve the trials, though they were never carried out (Kummervold et al., Reference Kummervold, Schulz, Smout, Fernandez-Luque and Larson2017; Okyere, Reference Okyere2015).
Vaccine trials, particularly for a disease such as Ebola, require several layers of trust. First, scientists trust in the willing participation of the volunteers in vaccine trials. Second, regulatory institutions trust participating researchers to adhere to the ethics and proposed methodology of their inquiry. Third, volunteers trust these scientists to conduct themselves professionally, report outcomes, and employ the results to reduce infectious disease. We explore these relationships to understand the breakdown of one of the most significant attempts to develop a vaccine during the period of the Ebola outbreak (2014–2016). Our conceptual framework employs the concept of a “tandem,” a mutual condition of trust and control (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). We focus particularly on the characteristics of the disease and the formation of linkages between groups in vaccine trials. Tandems are sites where trust and control are produced together, linking the personal, technical, and institutional domains of clinical trials, which play a crucial role in their success as well as in the process of developing scientific knowledge claims.
Our analysis begins with the central assumption that the milieu of trust occurs in a political environment that affects the relationships forged in clinical trials. For infectious disease, prior work by Ear and others shows that this political context impinges on the development of surveillance infrastructure. First, political challenges such as deficits in understanding and the differing priorities of the Indonesian government and the U.S. Navy led to the closure of the Naval Area Medical Research Unit 2 in April 2010 after 40 years of infectious disease surveillance and research (Ear, Reference Ear2014). Politics also played an essential role in the handling of the A/H1N1 outbreak in Mexico, where cooperation between the Mexican, Canadian, and U.S. governments led to a successful containment of the outbreak (Ear, Reference Ear2012). The process can also thwart efforts in infectious disease surveillance. In Mexico, party loyalties rather than qualifications determined appointments in epidemiology during the A/H1N1 outbreak, affecting the way the outbreak was handled. Youde’s (Reference Youde2013) examination of the global eradication of rinderpest in Somalia and Kenya suggests that the local political context is important as well. Finally, where the use of donor funds was used to curb infectious disease in Cambodia, institutional failures including poor governance and lack of political commitment hampered the effort to contain the 2004 H5N1 outbreak (Ear, Reference Ear2011).
This article explores the impact of political context and disease characteristics on the design and implementation of vaccine trials for infectious disease. We argue that (1) the establishment of trust and control begins long before the implementation of clinical trials, and (2) both political context and disease characteristics affect linkages between institutions and between scientists and volunteers—as well as the success or breakdown of the vaccine trials. Given the limits of qualitative research, this study is designed to develop theory rather than to test it: disease characteristics in a political context are crucial to the design and implementation of vaccine/clinical trials.
Tandems of trust and control
To conceptualize disease as a site of trust and control, we employ the concept of actors first developed in actor network theory. The term “actors” refers to both human and nonhuman entities in a network (Michael, Reference Michael2017). Actors exist in networks of relationships, and they are enabled by the network. Actors—people, organizations, even nonhumans—are not created by networks, but within the context of the network, they are made active (Mol, Reference Mol2010). They draw their ability to act from the network within which they are embedded, including its stability and the constraints it imposes. The Ebola vaccine trials were launched and forged within a network of relationships that included regulating institutions, scientists, volunteers, technologies, the research facility, and the Ebola virus disease (EVD) itself. All were enabled or activated by the vaccine trial network. Yet the disease had already received global publicity, identified and recognized as an agent with deadly and highly infectious characteristics.
Trust and control have often been studied in organizational research in which the focus has been on their relationship within and between organizations (Bijlsma-Frankema, Reference Bijlsma-Frankema2004; Bijlsma-Frankema & Costa, Reference Bijlsma-Frankema and Costa2005; Kerkhof et al., Reference Kerkhof, Vahstal-Lapaix, Caljé, Bijlsma-Frankema and Woolthuis2005; Sydow & Windeler, Reference Sydow and Windeler2003; Tyler, Reference Tyler2003). Defining the concepts of trust and control has been challenging for scholars because both concepts depict social relationships and processes that are highly intricate (Reed, Reference Reed2001).
Trust
Trust has been explored within various disciplines and defined in a multitude of ways. Common to most is that trusting others entails an assumption that others will behave in a particular way, which, in turn, will lead to an anticipated positive or negative outcome (Mayer et al., Reference Mayer, Davis and Schoorman1995). Hence, trust has been defined as “the willingness to accept vulnerability, based on positive expectations about another’s intensions and behavior” (McEvily et al., Reference McEvily, Perrone and Zaheer2003, p. 92). The willingness to be vulnerable exposes the trustor to the risk of being harmed by the trustee’s actions, thus associating trust with risk. Having positive expectations of the trustee entails the belief that the trustee is willing or has the integrity to fulfill commitments to the trustor and to demonstrate goodwill or kindness, and that the trustee has the ability or competence to undertake the tasks expected (Mayer et al., Reference Mayer, Davis and Schoorman1995). Trust, by implication, is relational and associated with risk: the trustee may disappoint the trustor (Boon & Holmes, Reference Boon, Holmes, Hinde and Gorebel1991; Luhmann, Reference Luhmann and Gambetta1988).
From the Simmelian perspective, trust consists of three elements: expectation, interpretation, and suspension (Möllering, Reference Möllering2001). Expectation refers to the final state of the trust process, in which the outcome is either a positive state (trust) or a negative state (distrust). Drawing from the everyday experience of the social actor’s lifeworld, one has some basis or “good reason” to trust another. This constitutes the interpretative element of trust. To move from interpretation to expectation, a mental leap of faith or suspension of doubt is required. This is the bracketing of the unknown, the possibility that the trustee might act otherwise and leave the trustor disappointed. Suspension, then, is the bracketing of this uncertainty and doubt embedded in the trust process (Möllering, Reference Möllering2001).
Control
Control, in an organizational context, refers to processes that regulate the behavior of members to achieve organizational goals (Cardinal et al., Reference Cardinal, Sitkin and Long2004; Das & Teng, Reference Das and Teng2001). Control may be formal or informal. Formal control refers to the systemic use of rules, policies, and procedures to regulate, monitor, and reward expected performance (Das & Teng, Reference Das and Teng2001). Informal social control refers to internalized goals, including the culture, norms, and values of an organization that promote desirable outcomes (Das & Teng, Reference Das and Teng2001). On the assumption that both formal and informal control play a role in the creation of scientific knowledge, the distinction between methodological and institutional control is crucial (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). Methodological control is central to the scientific process when research entails systematic procedures to ensure that both the process and the results are objective and consistent with positivistic ontology. In the context of vaccine trials, methodological control is exerted by the scientists in the formulation of the protocol, as well as in their use of appropriate methods, materials, and machines for collecting and analyzing data. All these serve to ensure that the “standard” procedures governing vaccine trials are performed, leading to a nonbiased result.
Institutional control stems from the oversight responsibility regulating organizations have over scientists in vaccine trials. Both protocols and an external regulating organization are significant in signaling the legitimacy of the vaccine trial process—that it will yield valid and reliable outcomes (Bijker et al., Reference Bijker, Sauerwein and Bijker2016).
Tandems
Bijker and colleagues (Reference Bijker, Sauerwein and Bijker2016) build on Möllering’s (Reference Möllering2005) trust/control duality to develop the concept of trust and control in tandem. The distinctive feature of this concept is the notion of substitution. They focus on the role of trust and control in the construction of scientific knowledge in clinical trials, with special emphasis on the trust/control dynamics between people, machines, and institutions. The argument is that trust and control work together to increase the legitimacy of scientific research and, more specifically, clinical trials. They posit that tandems are sites where trust and control are “coproduced,” linking the institutions, people, and technology used in clinical trials.
In this perspective, trust and control produce and presuppose each other. Their relationship is mutually reinforcing but not unidirectional: trust and control can substitute for each other. Simply put, a deficiency in one may be offset by an increase (either fully or partially) in the other (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). They liken the relationship between trust and control to a tandem bicycle: both riders power the bicycle together by their combined effort. But one rider may coast or freewheel while the other rider pushes harder to keep the bicycle moving.
Trust in clinical trials is not built on personal or private relationships but on the social structures embodied in the institutions that regulate the activities of those to be trusted. In vaccine trials, scientists and volunteers are not members of any common, formal organization, so control cannot be exercised through bureaucratic procedure. Trust formed among social actors in such a context is based on the assumption that the social structures exercise control by creating order.
Similarly, control cannot exist without trust. Much as social structures exert control, social actors still need to trust that other social actors will adhere to norms (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). We argue that in the Ghana vaccine trials, the trust/control nexus was distorted by the characteristics of Ebola, a deadly and highly infectious disease, even before the trials were approved. Much of the controversy surrounding the failed vaccine trials stemmed from (1) the belief that implementing the trials would lead to Ebola infections in Ghana; (2) the allegation that trials were already being carried out in secret; and (3) the contention that volunteers were lured by sums of money to participate in the trials (Kummervold et al., Reference Kummervold, Schulz, Smout, Fernandez-Luque and Larson2017). These conditions undermined community trust in the safety of the vaccine trials. First, their trust in the EVTs was hampered by the characteristics of Ebola. Second, based on newspaper publications and rumors, most Ghanaians believed that the trials had been approved and were underway. Government and regulatory institutions were perceived as failing them by allowing the trials to be implemented. Third, Ghanaians did not trust the intentions of scientists in carrying out the EVTs in a place where no case of Ebola had been confirmed, knowing that Ebola is a deadly disease. Fourth, following public outcry and concern over the safety of the trials, the Parliament of Ghana intervened and suspended the planned trials. Hence, the trust/control nexus in the planned EVTs was negatively impacted by both the characteristics of Ebola and the political process through which parliamentary action halted the approval process. Linkages between volunteers, scientists, and the regulating institutions could not be forged for the implementation of the EVTs, even when they were eventually approved.
Vaccine/clinical trials have been explored from both micro and macro perspectives in prior research. First, the use of payments to aggressively recruit and encourage the continuous participation of paid healthy individuals in clinical trials has been explored, with particular attention to the ways in which incentives blur the boundary of informed consent and downplay the risks of exposure (Abadie, Reference Abadie2010; Monahan & Fisher, Reference Monahan and Fisher2015). Some research focuses on the role of volunteers as active participants in clinical trials (Prescott, Reference Prescott2002; Scott et al., Reference Scott, Walker, White and Lewith2011). On an institutional level, studies have examined the impact of globalization, financial challenges, and the changing nature of research through the rise of contract research organizations in clinical trials (Jonvallen et al., Reference Jonvallen, Berg and Barry2011; Mirowski & Van Horn, Reference Mirowski and Van Horn2005). Others have explored the influence of pharmaceutical funding on clinical trials and the ways in which the industry masks corporate science as academic work with the aim of marketing pharmaceutical products through journal publications (DeVries & Lemmens, Reference De Vries and Lemmens2006; Sismondo, Reference Sismondo2008, Reference Sismondo2009; Wadmann, Reference Wadmann2014; Wynia & Boren, Reference Wynia and Boren2009). These companies manifest a Fordist preference for the production of rapid results over the well-being of volunteers and participants (Fisher, Reference Fisher2008, Reference Fisher2015; Hedgecoe, Reference Hedgecoe2014). Yet little or no attention has been paid to the impact of the characteristics of emerging infectious disease in a political context. While Ryan, Giles-Vernick, and Graham (Reference Ryan, Giles-Vernick and Graham2019, p. 6) make tangential reference to Bijker’s (Reference Bijker, Sauerwein and Bijker2016) “trust in tandem,” this study is the first to use the trust-control tandem in the study of epidemics.
A multiplicity of factors may account for vaccine/clinical trial failure. Vaccine/clinical trials are capital intensive. Thus, underfunded trials are unable to meet the stringent regulatory standards for each phase of the vaccine/clinical trial and can fail as a result (Getz, Reference Getz2015; Hwang et al. Reference Hwang, Carpenter, Lauffenburger, Wang, Franklin and Kesselheim2016). Two, criteria of inclusion/exclusion have been offered as an explanation of failure.
The criteria used for vaccine/clinical trials should result in enrolling a population that matches the intended patient population for the trials. For instance, inclusion that does not account for the presence of many comorbidities in a segment of the population may result in the removal of participants from the trial. This may cause failure through inappropriate trial populations (Heneghan et al., Reference Heneghan, Goldacre and Mahtani2017; Hill et al., Reference Hill, Preston and Roberts2008). Yet narrow inclusion criteria can lead to a long period of recruitment and ultimately an alteration of the protocol to recruit more participants. The effect is to have different populations before and after amendments of criteria and protocol (Getz et al., Reference Getz, Zuckerman, Cropp, Hindle, Krauss and Kaitlin2011; Lösch & Neuhäuser, Reference Lösch and Neuhäuser2008). The type of inclusion/exclusion criteria employed in a vaccine/clinical trial has an impact on the cost and duration of the trial, impacting the likelihood of the trial meeting the required enrollment levels (Babbs, Reference Babbs2014). Patient recruitment accounts for failed vaccine/clinical trials in which inadequate recruitment results in the eventual demise of the entire trial (Campbell et al., Reference Campbell, Snowdon, Francis, Elbourne, McDonald, Knight and Grant2007; Feller, Reference Feller2015).
The assumption underlying each of these explanations is that a well-designed trial protocol with enough funding will generally succeed. But we show that these factors are not by themselves decisive: the disease characteristics and the political context of the site are also important to implementation, especially in cases dealing with emerging infectious diseases. The trust-control tandem provides a framing that includes disease characteristics and the political context of the trial site as a way of understanding the larger context of vaccine/clinical trial failure.
Methodology
The study emanates from a secondary qualitative analysis of in-depth interviews that were collected as part of a larger project in which data were collected in two waves (2015 and 2016) on Zika and Ebola in Ghana (Accra, 2015; Hohoe, 2016), Kenya, Guinea, Mali, Brazil, Argentina, Mexico, and the United States. Though secondary analysis is common in quantitative research, it can be carried out in qualitative research for the purposes of original research or corroborating existing research (Hammersley, Reference Hammersley1997). Our choice of a qualitative study stems from the fundamental principles undergirding qualitative research—that is, through the meanings derived from their lived experiences and their interactions with each other, social actors construct the social world (Creswell, Reference Creswell2009). Researchers rely on social actors’ experience and interpretation of the phenomenon under study while paying attention to the social and historical contexts (Creswell, Reference Creswell2009). Qualitative research is useful in understanding the behavior of social actors and the interpretations they derive from their lived experiences (Babbie, Reference Babbie2010; Vanderstoep & Johnston, Reference Vanderstoep and Johnston2008).
Thus, in studying the failed EVTs, the use of the qualitative paradigm enabled us to explore the phenomenon from the perspectives and experiences of the community members. Hohoe and Kintampo were the two designated towns for the planned EVTs. Hohoe is located in the southeastern part of Ghana, while Kintampo is located in the center of Ghana, serving as a transit town between the northern and southern parts of the country. We collected data in Hohoe during the summer of 2016. We selected Hohoe because it had received extraordinary media attention because of the resistance put up by the community and members of Parliament (MPs) from the Volta Region. The community’s unwillingness to participate in an activity/process (vaccine trials) that is familiar to them was also a compelling reason to focus on Hohoe. Ghana was the only country we examined with a reported case of failed vaccine trials. Kintampo was not part of the larger project, hence the unavailability of data from the town. In addition, there was virtually no resistance and minimal media coverage of the proposed trials in Kintampo.
We conducted semistructured interviews lasting between one-half hour and one hour. The interviews focused on informants’ general knowledge of Ebola, their sources of information about the disease, the preventive measures they had adopted, and the network of relationships used in seeking, receiving, and providing information about Ebola and the vaccine trials. The interviews were conducted approximately one year from the time that the story of the planned EVTs was first published by Ghanaian media. Our emphasis was on understanding how the planned EVTs were initiated, as well as the community response, actions, and concerns. The choice of in-depth interviews enabled us to probe the responses of the interviewees deeply as well as capture their lived experience in their own words.
We conducted a total of 60 interviews (35 men, 25 women) using a combination of purposive, convenience, and snowball sampling. All respondents were residents of Hohoe who lived in the community at the time of the planned vaccine trials. They comprised 8 professionals (teachers, laboratory technicians), 11 health workers (nurses/clinicians), 5 elderly people (55 and above), 1 priest, and 16 students (from high school, the University of Health and Allied Sciences, and Kwame Nkrumah University of Science and Technology). About one-third (19) were in low-income occupations (street vendors, taxi drivers, market women, self-employed). All interviews were conducted by the first author in either Twi, Fante, English, or French, depending on the respondent’s preference.
Interviews were transcribed, then analyzed thematically using ATLAS.ti. Active codes were developed from the interview transcripts. These were categorized and developed into themes paying close attention to the respondents’ accounts of their experience of the planned EVTs and the behavior they exhibited at the time. Codes that shared the same or similar characteristics were put together to form a category. For example, all codes that had quotations, which included reasons why respondents were not willing to voluntarily enroll in the planned EVTs—particularly reasons having to do with the EVTs not being safe, volunteers being “paid” to participate in the EVTs, the fact that Ebola is deadly and yet there were planned EVTs—were put into a category labeled “mistrust.” For trust in EVTs, all codes which had quotations referring to reasons why the respondents will volunteer for the planned EVTs, particularly quotations indicating the planned EVTs were safe and not meant to transmit Ebola were placed in a category labeled “trust.” This approach of grouping codes that shared the same or similar characteristics was used to categorize the codes.
Based on the characteristics that defined a category, a central theme connecting the characteristics was developed. The first author determined the characteristics of the codes and assigned codes to the transcribed interviews. This was verified by the second author, after which discrepancies were resolved by discussion. Control as a defined category includes having oversight responsibility for others in the vaccine trial network and was gleaned from the content analysis of online newspapers, in which reference to the submission of the EVT protocol to the FDA and the power exercised by Parliament to suspend and reinstate the planned EVTs constituted control. Given that the planned EVTs did not take place, the control exerted by the scientists over volunteers in the context of the EVTs could not be captured. Other themes that were developed include fear of Ebola and attitudes toward regulating institutions.
In view of the political context of the planned EVTs, we did a content analysis of online newspaper publications in Ghana between 2014 and 2015. We paid particular attention to the accounts of the unfolding events surrounding the EVTs in both the local area and in Ghana as a whole. In the following two sections, we discuss media reports and parliamentary response to the trials and the nature of the community response.
Mistrust of Ebola vaccine trials and regulating institutions
The FDA in Ghana has oversight responsibility for approving vaccine trials, while the Ghana Health Service (GHS) is responsible for giving ethical clearance for vaccine trials. Protocols for two vaccine trials (Janssen Ebola Vaccine and GlaxoSmithKline Ebola Vaccine) were submitted to the FDA and GHS for the required approvals. However, media publication of the planned EVTs generated a major and far-reaching debate in the public sphere. Newspaper publications immediately questioned why the EVTs were being carried out in Ghana when there had been no reported case of Ebola (GhanaWeb, 2015c; Starr FM Online, 2015a). Some pointed out the danger that such trials would pose to volunteers (Bokor, Reference Bokor2015; Starr FM Online, 2015a; Modern Ghana, Reference Ghana2015g). Others described the planned EVTs as an affront to the dignity of Ghanaians as human beings (Joy FM Online, 2015; Starr FM Online, 2015a). These reports quickly caught the attention of Parliament. The opposition party condemned the planned trials, and its chairman for the Volta Region, where Hohoe is located, requested that the president of Ghana relocate the site of the planned EVTs to his own hometown, Bole, in the Northern Region (Asiedu, Reference Asiedu2015; Graphic Online, 2015d). Even members of the ruling party at the time issued a press statement condemning the planned EVTs and called on the minister of health to stop the vaccine trials from taking place (GhanaWeb, 2015a; Modern Ghana, Reference Ghana2015a; Starr FM Online, 2015b).
On the other side, publications featured a leading pharmacology professor in Ghana who chided parliamentarians for making misleading and unfounded statements about the planned EVTs and described the day that Parliament suspended the vaccine trials as “a sad day for science” (Appiah, Reference Appiah2015; 2015b; Modern Ghana, Reference Ghana2015f). Ghana, in his view, had lost a great opportunity as a nation to be part of the history of developing a vaccine for Ebola, which he viewed as an opportunity for Ghana to demonstrate its capacity to undertake such crucial vaccine trials and make a notable contribution to scientific knowledge (Graphic Online, 2015e; Quaicoe-Duho, Reference Quaicoe-Duho2015c, Reference Quaicoe-Duho2015d). Echoing the sentiments of the scientific community in Ghana, the director of Noguchi Memorial Institute for Medical Research, one of Ghana’s leading scientific research centers, also weighed in and criticized the suspension of the EVTs. Like the pharmacology professor, he attributed the suspension to ignorance and a lack of trust in Ghanaian scientific institutions, which had a sterling reputation (Graphic Online, 2015e). The parliamentary decision to suspend the EVTs was also criticized as usurping the powers of the FDA, which had been established by an act of Parliament (Public Health Act 2012 or Act 851). According to the pharmacology professor, the FDA was entrusted with the powers and mandate to undertake, regulate, and supervise vaccine/clinical trials based on its expertise. Thus, suspending the EVTs in spite of the conditional approval given undermined the authority of the FDA, which had previously undertaken and supervised over 50 vaccine/clinical trials (Quaicoe-Duho, Reference Quaicoe-Duho2015c).
In response to the pushback from Ghanaians and a display of institutional muscle, the minister of health announced the suspension of the planned EVTs, much to the chagrin of the FDA (Modern Ghana, Reference Ghana2015b; Graphic Online, 2015c; Quaicoe-Duho, Reference Quaicoe-Duho2015b). According to the FDA, it had reviewed the EVT protocol and given conditional approval to the scientists so they could begin recruiting volunteers pending final approval (Modern Ghana, Reference Ghana2015c; Quaicoe-Duho Reference Quaicoe-Duho2015a). According to the FDA’s assessment of the protocol, the EVTs were safe and posed no harm to Ghanaians (Graphic Online, 2015b). It was, however, startling for the FDA to learn of the Ministry of Health’s notice of suspension through the news media. In a statement to Parliament, the FDA noted that there was no official communication between the Ministry of Health and the FDA regarding the EVTs. Neither did the Ministry of Health contact the FDA for expert opinion on the EVT approval process it was undertaking (Modern Ghana, Reference Ghana2015h). The Ghana Academy of Arts and Sciences (GAAS) expressed dismay at the approval of the EVTs granted by the FDA to the scientists.
According to the GAAS, it was not aware of the Phase I trials that the FDA had approved to take place in Hohoe. According to its own assessment, the GAAS noted that the EVT that GlaxoSmithKline was seeking to carry out in Ghana was not safe. According to its technical committee, Ghana was not prepared for the EVTs. The GAAS was also concerned about the inclusion of children and the safety of the community where the EVTs were to take place, and it was not convinced that the protocol met the strict international standards of vaccine trials (Smith-Asante, Reference Smith-Asante2015). The actions of the various actors within the vaccine trial network demonstrate the many interests at play: the scientists collaborating with the FDA wanted the EVTs to be successfully implemented, both for their institutions and for the national prestige. The Ministry of Health sought to protect Ghanaians but undermined the work of the FDA by announcing the suspension of the EVTs a few days after they had been conditionally approved. The GAAS claimed that Ghana was not ready for the EVTs, in spite of the many vaccine/clinical trials that had already been conducted in the country. Its comments and actions were viewed as an affront to the scientific community and to the FDA in particular. For Parliament, the decision to eventually suspend the EVT stemmed from its rapid consensus that constituents would view any other course as risky. Though all the actors viewed their positions as protecting Ghanaians from the perceived risk associated with the EVTs, each institution approached it differently.
In a radio interview, the lead scientist of the planned EVTs expressed his frustration with the regulatory authority and simultaneously tried to persuade Ghanaians of the safety of the planned trials:
I have had a battle with the Food and Drugs Authority (FDA). I would be the first to take the vaccine. Because this is a political disease and maybe we have to find different ways of doing that. I don’t think I will be offering Ghanaians something that will be dangerous to them.
(GhanaWeb, 2015d; Graphic Online, 2015f)By describing Ebola as a “political disease,” we emphasize the immediate adoption of the EVD discourse for strategic political action. Though a single case of Ebola had yet to appear in Ghana, strategies and tests were to be negotiated by Parliament, the FDA, the GHS, scientists, and the affected communities. To scientists, EVD is a disease that ought to be tamed through the development of a vaccine, yet its character as a political disease meant that its absent presence would be negotiated by various stakeholders. In Parliament, Ebola had become a reality to be monitored and feared. To the FDA and the GHS, it remained a disease whose presence ought to be regulated under the most stringent conditions. To most Ghanaians, Ebola was a fearful but unknown reality whose actual presence would be unleashed by scientists. Within the vaccine trial network, the character of Ebola mutated through its encounters with the different actors in the network. From the community in Hohoe, to the Parliament House in Accra, to the laboratories of the scientists, and the offices of the regulating authorities (FDA and GHS), EVD manifested a shifting pattern that varies from location to location. The perceived presence or absent presence of Ebola in each of these locations in the vaccine trial network required negotiations and compromise in the media spotlight, with consequences for trust and control. Each encounter with the absent presence of EVD within the network was transformative and generative. It stoked fear that prompted some actors to embrace health-seeking behaviors while objecting to the actions of others. It instigated some actors (parliamentarians) to exercise caution, seek more information, and advocate the adoption of health-seeking behaviors. Other actors (the FDA and the GHS) exercised stringent controls in a bid to forestall any disaster. Still others (scientists) courted EVD in a bid to subdue it through vaccine development.
As a political disease, Ebola became a disease of contention among the two major political parties in Ghana as each party sought to make political gains. The opposition party at the time criticized the president of Ghana, John D. Mahama, for abandoning the economic woes of Ghanaians and focusing too much attention on Ebola, to the point of agreeing to make Ghana’s capital, Accra, the host of the United Nation’s Mission for Ebola Emergency Response (UNMEER) (Boadu, Reference Boadu2014). The ruling party at the time criticized the opposition party for being so desperate for power that it sought the infectious disease of Ebola for use as a tool of propaganda to discredit the sitting government (Modern Ghana, Reference Ghana2014). The opposition party, for its part, accused the government of sidelining the legislature in its decision to host UNMEER. The majority in Parliament noted that the executive can make decisions without having to involve all the arms of government. The Speaker of the House eventually invited the minsters of foreign affairs and health for questioning to ascertain the circumstances leading to the decision to host UNMEER in Ghana and to appraise the implication of the decision for Ghanaians (Smith-Asante, Reference Smith-Asante2014). The actions of the political parties show the extent to which Ebola discourse was the common currency of vilification, without any verified instance of the disease.
In the wake of the heated public debate on the planned vaccine trials, the Parliament of Ghana ordered the FDA and the GHS to halt the approval process until the minister of health and the principal investigator of the vaccine trials appeared before Parliament to answer questions and explain the nature of the trials. Owing to the continuing objections of Ghanaians, coupled with the specter of what could happen if Ebola became widespread, Parliament intervened and suspended the approval process pending more information on the proposed trials. Following its interrogation of the minister of health and the principal investigator of the trials, approval was granted, and a campaign of public education was recommended. This campaign would convince the public that (1) the planned trials were safe, (2) they would not introduce EVD into Hohoe or Ghana as a whole, and (3) the scientists would only use an attenuated version of the virus without risk. The rationale and choice of the country as a site for the planned EVTs would be explained before the trials commenced.
In actor network terms, the characteristics of Ebola distorted the trust/control nexus in the relationship between the scientists and the regulating institutions. The scientists sought approval from the substantive regulating institutions (the FDA and the GHS), trusting these institutions to grant them regulatory approval upon reviewing the planned EVT protocol to ensure that it met required standards of vaccine trials. The FDA and the GHS, in turn, exerted control through the approval process while trusting that the scientists would conform to approved norms of vaccine trials.
In the approval process, a tandem of trust and control had developed between the scientists and the regulating institutions within the context of the EVT network. But the characteristics of the disease had been drawn into sharp relief by the media and thereby enrolled a new regulatory actor in the EVT network, the Parliament of Ghana. As an actor embedded in the EVT network, Parliament had concerns about the planned EVTs based on media reports of the characteristics of the disease and consequent public objections. Members began to doubt the safety of the planned trials, suspending the plan until more information could be collected and the safety of the planned EVTs could be ensured. Prior to the collection of information, neither trust nor control could be produced in the relationship between government and science. By this time, elected representatives could not allow Ghanaians, even willingly, to accept vulnerability by taking part in the planned EVTs. The characteristics of Ebola fractured the usual trust relations between the scientists, the regulatory agency, and Parliament. Project scientists and sponsors of the vaccine trials withdrew the planned EVTs even though Parliament and the FDA eventually approved them. According to the clinician on the EVT project, this was as a result of the delay caused by the suspension. The planned EVTs were being carried out on a quota basis, and once the sponsors got the required quotas from the other participating countries, they lost interest in Ghana.
Trust operates at a complex level in vaccine trials and is tied to the level of perceived risk. Risk perceptions do not solely emanate from a single event, such as the announcement of a trial. Neither do they stem simply from uncertain medical technologies or mistrust in expert knowledge but from a more generalized notion of risk in the populace (Brownlie & Howson, Reference Brownlie and Howson2005). Therefore, trust is built on community experience and perception of the risks. This implies that trust does not simply evolve from the nature of the information being disseminated or the mode of transmission, but also from the larger sociopolitical context within which volunteers, health professionals, and health authorities interact during the vaccination process. The connections between knowledge, risk perception, and anxiety are such that for health systems to function effectively, trust must begin at a systemic level (Brownlie & Howson, Reference Brownlie and Howson2005), but it must also operate at the interpersonal and sociopolitical levels (Bester, Reference Bester2015). The lack of trust in the Ebola vaccine trials in Hohoe went beyond the interpersonal level, to be discussed in the following section, and included this wider political context.
The construction of scientific knowledge in vaccine/clinical trials is shaped by the specific combination of trust and control that drives the sociotechnical machinery at the heart of knowledge production in vaccine trials (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). The trust relationship between regulating institutions and scientists ensures that scientists conform to approved standards in vaccine trials. Having the approval of the regulating institutions, scientists are normally able to attract volunteers by gaining their trust while conforming to the ethical standards of the clinical trial process. The methodological controls embedded in the scientific process of conducting and using technology and procedures in trials is claimed to eliminate bias and guarantee the objective results that will eventually lead to the development of a vaccine or drug (Bijker et al., Reference Bijker, Sauerwein and Bijker2016). The trusting relationship between the scientist and the regulatory authorities was fractured by the characteristics of Ebola. For the scientists and community volunteers, these characteristics hampered the formation of the tandems. Thus, the construction of scientific knowledge in the planned EVTs could not take place. The characteristics of Ebola marred the coproduction of trust and control between the volunteers and the scientists, as well as between regulating institutions and scientists. This ruptured the linkages between volunteers, scientists, and the regulating institutions, leading to a breakdown of the EVT network.
In this section, we showed that when the Ebola discourse emerged in the vaccine trial network, the characteristics of the disease became an important determinant of trust and control. We turn now to the expectations that community members had in the context of the vaccine trials and the dynamics that determined their support for these trials and willingness to accept vulnerability. Here, too, the perceived characteristics of disease alter the trust and control dynamics in the relationships. In the context of the Ebola vaccine trials, these shifted the relationships between volunteers, regulating institutions, and research scientists.
Fear of Ebola
The identity of Ebola as an object of media attention produced not just worry but often extreme fear and avoidance of people or objects perceived to be connected to the disease. At the center of the outbreak in West Africa in 2014, many fled communities with reported cases of Ebola. Community members attacked volunteers of humanitarian aid agencies as they disinfected the houses of patients and aided in their burial. Treatment facilities were even attacked by community members who feared the facilities would spread Ebola in the community. Some people who fell sick during the outbreak refused to seek medical treatment at hospitals or clinics lest they might be declared Ebola patients and suffer the psychological burden of community stigmatization. The morbid fear of EVD went beyond the actual locations of the outbreak. In distant places, people were concerned they could be infected given the rapid rate at which the disease was spreading across national borders. This motivated them to adopt a variety of health-seeking and avoidance behaviors in the interest of preventing infection.
In the context of the Ebola vaccine trials, Hohoe community members, health workers, and regulatory institutions quickly developed concerns about the intent, procedures, and origins of the proposed trials. They did not trust its safety and thus had reservations about participating in the trials. This impaired the network of relationships required for the effective conduct of the trials and eventually led to their cancellation. The fear that Ebola generated in people and its subsequent effect on the tandems of trust and control in the vaccine trial network are discussed in this section, with a focus on how the characteristics of disease may be a site of trust and control.
Knowledge and perceptions of the characteristics and risks of a particular disease affect receptivity toward vaccine trials. Prior to hearing reports on the planned trials, Ghanaians learned of Ebola from radio and television broadcasts, printed newspapers, and online news outlets, as well as social media. Ghanaian media published stories virtually daily on the Ebola outbreak in Sierra Leone, Liberia, and Guinea, focusing on the death toll, the dangers associated with the disease, and its rapid rate of infection in the affected countries. These newspapers also reported incidents of Ebola in Ghana. All turned out to be suspected isolated cases of hemorrhagic fevers that subsequent tests indicated were not Ebola. Yet throughout this period, informal rumors among the population and the media reportage stirred up fear among Ghanaians. The Ministry of Health and the interministerial committee responsible for containing an EVD outbreak in Ghana organized news conferences to discredit rumors and assuage the fears of Ghanaians.
These tactics were used to educate Ghanaians on the characteristics of Ebola as well as its risk factors and avoidance procedures, but the disease remained at the forefront of public consciousness. People talked with friends and family, sending text messages and discussing what kinds of activities they would and would not do to avoid getting infected. Our respondents consistently viewed Ebola as a disease that is extremely painful, highly infectious, and without any known cure. This knowledge of the characteristics of Ebola generated the fear of contracting Ebola among community members and some health workers. A 24-year-old self-employed male commented,
As for me, I was seriously scared because … they say if you have body contact small, you could be affected and those kind of things. I was seriously scared.
Avoidance procedures were repeated from person to person, affecting community interaction so much that many people were reluctant to shake hands—an important component of the Ghanaian culture of greeting and exchanging pleasantries. Handshake avoidance was a small but instant signal that Ebola was already a danger, even before the trials were announced, and made it difficult for community members to trust their safety.
Why did residents distrust the safety of the trials? Referring to the Ebola outbreak in parts of West Africa, a 62-year-old retired male education officer expressed his doubts about the intentions of the vaccine trials:
Ebola which has been killing a lot of people, they said they want the center to be here. Ebola center, they said people should come and they should be vaccinated. And then if the virus is killing a lot of people in other parts of West Africa, why is it that the center is not established there, and you want to establish it in Ghana? You want us to die?
For this respondent, the thought of having an EVD outbreak elsewhere and having the planned trials in Ghana was inconceivable. Thus, moving ahead with the planned trials simply meant that the authorities wanted people to die, given the high death rate associated with infection. This, for him, explained why people had concerns and rejected the planned EVTs. As our respondents expressed repeatedly, the central point of vaccine trials was to introduce the Ebola virus into their community, prior to treating it with an uncertified vaccine with unknown efficacy. As a result, they vehemently opposed it. Simple logic suggested vaccine trials should occur in communities where Ebola was already present. As a 35-year-old male mechanic explained,
They said they wanted to bring some antivirus into our town here and even me myself, I was against it … I wasn’t happy about it.…they said they have gotten the drug for the virus and they want to see if it is really effective. So that means when you go in …so they needed volunteers …ok fine. So that means when you go in you have to be infected by the Ebola virus before they give you the vaccine to cure it. I said, ah so this one … let’s say if the drug doesn’t work that means we are going to be killed and I even said ok, fine these things most at times the whites when they do these drugs, they test it with animals. They will use these animals, rabbits, rats, dogs to test the virus to see if it is really effective. But to use human beings to test it, I don’t understand why they should do that.
This respondent’s mistrust stems from his understanding that volunteers would be infected with the Ebola virus and then treated with a vaccine of unknown efficacy. To make matters worse, the trials were going to be conducted on humans instead of animals. Although the purpose of vaccine/clinical trials is to ascertain the efficacy of drugs/vaccines in human populations, this respondent and other community members had a preexisting understanding that trials are meant to test drugs on animals. Only when proven effective would they be prescribed for humans.
Though the risk of the disease and the risk of disease through a vaccine trial are not the same, community members associated the two. They were aware that taking part in vaccine trials came with a certain level of risk. But in the case of Ebola, the horrific symptoms and certain death made it difficult for community members to dissociate the risk of the disease from the risk of the vaccine trials. Neither did they trust the regulating institutions to duly regulate the activities of the scientists to ensure safety. A common story was that collusion existed between scientists and politicians. A 31-year-old female teacher told us,
I don’t understand why they should do that. It came to a time that most of us are saying that it is because our MP took some money … she was only interested in taking her own part of the 10%. If we are dead, she will take her family away and then we that voted for her we will just die. So that thing made some of us hated her a lot because … the sickness that we heard, was not the type that we should joke with. It is the type that everybody should prevent himself or herself from it. So, when you are saying that they should come and test the vaccines around the area, why do you even accept it in the first place? Why should you even accept that in the first place? Does it mean you don’t think about the people that you are ruling?
While bribery and payoffs were often mentioned, it was often unclear from our interviews who would be paying. Sometimes the perception seemed to be that scientists who sought to carry out the trials were paying the leaders of the community to grant them access to the community. They might also be paying the regulatory authorities to approve their project. Hohoe is not a community new to vaccine/clinical trials. It houses the Onchocerciasis Chemotherapy Research Centre in Ghana, where clinical trials for onchocerciasis and malaria vaccines have been conducted. These occurred in Hohoe and surrounding villages without any resistance from the community (ClinicalTrials.gov, 2017, 2019; Conteh et al., Reference Conteh, Patouillard, Kweku, Legood, Greenwood and Chandramohan2010; Keiser et al. Reference Keiser, Reynolds, Awadzi, Ottesen, Taylor and Nutman2002; Kweku et al., Reference Kweku, Liu, Adjuik, Binka, Seidu, Greenwood and Chandramohan2008; Maïga et al., Reference Maïga, Djimdé, Hubert, Renard, Aubouy, Kironde, Nsimba, Koram, Doumbo, Le Bras and Clain2007; Rogers et al., Reference Rogers, Atuguba, Oduro, Hodgson and Koram2006).
In these cases, they were made aware of the risks associated with these vaccine/clinical trials and trusted the scientists to follow standardized procedures in medical research to protect them from harm. They also trusted the regulating institutions to duly exercise their oversight responsibility. But these diseases were not fatal: their symptoms were known and could be expected. The basis of this trust stemmed from the social norms of the community, which accepted medical personnel as experts authorized to undertake actions for the good of the community on known diseases. These norms guided the institutional safeguards set in place to protect the community (Dixion-Woods and Tarrant, Reference Dixion-Woods and Tarrant2009). Thus, the community’s encounter with medical personnel was devoid of fear. They accepted that the regulatory system would ensure the safety of the research and that the scientists would conform to safety standards. This reduced the risks involved thereby making it safe for them to participate (Dixion-Woods & Tarrant, Reference Dixion-Woods and Tarrant2009).
But Ebola vaccine trials, with all of the horrifying characteristics of the disease, called for a discussion of intentions, payments to leaders, and the regulatory authorities. A primary theme emerging from our interviews was a violation of the community sense of fairness—that the government had unfairly targeted them for the planned trials of a deadly disease.
The persistent question they asked was, “Why Hohoe?” Why had their particular community been designated as the site for the planned EVTs? Some suggested that the government wanted to wipe out the residents of Hohoe with the planned EVTs. An elderly retired female teacher summed up strong community sentiment by noting,
[People] said the present government just wanted to disturb them and then kill them before the election. So, people even said that that it was the planned thing. It was a planned thing that the government is bringing in to kill them before 2016 election.
Informants were seeking to make sense of the unnerving fact that their community has been designated for the planned EVTs in the absence of adequate information. The official explanation that Hohoe was chosen because of its preexisting onchocerciasis research center was insufficient. That center was for a different disease. Why must it be in a location where there was a center for a different disease? There must be some reason the government might want to eliminate the community. But even this was hard to believe, since Hohoe was known as one of the strongholds of the ruling party at the time. It was simply not common sense that the government would want to wipe out its primary electoral base.
Thus, an alternative political interpretation arose wherein community members raised doubts about the integrity of local leaders. Why would these leaders, from the same political party they supported, agree to the planned trials in their community knowing that Ebola is a deadly disease? The idea of official bribery emerged as a readily available explanation for this outrageous behavior. Stories circulated in the community that the chief, the district chief executive (DCE), the MPs for the constituency, and even the national government had been bribed to agree to the planned EVTs. They registered their resentment toward community leaders through call-in sessions on the local radio stations. As a 50-year-old market woman told us,
They were insulting the DCE and the MP and even the government… . they say our chief too they give him some percentage of the money and the MP too the DCE too … so they were insulting them. People call the FM station and talk rubbish to them on air.
For community members, the scourge of Ebola embedded in the planned EVTs could only descend on the community with the approval of leaders who deserved their scorn. The community’s notion of the EVTs, coupled with the fear that Ebola elicited, affected their perception and evaluation of the process. This undermined the level of trust in the scientists involved in the EVTs, heightened by the community’s perception of the tokens that were to be given to those who volunteered. A 27-year-old seamstress expressed this sentiment:
I’m always warning people. Even those I don’t know. I always warn them against taking vaccination. They can give that vaccination and after that you will get Ebola. Because they said, they want to give the vaccination with money and phone.
For the community members, the scientists were “luring” them to participate in the vaccine trials by offering them 200 Ghana cedis—approximately two days’ wages—and a mobile phone. A clinician on the planned EVTs explained that the amount of 200 Ghana cedis was not really a compensation meant to “lure” the community members. He described it as lost earnings, payment for time lost by taking part in the clinical trials. But the mobile phone was meant to be used to call the EVT center in the event of an emergency after the volunteers were injected with the candidate vaccine. This seemed suspicious, indeed. The very characteristics of Ebola and its associated risks impacted the trusting relationships that should have been forged between community members and the scientists.
In the context of vaccine trials, networks of relationships are laden with trust and control. As Bijker and colleagues (Reference Bijker, Sauerwein and Bijker2016) argue, tandems of trust and control link the personal, the institutional, and the technical in the construction of knowledge. These are the elements in the relationships between scientists and volunteers, the regulating institutions and scientists, and the technology employed in vaccine trials. Here the characteristics of Ebola (horrifying symptoms and the absence of any cure) became a key component of the network of relationships, thwarting all positive expectations of the politicians and scientists involved for most community members. They overwhelmingly sought to avoid vulnerability by avoiding the trials. The refusal of the volunteers to participate in the vaccine trials meant the scientists could not exercise control over the volunteers according to the vaccine trial protocol. Once Ebola emerged as an enabled actor in the trial network, its deadly and infectious properties undermined the relationship between scientists and community members.
Discussion
The absence of a trusting relationship between the community members and the scientists decreased support for vaccine trials, while the parliamentary ban delayed the trials until they were no longer needed by international agencies. Bester’s (Reference Bester2015) examination of childhood vaccinations shows that that trusting relationships between volunteers, parents, and health care workers increased vaccine uptake. This was achieved when health care workers were able to provide volunteers and parents with adequate information about the vaccine to be administered and dispel fears and concerns in a timely fashion—quite the opposite of the Ghanaian case. Since there was insufficient information on the planned vaccine trials prior to the emergence of a community consensus on risk, community members were reluctant to volunteer. The paucity of information on the planned EVTs heightened fears and concerns and eroded their trust in the vaccine trial process and the scientists. Morris and Bàlmer (Reference Morris and Bàlmer2006) argue that the relations between volunteers and researchers are interactions, negotiated in real time, and should be considered an active social situation and not a passive one. Indeed, following the announcement of the trials, the community members showed themselves to be active participants and not passive “guinea pigs.”
Potential volunteers navigate unfamiliar and unknown social territories until they forge relations with those they can trust. At the peak of the Ebola outbreak in Sierra Leone, trust was invested in health systems, and vaccine trials took place in the northern region. The Ebola vaccine trial team employed a combination of community engagement strategies spearheaded by social scientists and leading community members in building trusting relationships (Enria et al., Reference Enria, Lees, Smout, Mooney, Tengbeh, Leigh, Greenwood, Watson-Jones and Larson2016). Unlike the Ghana case, they worked closely with the community, listened to and addressed their fears and concerns, dispelled rumors about the EVTs through active dialogue rather than “correcting misinformation,” and involved those who had volunteered and taken part in the vaccine trials in their outreach to the community (Enria et al., Reference Enria, Lees, Smout, Mooney, Tengbeh, Leigh, Greenwood, Watson-Jones and Larson2016).
Yet the failed trials in Hohoe were not simply the result of local relationships. As we have argued, local problems interacted within the larger Ghanaian political context as national politicians quickly found ways of interpreting trials for political advantage, which further reduced the trust that had existed in prior clinical trials for onchocerciasis and malaria vaccines. During the flood of media attention, national political actors became involved in what would normally be purely regulatory processes. Preexisting beliefs about bribery and politics yielded support for various accounts that were not raised in previous trials, and the site of the trials became a source of controversy as community members questioned why Hohoe in particular had been selected. Since there seemed no good reason, it was plausible that vaccine trials could be used to eliminate groups or line the pocketbooks of leaders. Less common, but apparent in a few interviews, were religious interpretations:
Pastors were saying that according to the Bible during the end time, there will be an outbreak of serious diseases of which we are not going to know their cure, their vaccines and stuffs. So, they were saying it will lead to the end time so we the youth we should change or we as this generation we should change and attend to God so that if the rapture comes close, we wouldn’t find ourselves wanted.
This 23-year-old male student felt that Ebola might be considered the “disease without cure,” signifying the eschatological prophesy.
In a “post-trust” society, where trust and confidence in health authorities are dwindling (Bouder, Reference Bouder2015; Löfstedt, Reference Lofstedt2005), there are new calls for the voice of the patient, including healthy individuals who volunteer to receive vaccines or participate in trials, to be “heard, considered and addressed” (Holt et al., Reference Holt, Bouder, Elemuwa, Gaedicke, Khamesipour, Kisler, Kochhar, Kutalek, Maurer, Obermeier, Seeber, Trusko, Gould and Rath2016, p. S146). This implies that vaccine communication ought to be a series of interpersonal interactions in which volunteers are encouraged to voice their concerns in their own words about the safety and risks of the vaccine, their beliefs and concepts of disease, and their religious or cultural norms regarding disease (Holt et al., Reference Holt, Bouder, Elemuwa, Gaedicke, Khamesipour, Kisler, Kochhar, Kutalek, Maurer, Obermeier, Seeber, Trusko, Gould and Rath2016). A key feature missing in the planned Ghana trials was the voice of the community members before the announcement was made. The community was not adequately involved in the processes leading up to the vaccine trials. Their fears and concerns were not adequately heard and addressed. Their notions of risk, disease, and perceptions of the disease and vaccine entities were not factored into the trial communications. The information and communication reflected a top-down model of scientific and medical expertise rather than a two-way communication model. The vaccine communication was not tailor-made to fit the specific context addressing the risks and safety of the trials as perceived by volunteers.
This case study is limited by the data available and the linguistic abilities of the coders in a variety of languages in which trust and trustworthiness are implied but not directly mentioned in the data. Further, the study was originally designed to examine perceptions of Ebola rather than the vaccine trials, which emerged during the course of the study and was not explored to the same extent among all respondents. Nonetheless, the results suggest the importance of examining both the political context and the characteristics of emergent infectious diseases in future studies of vaccine trials as well as the implementation of trials. In the context of vaccine trials for infectious disease, we have argued that the perception of disease characteristics is crucial to their success, and by extension the construction of scientific knowledge. These perceptions determined the creation and breakdown of relationships in the vaccine trial network, rooted in a variety of information and communication practices that were influenced by the national political context. The perspective of disease as an actor enabled within a network implies that vaccine trials ought not to be designed and implemented in similar fashion without regard for the preexisting knowledge and beliefs about disease. The design and implementation of vaccine trials is far more complex than laboratory studies in which characteristics of the disease primarily determine how scientists relate to the causative organism.
This perspective also has implications for vaccine communication. Vaccine trials for highly infectious diseases require a communication model that includes an active exchange between volunteers and scientists at an early point in their planning. Such an exchange requires constant interaction between the vaccine trial team and the community with the involvement of its leaders. These interactions will enable both the volunteers and the scientists to negotiate the risks associated with disease and participation in the trials. It will also afford scientists the opportunity to have a better understanding of the fears of the volunteers while assuaging these concerns and simultaneously gaining their trust. This active exchange component will result in communication that is “trust-based and science-informed” (Holt et al., Reference Holt, Bouder, Elemuwa, Gaedicke, Khamesipour, Kisler, Kochhar, Kutalek, Maurer, Obermeier, Seeber, Trusko, Gould and Rath2016, p. S146).
Appendix 1
Table 1 Timeline of EVD major events
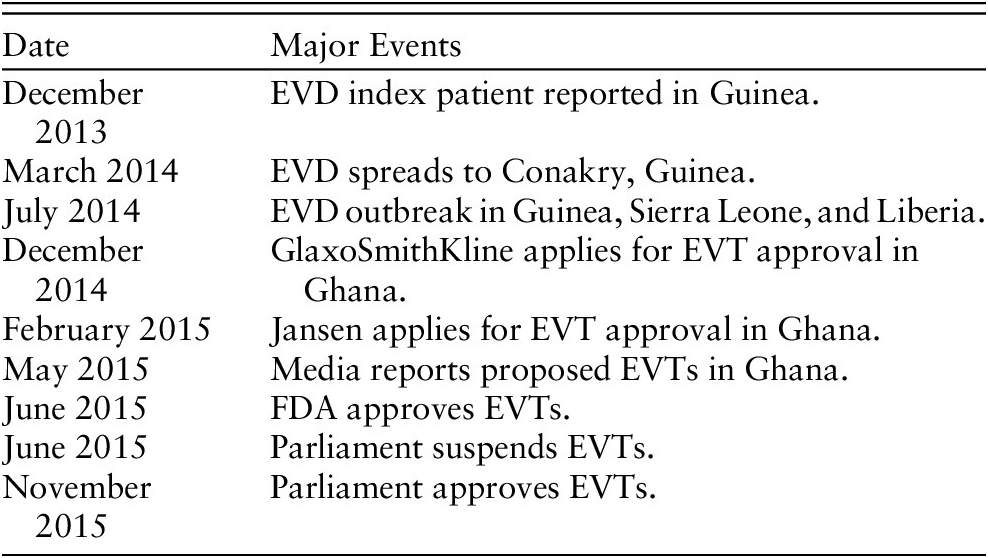
Appendix 2
Ebola Interview Guide Hohoe, Summer 2016
1. Kindly tell me about yourself (Probe for age, occupation, marital status, number of children etc.)
2. When was the first time you heard the word ebola? (Probe for the context within which the respondent heard the word ebola)
3. What were your sources of information on ebola? (Probe for the media outlets – TV/Radio stations; probe for the credibility of the information they got and why they deemed it credible)
4. Establish the respondent’s uses of the following if they were sources of information for ebola: the internet (which webpages they browsed for information); facebook, whatsapp, online news sources, other social media.
5. Explore any conversations or stories they remember from their interactions with their families and circles of friends (include work colleagues and neighbours) during the outbreak. (Establish the context of the conversation or story and where it took place).
6. How well informed were you about ebola during the outbreak? (Probe if the respondent was adequately or partially informed)
7. What precautionary measures did you take to ensure you were not infected? (Probe for specific changes the person made or carried out during the outbreak)
8. What are the symptoms associated with ebola?
9. What are the stories/jokes you heard about ebola (origin and nature of ebola)?
10. Did you know anyone (personally) who had ebola? (Probe for rumours associated with this as well)
11. How do people get ebola? How does it spread from one person to another?
12. Would you board a car with anyone infected with ebola?
13. What measures did your church/work take to ensure the prevention of ebola? (Probe if EVD was discussed at the formal level in the church or the workplace)
14. Would you volunteer to help in the wake of an outbreak? (Establish the reasons why)
15. What stories did you hear from other ministers/pastors about EVD?
16. What measures did government put in place at the time?
17. In your estimation, were the measures taken by government adequate or they could have done more?
18. What would you suggest as the best approach in solving the ebola outbreak?
19. What are your thoughts on the global response to EVB outbreat? Was it adequate? Why?
20. What did the AU/ECOWAS do during the time of the outbreak? Could they have done more?
21. What are your thoughts on Ghana opening its borders during the EVD outbreak while other countries closed their borders?
22. Those who die of ebola are buried by health professionals. The families are not allowed to go close to the corpse or burry it. In the event that someone in your family dies of ebola, would you allow the health professionals to handle the burial of such a person? (Probe for the respondents’ reasons for allowing or declining to services of the health professionals in burying the family member)
23. What is the most common information source you turn to for information about vaccines?
24. What did you hear about the government’s attempt to carry out EVD vaccinations in Hohoe? (Probe for sources of information)
25. Were you informed? (Probe for information passed on via community leaders, health professionals, church leaders etc.)
26. What are your thoughts on the government’s approach to the vaccination process? (Was it right or wrong?)
27. What compensation did the government offer during the proposed vaccination; was it adequate?
28. What stories did you hear about the proposed vaccination?
29. Was the vaccination carried out eventually?
30. What made government put a stop to the proposed vaccination process?



