INTRODUCTION
Digenean trematodes using marine invertebrates as intermediate hosts are widespread in shallow-water ecosystems where dense flocks of waterbirds and fish, their final hosts, congregate (Mouritsen and Poulin, Reference Mouritsen and Poulin2002). To understand spatial and temporal patterns of these digenean trematodes in marine intermediate host populations, knowledge of the factors controlling their transmission ecology is required. Transmission between first and second marine intermediate hosts is a small-scale phenomenon usually mediated by free-living lecitotrophic larvae (cercariae) with a short life-span (Marcogliese, Reference Marcogliese and Rohde2005). Once released into the environment, the free-living larvae encounter a multitude of factors which may impact their survival and infection success. While natural abiotic factors like water temperature, salinity and oxygen content (Pietrock and Marcogliese, Reference Pietrock and Marcogliese2003; Poulin, Reference Poulin2006), as well as anthropogenic pollutants (Khan and Thulin, Reference Khan and Thulin1991; Lafferty, Reference Lafferty1997), are well known to affect the transmission of free-living parasite stages, little is known about the role of the ambient fauna. Studies of trematode-infected freshwater snails have demonstrated that an endosymbiontic worm associated with the snail up-stream host is a cercarial predator that may reduce the survival of emitted cercariae (Christensen, Reference Christensen1979). Also, predators intimately associated with downstream hosts may reduce the survival of approaching infective propagules and thus reduce transmission of parasites to the target host. For example, an epibiotic sea anemone attached to the cockle Austrovenus stutchbury preys on cercariae approaching the cockle down-stream hosts (Mouritsen and Poulin, Reference Mouritsen and Poulin2003). Apart from epi- and endobionts intimately associated with host organisms, most parasite-host systems will be surrounded by many other organisms within the host space that potentially could interfere with transmission of parasites. To generalist predators consuming food items within the size range of cercariae, such parasite larvae might be a valuable food resource. Other organisms such as filter feeders may accidentally kill cercariae being inhaled together with regular food items. However, little is known about the effect of ambient fauna within the host space on parasite transmission.
In this study, laboratory mesocosm experiments were used to test whether selected ambient organisms occurring within the host space can cause a reduction in the parasite load of a down-stream host by interfering with the transmission of free-living trematode cercariae. A common marine parasite-host system of eastern North Atlantic shores is used to test this. The common cockle Cerastoderma edule is an abundant infaunal bivalve, hosting a variety of macroparasites (de Montaudouin et al. Reference de Montaudouin, Kisielewski, Bachelet and Desclaux2000; Russell-Pinto et al. Reference Russell-Pinto, Gonçalves and Bowers2006; Thieltges and Reise, Reference Thieltges and Reise2006) with the digenean trematode Himasthla elongata being one of the dominant species (Thieltges and Reise, Reference Thieltges and Reise2006). Its first intermediate host is the periwinkle Littorina littorea. Cercariae, which are shed from the snail hosts into the water, enter the cockles mainly via their filtration current and penetrate the foot from the mantle cavity (Jensen et al. Reference Jensen, Castro and Bachelet1999).
MATERIALS AND METHODS
Parasites, hosts and ambient organisms
Cercariae of Himasthla elongata were obtained from periwinkles (Littorina littorea) collected in the vicinity of the Marine Biological Station at Rønbjerg (Limfjord, Denmark). After collection, the snails were put in bowls filled with seawater and exposed to light for several hours. Snails shedding cercariae of H. elongata were kept in an aerated flow-through aquarium. Cercariae for the experiments were obtained by incubating a pool of 30–50 snails in bowls filled with seawater under light for a maximum of 3 h (=max. age of cercariae).
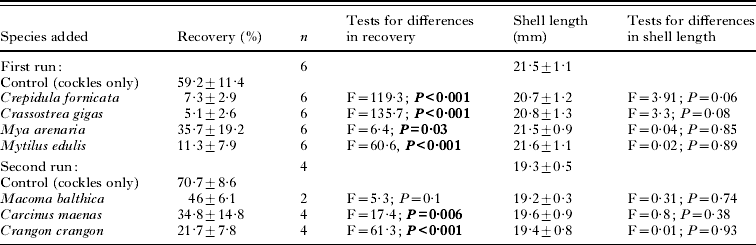
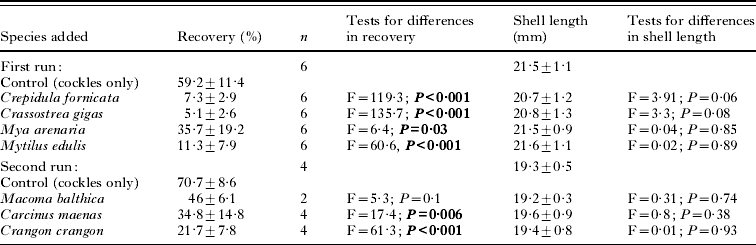
Cockle (Cerastoderma edule) hosts (18–22 mm shell length) were collected from a site with an uninfected cockle population (no first intermediate host present, and checked by dissecting 50 cockles) on tidal flats of the Wadden Sea (Sylt, Germany). Baltic clams (Macoma balthica) (15–25 mm shell length) were collected on tidal flats of the Wadden Sea (Skallingen, Denmark). Soft-shelled clams (Mya arenaria) (50–100 mm shell length), oysters (Crassostrea gigas) (70–80 mm shell length) and slipper limpets (Crepidula fornicata) (20–40 mm shell length) were collected in the shallow subtidal of the Limfjord (Denmark). Blue mussels (Mytilus edulis) (50–60 mm shell length) were obtained from subtidal mussel cultures in the Limfjord (Denmark). Shrimps (Crangon crangon) (15–25 mm body length) and juvenile crabs (Carcinus maenas) (8–15 mm carapax width) were collected with a push net in the vicinity of the Marine Biological Station in Rønbjerg (Limfjord, Denmark). All organisms were kept in the experimental set-ups for 1–2 days prior to the experiments.
Mesocosm experiments
Buckets (26×24 cm) were filled with 12 cm of sediment and 6 l of filtered seawater (salinity approx. 30 psu), constantly aerated and placed on a bench in a completely randomized design. Light was applied from above and water temperature was kept at 20°C. The first run of the experiments consisted of 5 treatments: (1) 3 cockles only, (2) 3 cockles plus 2 Mya arenaria, (3) 3 cockles plus 3 Mytilus edulis, (4) 3 cockles plus 3–4 stacks of 3–4 Crepidula fornicata and (5) 3 cockles plus 3 Crassostrea gigas. All treatments were replicated 6 times. A second run consisted of (1) 3 cockles only, (2) 3 cockles plus 20 Carcinus maenas, (3) 3 cockles plus 10 Crangon crangon and (4) 3 cockles plus 15 Macoma balthica. Cockle, C. maenas and C. crangon treatments were replicated 4 times while the M. balthica treatment was replicated only twice. To each of the above treatments 300 cercariae (counted under a dissection microscope) were added. After 48 h all cockles were dissected and the number of metacercariae of H. elongata counted using a dissection microscope.
Mechanisms of interference
Several ways were used to indentify potential mechanisms underlying an interference of cercarial transmission by the various organisms. Five crabs and shrimps were observed in small separate containers under a dissection microscope after addition of cercariae. Cercariae of H. elongata are large (body length 340–720 μm, see Werding, Reference Werding1969) and visible to the naked eye, allowing for easy observation on predation by crabs and shrimps. As H. elongata is known to use bivalves as second intermediate hosts but never crustaceans (Lauckner, Reference Lauckner and Kinne1983) we did not investigate crabs and shrimps for potential infections. One individual of slipper limpets and 1 individual of the bivalves added to each mesocosm was dissected and the entire tissue searched for parasites under the dissection microscope to check for infections. As C. fornicata, C. gigas and M. arenaria have been reported to be free or only marginally infected with H. elongata in the field (Krakau et al. Reference Krakau, Thieltges and Reise2006; Thieltges et al. Reference Thieltges, Krakau, Andresen, Fottner and Reise2006) we did not expect infections to occur. Individuals of M. balthica and M. edulis used for the experiments were free of H. elongata infections as checked by dissections of 20 individuals prior to the experiments. Both species are well known to be host to H. elongata (Lauckner, Reference Lauckner and Kinne1983; Thieltges et al. Reference Thieltges, Krakau, Andresen, Fottner and Reise2006) and we expected infections to occur. Due to the knowledge available from the literature and mentioned above, we decided to investigate only a fraction of the ambient organisms. In addition to the dissections, we observed 5 individuals of limpets and bivalves in small containers after adding cercariae. As cercariae of H. elongata can be observed with the naked eye (see above), it could be determined if and how the cercariae entered the organisms.
Statistics
For all experiments a recovery rate was determined by calculating the proportion of the 300 added cercariae recovered as metacercariae in all 3 cockles per replicate. Differences in the recovery rate between treatments were analysed using t-tests. All recovery rates were arcsine-transformed prior to the analyses to meet the assumptions of parametric tests. Potential differences in cockle size between treatments were checked with t-tests for each treatment versus the appropriate cockles-only treatment. To calculate the rate of reduction of parasite load (no. of metacercariae) in the cockles caused by the different organisms, the recovery rate of the cockles-only treatments (control) was set to 100% and the recovery rate observed in the treatments was subtracted.
RESULTS
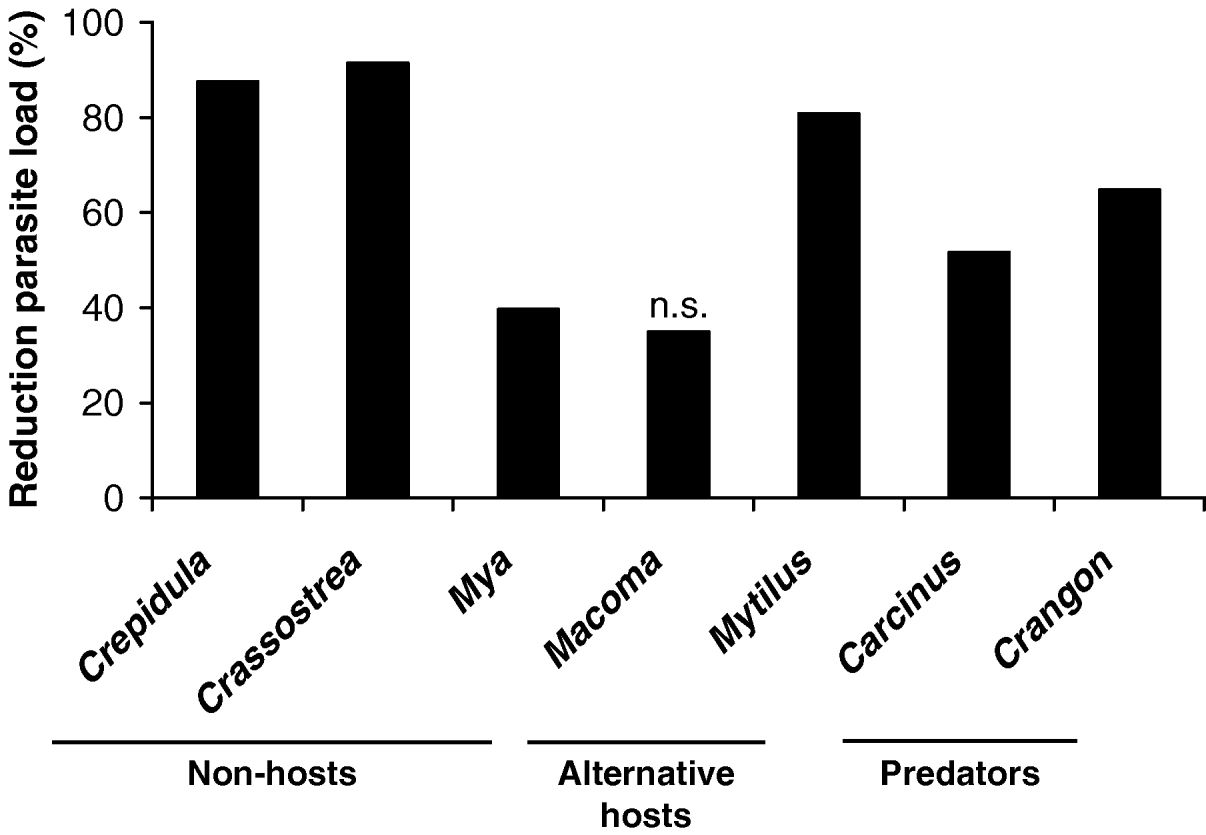
Almost all ambient organisms reduced the recovery rate of the cercariae in the cockles (Table 1; Fig. 1). Cockles incubated with other macrozoobenthic organisms acquired between 35 and 91% less metacercariae than cockles kept alone (Fig. 1). The difference in recovery rates between cockles only and all other treatments was significant in all tested organisms but Macoma balthica, where the effect was only marginally significant, probably due to the low number of replicates (Table 1). There was no difference in cockle size between treatments and controls (Table 1). Crabs (Carcinus maenas) and shrimps (Crangon crangon) were observed to actively prey on cercariae. The relatively large cercariae (body length up to 720 μm, see Werding, Reference Werding1969) were approached by them, caught with the claws and ingested. In slipper limpets (Crepidula fornicata), soft-shelled clams (Mya arenaria) and oysters (Crassostrea gigas) cercariae were taken in with the filtration current but the organisms were not infected with metacercariae. In contrast blue mussels (Mytilus edulis) and the Baltic clam (Macoma balthica) that also inhaled cercariae with their filtration current became infected with metacercariae.
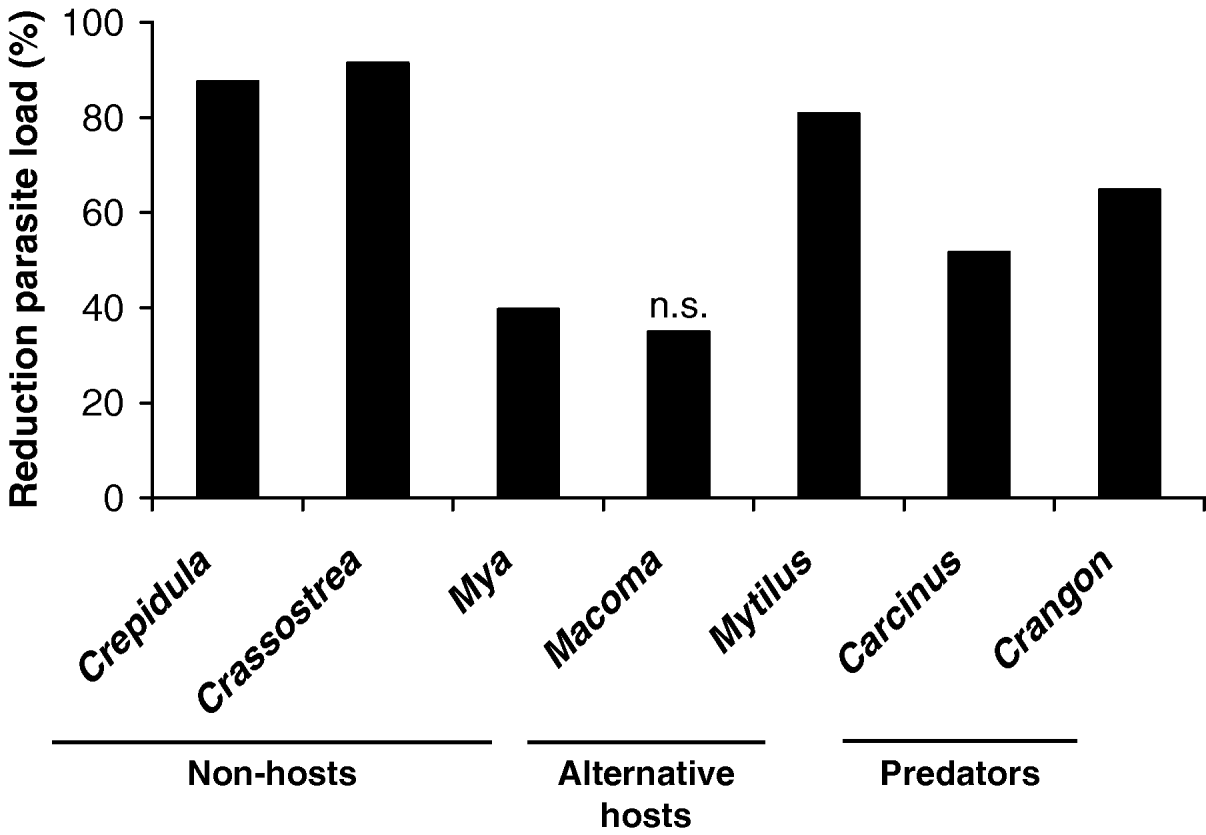
Fig. 1. Reduction of parasite loads (%) in cockles by different ambient organisms kept together with the cockles in laboratory experiments. Only 1 organism (Macoma balthica) did not show a significant effect (n.s.). n=2–6.
Table 1. Recovery (%)±s.d. of cercariae (as metacercariae) in the cockles, number of replicates (n) and mean shell length (mm)±s.d. of cockles in the different single species and cockles only (control) experiments
(Results of t-tests comparing recovery and shell length in single species with the respective controls are given. Significant results are in bold.)
DISCUSSION
Adding various ambient organisms to the mesocosms resulted in a significant reduction of parasite load in the cockle hosts in almost all tested organisms. Although similar in effect, the underlying mechanisms of this reduction were different, and 3 functional types of ambient organisms were involved: predators, non-host filter feeders and alternative hosts to the target host.
The predators Carcinus maenas and Crangon crangon were actively preying on cercariae. Both species are efficient benthic predators mainly preying at the sediment surface (Pihl and Rosenberg, Reference Pihl and Rosenberg1984; Pihl, Reference Pihl1985). As H. elongata cercariae are positively geotactic (Werding, Reference Werding1969) they could easily be preyed upon by the predators. The parasite species uses bivalves as second intermediate hosts but never crustaceans (Lauckner, Reference Lauckner and Kinne1983). Hence, the crabs and shrimps did not act as alternative hosts. Apart from consumption of cercariae, crabs and shrimps may also affect cercarial transmission through interference. In the presence of the two predators cockles may become repetitively disturbed by the organisms moving around. Cockles might retract their siphons and stop filtrating. This may result in a lower total filtration rate and ultimately in a lower parasite load. However, if this is actually the case, and what the magnitude of such disturbance interaction might be, has to be studied in more detail.
The filter feeders Crepidula fornicata, Mya arenaria and Crassostrea gigas were filtering cercariae but were not infected, as subsequent dissections revealed, and hence they acted as decoy organisms for cercariae. In C. fornicata, the extensive mucus net produced for the feeding process (Werner, Reference Werner1953) may prevent an infection by trapping and immobilizing cercariae. The oyster C. gigas does not have a foot, the preferred infection site of H. elongata cercariae (Thieltges and Reise, Reference Thieltges and Reise2006) which may hinder infection attempts. There may also be host-elaborated substances that might influence cercariae as suggested by Cheng et al. (Reference Cheng, Schuster and Anderson1966). Why Mya arenaria is not infected remains unclear but in the field infection levels with H. elongata are also extremely low (Thieltges et al. Reference Thieltges, Krakau, Andresen, Fottner and Reise2006), suggesting that it is not suitable as a host. Slipper limpets C. fornicata and oysters C. gigas are never infected by H. elongata in the field (Krakau et al. Reference Krakau, Thieltges and Reise2006; Thieltges et al. Reference Thieltges, Krakau, Andresen, Fottner and Reise2006). Hence, field data are in line with our findings and clearly suggest all three species to be non-hosts interfering with transmission.
Alternative hosts were the filter- or deposit-feeding bivalves Mytilus edulis and Macoma balthica. Although not statistically significant in the case of M. balthica, the presence of both species reduced parasite load in the cockles. Both were infected by inhalation of cercariae and, subsequently, metacercariae could be observed in their tissue. H. elongata is known to infect both species in the field, although with lower infection levels than observed in cockles (Lauckner, Reference Lauckner and Kinne1983; Thieltges et al. Reference Thieltges, Krakau, Andresen, Fottner and Reise2006).
The actual strength of the different mechanisms observed will depend on various factors. First of all, the species identity certainly matters with different ambient organisms differentially affecting cercarial transmission. The size of ambient organisms may also be important. For example, large crabs may be unable to catch small prey items like cercariae. In contrast, larger bivalve filter feeders may inhale more cercariae than smaller ones as a result of size-dependent pumping rates. Behaviour may also play a role in the case where ambient organisms are more or less active during times of high cercarial release. Another major factor is certainly the density of the ambient organisms. With increasing density the observed effects should increase too, similar to observations in freshwater parasite-host systems (Christensen, Reference Christensen1979). Hence, it is not the presence of ambient species alone but the species composition and relative abundance that determines the magnitude of interference of ambient organisms with transmission.
From the perspective of the cockle host, the type of mechanism does not matter much because all three mechanisms result in a reduction of the parasite burden. Although the ambient organisms in our experiments were not directly associated with the cockle hosts, ambient fauna within the host space generally seems to protect the down-stream hosts against parasites. In contrast, from the perspective of the parasites, the type of mechanism matters a lot. When predators and non-host filter feeders remove infective stages from the system, cercariae are lost from the local population and this may result in lower levels of parasitism, not only in second intermediate hosts but also in the subsequent final hosts. When cercariae end up in alternative hosts, the situation is different. The infective stages remain within the local system and may still allow completion of the life-cycle. It may also be that alternative hosts open additional ways of transmission to, for example, different final hosts, thus actually increasing the infection success of the parasites.
Our results demonstrate that ambient macrozoobenthic organisms within the host space may play an important role in the transmission success of trematode larvae in their down-stream hosts in benthic marine systems. Hosts usually do not occur alone, they are rather part of complex communities with often high densities of the constituting species (Nybakken, Reference Nybakken1997). The predators, non-host filter feeders and alternative hosts used in our experiments all co-occur in coastal waters of the eastern North Atlantic and the densities in the mesocosms were not artificially high (Reise et al. Reference Reise, Herre and Sturm1994; Thieltges et al. Reference Thieltges, Strasser and Reise2003; Diederich et al. Reference Diederich, Nehls, van Beusekom and Reise2005). Hence, the effects observed in our laboratory experiments are highly likely to occur in the field. It is also likely that the effects of the different macrozoobenthic organisms are additive and thus increase with the species diversity of the ambient fauna. Considering the strong effects observed in our experiments, ambient fauna may thus act as a buffer against parasite outbreaks in coastal environments. However, the strength of this effect will not so much depend on the diversity of the ambient fauna but more on the species composition and relative abundance of the ambient community. A single species with strong interference effects will certainly have a much stronger total effect on parasite transmission when occurring at high densities compared to a diverse community at very low densities. Likewise, the density of con-specifics will also be important as higher densities of con-specifics also dilute the cercarial infective stages and reduce infection levels in the down-stream hosts (Thieltges and Reise, Reference Thieltges and Reise2007). Hence, the net effect of the ambient fauna on parasite transmission will result from a complex interplay of species composition and the density of ambient organisms as well as con-specifics. In some complex communities the resulting interference effects may be of high relevance to the hosts. Ambient communities consisting of efficient interference organisms occurring at high densities should cause particularly strong effects and may significantly release host populations from parasite burden. Such a reduced exposure to pathogens could be one of the mechanisms by which complexity in biotic communities could induce community stability. This relates to findings in other systems where dilution effects due to ambient organisms depending on species identity and density have been reported (Ostfeld and Keesing, Reference Ostfeld and Keesing2000; Keesing et al. Reference Keesing, Holt and Ostfeld2006). Field experiments manipulating the species composition and density of the ambient fauna will be a promising approach to investigate the effect of ambient marine diversity on parasite transmission and to determine the magnitude of the effect in the field.
This work was supported by a fellowship to D. W. T. within the Postdoc-Programme of the German Academic Exchange Service (DAAD).