Published online by Cambridge University Press: 09 October 2003
Schistosoma haematobium and S. intercalatum readily hybridize with each other producing generations of viable hybrid offspring. Experiments were designed to investigate the infectivity and viability of the S. haematobium×S. intercalatum F1 and F2 hybrid larvae in their two intermediate snail hosts compared with the parental species. Analysis of the data obtained suggested that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid miracidia were more infective to Bulinus truncatus than to B. forskalii, and also more infective to B. truncatus compared with the parental S. haematobium miracidia. This hybrid was also observed to have a greater cercarial productivity from both intermediate hosts and these cercariae were shown to be more infectious and to have a longer longevity compared with the cercariae of S. haematobium, S. intercalatum and the S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae. The S. haematobium [female]×S. intercalatum [male ] F1 hybrid was shown not to be very successful in all stages of the investigations. The results indicate that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid may have many reproductive advantages over the reciprocal hybrid and the parental schistosome species. The significance of the results is discussed in relation to the epidemiological consequences occurring in Loum, Cameroon, and other areas where S. haematobium and S. intercalatum are sympatric and able to hybridize.
Schistosomes have a wide geographical distribution causing the most significant helminth disease of mankind (Doumenge et al. 1987). There is an extremely close host–parasite relationship between larval schistosomes and their snail hosts. One effect of this very specific association is that a population of schistosomes may be better adapted to its local race of snail than it is to other races of the same species (Rollinson & Southgate, 1985; Gandon & Michalakis, 1996; Morand, Manning & Woolhouse, 1996). This leads to segregation of populations of the parasite with reduced genetic interchange between one group and another. Therefore the distribution of the different species is dependent on the distribution of their specific intermediate snail hosts (Ratard et al. 1990).
The distribution of schistosome species in Cameroon, apart from S. intercalatum, is largely influenced by the distribution of the intermediate snail hosts whose distribution, in turn, is dependent on environmental and ecological factors. Many parasitological surveys have been reported on throughout Cameroon, mainly over the last 30 years (van Wijk, 1969 a, b; Tchuem Tchuenté et al. 1997). From these surveys it can be seen that schistosomiasis in Cameroon has undergone many changes in dynamics, prevalence and the distribution of the different species. There have also been many changes to the environmental ecology of the area due to progressive intrusion of man into the rain forests and the domestication of large areas of land. This has in turn influenced the distribution of the intermediate snail hosts, hence influencing the distribution of schistosomiasis.
The distribution and prevalence of S. intercalatum, was historically restricted to a small number of foci in the equatorial rain forest, in spite of the fact that its intermediate host, B. forskalii, is a very widely distributed planorbid. The factors effecting the distribution of S. intercalatum are not fully understood. The biology of B. forskalii, its susceptibility to infection, the environmental requirements of S. intercalatum and the low intensity of infection, may all influence the distribution of S. intercalatum. It has also been suggested that the interactions of other species of schistosome, such as S. mansoni and S. haematobium with S. intercalatum, leading to parthenogenesis or hybridization, respectively, could be causing the progressive replacement of S. intercalatum in areas of sympatry (Mutani, Christensen & Frandsen, 1985; Tchuem Tchuenté et al. 1993, 1996, 1997; Southgate, 1978; Southgate et al. 1995; Southgate, Jourdane & Tchuem Tchuenté, 1998).
There have been a number of studies concerning hybridization of schistosomes in the laboratory (Le Roux, 1954; Taylor, 1970; Wright & Southgate, 1976; Wright & Ross, 1980). S. haematobium and S. intercalatum are known to hybridize naturally and the mating behaviour between S. haematobium and S. intercalatum and their consequences have already been studied (Southgate, van Wijk & Wright, 1976; Wright & Southgate, 1976; Southgate et al. 1982; Cosgrove & Southgate, 2003). Southgate et al. (1982) showed that S. haematobium males are more successful than S. intercalatum males at pairing, and this was thought to be one possible explanation of the replacement of S. intercalatum by S. haematobium, and it has been argued that introgressive hybridization between these two species is one factor restricting the distribution of S. intercalatum in areas of sympatry (Southgate, 1978). Previous surveys in Loum over a period of about the last 25 years have suggested that S. haematobium has completely replaced S. intercalatum through introgressive hybridization, possibly associated with an interspecific competitive exclusion mechanism (Southgate et al. 1982; Tchuem Tchuenté et al. 1996). Moreover, Morand, Southgate & Jourdane (2002) showed, using a mathematical model, that the unequal sex ratios in schistosome populations in favour of the male sex, and the greater ability of male S. haematobium worms to pair with female worms than male S. intercalatum, are critical factors in driving the outcome of this interaction, and predicted that S. intercalatum would be replaced by S. haematobium and the hybrid through introgression, which recent studies have proved (Webster, Southgate & Tchuem Tchuenté, 2003).
One of the most important characteristics of the hybrids is that they are able to infect both of the parental intermediate host snails, which puts the hybrid at an advantage over the parental species. The aim of this paper was to investigate and compare several aspects of; the intermediate host infectivity, viability and compatibility of S. haematobium, S. intercalatum to B. truncatus and B. forskalii, respectively, and the S. haematobium×S. intercalatum F1 and F2 hybrids to both these intermediate hosts, and also the output, infectivity and longevity of the S. haematobium×S. intercalatum F1 hybrid, S. haematobium and S. intercalatum cercariae. The significance of the results is discussed in relation to these interactions between the species, being another factor influencing the replacement of S. intercalatum by S. haematobium.
S. haematobium was isolated from schistosome eggs filtered from 5 urine samples collected from schoolchildren from Barombi Mbo, Cameroon in 1999. The eggs were amalgamated and hatched and 50 B. wrighti were exposed in groups of 5 to 25 miracidia per group: 16 B. wrighti became infected, and female golden hamsters, Mesocricetus auratus were exposed to 200 cercariae each using the paddling method to establish the isolate in the laboratory, which was then subsequently maintained in the laboratory by passage through hamsters and B. wrighti.
S. intercalatum was isolated from 100 B. forskalii collected in 1998 from Edea, Cameroon by Dr L. A. Tchuem Tchuenté. In total, 26 snails were found to be naturally infected with S. intercalatum, and cercariae from these snails were used to establish the infection in the laboratory and the isolate was maintained by passage through mice and B. wrighti.
Snails were bred from wild-caught B. truncatus from Loum, Cameroon. Healthy snails, all of approximately the same size (~5 mm in diameter, and approximately 4 weeks old) were used.
Snails were bred from wild-caught B. forskalii from Edea, Cameroon. Healthy snails, all of approximately the same size (~8 mm in length and approximately 4 weeks old) were used. Due to the difficulties of breeding B. forskalii in the laboratory, some of the experiments are represented by fewer snails compared with the numbers of B. truncatus used.
Studies on S. haematobium and S. intercalatum and their hybrids have been carried out in the laboratory (Southgate et al. 1976). In 1999 laboratory hybridization experiments were repeated using single miracidium snail infections to produce S. haematobium [male ]×S. intercalatum [female] and S. intercalatum [male ]×S. haematobium [female] hybrids.
Single S. haematobium and S. intercalatum miracidia were obtained from laboratory animals in the first passage as above, by the technique described by Taylor (1970). Fifty B. wrighti were exposed individually to 1 S. haematobium miracidium each and 50 B. wrighti snails were exposed individually to 1 S. intercalatum miracidium each, so that each infected snail would produce single-sex cercariae. The snails were maintained in trays with ‘snail-conditioned water’ at about 25 °C. They were carefully monitored and fed dry lettuce ad libitum. At 25 days post-exposure the snails were placed into individual pots containing clean snail-conditioned water and exposed to a light source to stimulate them to liberate cercariae. After 2–3 h the pots were examined under a binocular microscope for the presence of cercariae and any snail found to be infected was isolated and given an identity number. Any snails that did not produce cercariae were examined every other day for the next 10 days to identify any additional infected snails. All infected snails were kept individually in a pot and the water changed every other day. A control animal was infected with the cercariae from each infected individual snail to facilitate identification of the sex of the cercariae by examining the adult worm at a later date. Control hamsters were used for S. haematobium and control mice were used for S. intercalatum. To produce the hybrid schistosomes, hamsters were exposed to cercariae shed from 2 known B. wrighti, one shedding S. intercalatum cercariae and the other S. haematobium cercariae. Each cross was blind, that is, at the time of the double infection the sexes of the cercariae were unknown. Approximately 100 cercariae of each species were used in each infection. After the patency date of the control infections, 60–70 days for S. haematobium and 45–50 days for S. intercalatum, the animals were culled, perfused and the worms obtained were examined under a binocular microscope to determine their sex. Thus, the sex of the cercariae being produced by each individual snail was determined, allowing identification of animals that were infected with schistosomes of different species and different sexes. For the experimental animals that had been infected with S. haematobium and S. intercalatum of different sexes, a time was selected some weeks after the patency date of both S. haematobium and S. intercalatum thereby allowing the adult worms to pair, mature and produce eggs in quantity. At this date the animals were culled and eggs from the livers were hatched to produce F1 hybrid miracidia and the hybrid parasites were passaged to produce F1 and F2 generations of all stages of the life-cycle as described by Taylor (1970). The hybrid parasites were passaged through hamsters and the intermediate molluscan hosts B. truncatus and B. forskalii and were used in the experiments below.
Experiments were designed to compare the compatibility of S. haematobium, S. intercalatum, and the S. haematobium [male ]×S. intercalatum [female] and S. haematobium [female]×S. intercalatum [male ] hybrids with their respective intermediate hosts B. truncatus and B. forskalii, and also to compare the cercarial viability and infectivity, resulting from these host–parasite interactions.
Miracidia were hatched from livers from infected laboratory animals as described by Taylor (1970). Miracidia were collected individually using a small pipette less than 60 min after they had been hatched. Snails were individually exposed to 5 miracidia each as in Table 1, in a well of a Disposo-Tray containing 1 ml of snail-conditioned water. They were left overnight in the light, at a temperature of 26–28 °C, before being transferred to polypropylene trays and fed ad libitum with lettuce. The water temperature was maintained at 26 °C for the duration of the experiment, and the snails were monitored carefully, keeping them as healthy as possible.

Miracidia were hatched from 3 animal livers for each of the snail infections A–J in Table 1 and the snails were kept and monitored in 2 batches of 50 for the B. truncatus and 2 batches of 25 for the B. forskalii.
From day 20 post-infection, each group of snails was exposed to light in 50 ml pots of snail water every second day. The water was examined using a binocular microscope for the presence of cercariae and the day post-infection when the snails were found to be shedding was recorded for each group, together with the number shedding. Non-shedding snails at the onset of patency were examined on consecutive days to identify any additional infected snails. The day post-infection when all the infected snails had started to shed was also recorded and the total number of snails that were infected per group was recorded to give a percentage infection rate. Also, the deaths of any snails from each group and the general health of the snails was recorded.
The data recorded on the patencies were analysed using a simple linear regression and the data recorded on the infectivities and deaths of the positively infected snails were statistically analysed using a generalized linear model with a binomial error distribution with logit link, to test if there were any significant differences between the parental parasites and their hybrids in the 2 different intermediate snail hosts. The differences were presented on approximate t values with 2 degrees of freedom.
At day 35 post-infection, infected snails from groups A–F were isolated and if there were more than 20 infected snails per group, 20 snails were selected at random. Each snail was kept in an individual pot containing 25 ml of clean ‘snail water’. All the snails were kept in the same area of the laboratory at the same temperature of 26 °C and received the same light intensity and periods of light and darkness (approximately 12 h each). Every 24 h the water in the pot was swirled and a 1 ml aliquot of the water was taken and put in a watch glass using a pipette. Ten drops of Lugols solution were added to the 1 ml sample and viewed under a binocular microscope. The Lugol solution kills and stains any cercariae in the water allowing the number of cercariae per ml of water to be calculated. This was repeated for each pot 3 times and the mean of the 3 counts, and hence the total number of cercariae per 25 ml sample, was calculated for each snail. This was repeated for all the snails and a mean count per snail for that 24-h period was recorded for each group. After each set of counts the water in the pots was changed so that the cercarial production of the snail host could be counted for each 24-h period. The mean daily cercarial output for each group of snails was recorded from days 35 to 59 post-infection. The snails were observed for shedding every 7 days after day 59 post-infection, until all the snails had died or they had ceased shedding.
The mean cercarial production from days 35 to 59 post-infection and the duration of cercarial production, was statistically analysed for each group of snails A–F using linear regression on log-transformed values. These values were analysed to assess the differences between the hybrid and the parental cercarial production from B. truncatus and B. forskalli.
The snail infections A–E in Table 1 were repeated, and at day 35 post-infection each group of snails was exposed to light in 50 ml pots of snail water to stimulate them to shed. Thirty min post-shedding the snails were removed from the pots leaving cercariae-infected water. Using a Pasteur pipette and binocular microscope, cercariae were individually counted into animal infection pots containing 100 ml of snail-conditioned water. Cercariae were counted into 8 pots for each snail infection A–E (200 cercariae per pot). Twenty hamsters were placed separately in 4 of the animal infection pots from each of the snail infections A–E and individually exposed, by the paddling technique to the cercariae (Table 2, A–E1). The remaining pots containing the cercariae from each of the snail infections were kept undisturbed in the snail room at 26 °C at the same light intensity for 24 h. A further 20 hamsters were each placed separately, in these remaining pots and exposed to the cercariae as described previously (Table 2, A–E2). This experiment was not carried out using the S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae shed from the B. forskalii as there was not an adequate number of cercariae produced by this infection.

Each hamster was culled 1 week after the pre-patent period of the schistosomes with which it had been infected (Table 2). The patencies of the schistosome parasites had been determined in previous experiments (personal observations). All the schistosomes were perfused and dissected from the hamster and the number of schistosomes collected from each individual hamster was counted and recorded. From this the percentage worm return from each of the infections A–E could be calculated and the data statistically tested using a binomial logit link model to compare the longevity of the hybrid cercariae with that of the parental cercariae from the respective intermediate snail hosts.
These results indicate that there are differences between the patencies of S. haematobium, S. intercalatum and the S. haematobium×S. intercalatum hybrids in the intermediate snail hosts B. truncatus and B. forskalii (Table 3).
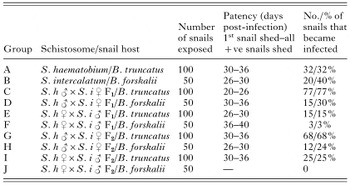
The 2 parental schistosomes, S. haematobium and S. intercalatum, readily infected B. truncatus and B. forskalii, respectively, with S. haematobium having a patency of 30–36 days in B. truncatus, which was slightly longer than that of S. intercalatum in B. forskalii, that showed a patency of 26–30 days.
There were also differences observed between the patencies of the hybrids in both the intermediate hosts and the parental species in their respective intermediate snail hosts. Both the hybrid crosses developed in both the parental intermediate snail hosts, B. truncatus and B. forskalii. The first cercarial production of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid occurred at day 20 post-infection for the B. truncatus and at day 30 post-infection in the B. forskalii. All the infected snails were found to be shedding by day 26 post-infection in the B. truncatus and by day 36 for the B. forskalii. The differences in patency of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid compared to the parental species in the respective intermediate hosts, proved to be significantly different with t=−49·70, P<0·001 for B. truncatus, and t=8·13, P=0·015 for B. forskalii.
The S. haematobium [female]×S. intercalatum [male ] F1 hybrid readily infected B. truncatus but did not infect B. forskalii quite as easily. The first cercarial production occurred at day 26 post-infection for the B. truncatus and at day 36 post-infection in the B. forskalii. All the infected snails were found to be shedding by day 30 post-infection in the B. truncatus and after day 40 for the B. forskalii. The longer patency of the S. haematobium [female]×S. intercalatum [male ] F1 hybrid compared to the parental species in the respective intermediate snail hosts, proved to be significantly different with t=−34·16, P<0·001 for B. truncatus and t=26·13, P=0·015 for B. forskalii.
These data show that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid readily infects both B. truncatus and B. forskalii, having a shorter pre-patent time in B. truncatus and longer pre-patent time in B. forskalii compared with the parental species in their respective intermediate hosts. The S. haematobium [female]×S. intercalatum [male ] F1 hybrid readily infected B. truncatus but did not so readily infect the B. forskalii, and also had a longer patency than all the other schistosome parasites in this intermediate host.
The hybrid parasites were also passaged through to the F2 generation. In this generation the S. haematobium [male ]×S. intercalatum [female] F2 hybrid was observed to still readily develop in both B. truncatus and B. forskalii, but the pre-patent periods were the same as seen with the 2 parental species in their respective hosts. The S. haematobium [female]×S. intercalatum [male ] F2 hybrid was observed to develop readily in the B. truncatus intermediate host with the same pre-patent time as that seen for S. haematobium in B. truncatus; however, this F2 hybrid did not manage to develop in B. forskalii.
It was observed that there was an increased temperature of about 3 °C in the snail room at the time of these experiments, which could have affected the patencies of the snails, nevertheless all snails were kept under the same conditions so the data could be compared between the different groups.
The results of the infectivity of the different schistosome miracidia to B. forskalii and B. truncatus are shown in Table 3.
The data show differences in the percentage of snails that became infected when exposed to the different parasites: 32% of the B. truncatus exposed to S. haematobium became infected and 40% of the B. forskalii exposed to S. intercalatum became infected. The S. haematobium×S. intercalatum hybrids were observed to be able to infect both the intermediate snail hosts of the parental schistosomes; however, the infectivities were seen to vary between the parasites and snail hosts. Overall, 77% of the B. truncatus and 30% of the B. forskalii, and 68% of the B. truncatus and 24% of the B. forskalii exposed to the S. haematobium [male ]×S. intercalatum [female] F1 and F2 hybrids respectively, became infected.
These data show that a considerably higher percentage of the B. truncatus became infected when exposed to these hybrids compared to when exposed to S. haematobium, and a considerably lower percentage of the B. forskalii became infected when exposed to these hybrids compared with when exposed to S. intercalatum. These differences proved to be statistically significant with t=6·13, P<0·001 for B. truncatus and t=−1·05, P=0·296 for B. forskalii.
For the S. haematobium [female]×S. intercalatum [male ] hybrids the infection rates of the intermediate snail hosts were observed to be considerably lower than that for the parental and the reciprocal hybrid schistosomes. Only 15% of the B. truncatus and 3% of the B. forskalii, exposed to the S. haematobium [female]×S. intercalatum [male ] F1 hybrid became infected, and there was an increase to 25% in the infectivity in the F2 generation of this hybrid to B. truncatus but no B. forskalii became infected. These differences proved to be statistically significant with t=−2·78, P=0·005 for B. truncatus and t=−3·55, P<0·001 for B. forskalii.
There was no considerable difference observed between the mortality of the B. truncatus infected with the hybrids and those infected with S. haematobium; however, it was noted that the death rate of the B. forskalii was higher when infected with the hybrids, compared to when infected by S. intercalatum. The death rates may be due to infectivity of the parasites or possibly to the condition of the snails at the time of exposure. It was observed that there was an increased temperature of about 3 °C in the snail room at the time of these experiments, which could have affected the death rates of the snails. Nevertheless, all snails were kept under the same conditions. Data of the deaths of positively infected snails up to day 60 days post-infection can be seen in Table 4. There was no statistically significant difference found between the deaths of the same species of intermediate snail hosts infected with the different parasites.

The results of the cercarial production counts are summarized in Table 5. The average number of cercariae per 25 ml of ‘snail water’ per snail was recorded every 24 h from day 35 post-infection, as at this time all the snails were patent. From this, the mean total daily and total cercarial production per snail from days 35 to 59 post-infection was calculated and statistically compared for each of the different schistosomes in the two different snail hosts. The duration of cercarial production of each parasite from the different intermediate hosts was also recorded to differ.

Except for the S. haematobium [male ]×S. intercalatum [female] F1 hybrid in B. truncatus, which continued to produce large numbers of cercariae up until 13–14 weeks post-infection, the numbers of cercariae produced by the snails after day 59 started to decline until no cercariae were produced or all the snail hosts had died.
These results indicate that the output of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae from B. truncatus was maintained for a significantly longer period (t=50·09, P<0·001) and approximately the same from B. forskalii compared to that of the parental species. The cercarial production was significantly higher from both B. truncatus and B. forskalii compared to that of the parental schistosomes in the respective snail hosts (log(1·89)=0·64, t=25·57, D.F.=38, P<0·001 for B. truncatus, and log(1·65)=0·50, t=16·0, D.F.=38, P<0·001 for B. forskalii).
These data also suggest that the output of the S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae from B. truncatus was maintained for a significantly shorter period (t=−32·78, P<0·001) compared to S. haematobium, but the duration of cercarial production could not be analysed for this hybrid from B. forskalii as all the snails died before any of them ceased shedding. The cercarial production per snail was significantly lower compared with that of the parental species in the respective snail hosts (log(0·33)=−1·12, t=−17·6, D.F.=33, P<0·001 for B. truncatus, and log(0·18)=−1·67, t=−25·27, D.F.=20, P<0·001 for B. forskalii).
This indicates that the cercarial output of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid from both B. forskalii and B. truncatus is significantly higher and maintained for a longer period compared to the output of the S. haematobium [female]×S. intercalatum [male ] F1 hybrid and the parental schistosome cercariae from these intermediate hosts, possibly placing this hybrid at a reproductive advantage.
The data obtained to compare the infectivity of S. haematobium, S. intercalatum and the S. haematobium×S. intercalatum F1 hybrid cercariae are shown in Table 6. There was a considerable difference observed in the worm return from the laboratory animals exposed to the cercariae immediately post-shedding and 24 h post-shedding for each of the schistosome parasites.

The overall worm return from the hamsters exposed to S. haematobium cercariae immediately post-shedding was 28%, which dropped to 14% for the worm return in animals exposed to the 24-h-old cercariae. There was also a decrease in worm return observed for the S. intercalatum cercariae with a worm return of 23% for cercariae that infected immediately post-shedding, and 8% for cercariae 24 h old.
The worm return from the animals infected with the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae shed from the B. truncatus immediately after shedding was high at 66% and did not change noticeably for the cercariae that were 24 h old, being 58%. The worm return was also high from the animals infected with the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae shed from the B. forskalii snails. There was a 41% return from cercariae that infected immediately post-shedding, and a reduction to 32% return from the 24-h-old cercariae. The worm return in animals infected with the S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae from B. truncatus was low, with only a 16% worm return for cercariae that were used for infections immediately post-shedding and only 1% for cercariae 24 h old.
The worm return from the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae shed from B. truncatus and B. forskalii was much higher compared with that of the parental species, and the worm return from S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae was much lower than the parental species. Thus the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae appeared to be more viable compared with the reverse cross, and had a greater infectivity and longevity than the parental species.
The results show that over 24 h the parental and the S. haematobium [female]×S. intercalatum [male ] F1 hybrid lost viability: 14% were lost for S. haematobium and 15% lost for S. intercalatum and the S. haematobium [female]×S. intercalatum [male ] F1 hybrid. However, there was no considerable loss of viability over 24 h observed for the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae shed from both the intermediate snail hosts.
These differences in the longevity of the different cercariae proved to be statistically significant with P=0·04 and P=0·015 for S. haematobium cercariae compared to the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae and the S. haematobium [female]×S. intercalatum [male ] F1 hybrid shed from the B. truncatus, respectively and P=0·008 for S. intercalatum cercariae compared to S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae shed from the B. forskalii.
Observations of the cercarial behaviour showed that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid tended to aggregate in the zone of water near the surface of the pot, which is a characteristic of S. intercalatum cercariae. The reverse cross did not show this behaviour and the cercariae were spread throughout the water.
One of the characteristics of schistosomes is their high level of intermediate host specificity (Rollinson & Southgate, 1985). Several studies have been carried out on the compatibility of different schistosome species and strains to their different intermediate molluscan hosts, to evaluate if the intermediate host–parasite relationship influences the prevalence of schistosomiasis and transmission of schistosomes (Taylor, 1970; Tchuem Tchuenté et al. 1999). Laboratory studies have shown that hybridization readily occurs between S. haematobium and S. intercalatum with viable hybrids being produced from both crosses, but the S. haematobium [male ]×S. intercalatum [female] cross is more viable (Southgate et al. 1976). The reverse cross was originally reported to be non-viable; however, more recent studies have been able to produce this cross with successful F1 and F2 generations (personal observations).
Whereas the two parental species can develop only in their specific intermediate hosts (S. haematobium, in B. truncatus and S. intercalatum in B. forskalii) the hybrids between these two schistosome species can develop in both intermediate host species (Southgate et al. 1976). The compatibility studies between the parental species and the hybrids to these intermediate hosts in this paper have produced interesting comparative results, which could be used to explain the prevalence of these schistosomes in certain areas of Africa.
The data suggest that the patency of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid is shorter in B. truncatus but slightly longer in B. forskalii compared with the parental species in their respective intermediate hosts. However, the reverse cross, S. haematobium [female]×S. intercalatum [male ] F1 hybrid had a patency longer than the more viable hybrid and S. intercalatum in B. forskalii, but was shorter to that of S. haematobium in B. truncatus. This ability to develop in both the intermediate snail hosts and the shorter patency period and hence faster reproduction rate of the S. haematobium×S. intercalatum F1 hybrid in B. truncatus could present this schistosome with a reproductive advantage over the other schistosomes in nature.
The data from the infectivity experiment indicate that the S. haematobium [male ]×S. intercalatum [female] F1 and F2 hybrid miracidia are more infective to B. truncatus than to B. forskalii. This hybrid also appears to be more compatible with B. truncatus than S. haematobium, but less compatible with B. forskalii than S. intercalatum. The S. haematobium [female]×S. intercalatum [male ] F1 hybrid miracidia showed a very low viability to both the intermediate hosts, and the F2 hybrid showed a loss of infectivity to B. forskalii compared with that of B. truncatus, and this could be evidence of some impairment of the viability of the F2 miracidia in B. forskalii.
There were also some interesting comparisons of the cercarial production of each of the schistosomes from the intermediate snail hosts. There was a considerable difference in the mean number of cercariae produced per snail that were exposed to the hybrid and the parental miracidia. The average cercarial S. haematobium [male ]×S. intercalatum [female] F1 hybrid production, per B. truncatus was over twice that of S. haematobium cercariae production from B. truncatus. There was also an increase in the average cercarial production of this hybrid per B. forskalii, compared with that of S. intercalatum from B. forskalii. The average cercarial S. haematobium [female]×S. intercalatum [male ] F1 hybrid production was very low compared with all the other schistosomes from both the intermediate snail hosts.
The length of time that the intermediate hosts kept producing cercariae was also longer for the S. haematobium [male ]×S. intercalatum [female] F1 hybrid compared with the parental species and the less viable hybrids, hence the total production of the S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae from the B. truncatus and B. forskalii in their life-time could be much higher compared with the parental schistosomes and the reciprocal hybrid. This together with the possible higher infectivity of the S. haematobium [male ]×S. intercalatum [female] hybrid to the two snail hosts could enhance the reproductive advantages of this hybrid.
The S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae show higher infectivity of the definitive hamster host giving a higher worm return compared with the parental and the S. haematobium [female]×S. intercalatum [male ] F1 hybrid cercariae. The S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae also appear to retain their infectivity for a longer period of time after shedding from the snail compared to the other schistosomes. The S. haematobium [female]×S. intercalatum [male ] F1 hybrid shows a very low worm return in the hamsters and the cercariae do not stay infective for long after shedding from the snail. No significant difference in longevity of the hybrid cercariae from the different snail hosts was observed.
In summary, the data reported in this paper suggest that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid is more viable and has a greater fitness compared to the S. haematobium [female]×S. intercalatum [male ] F1 hybrid, and the S. haematobium [male ]×S. intercalatum [female] F1 hybrid has enhanced characteristics in phases of its life-cycle which could be expressed as ‘hybrid vigour’. The reduced infectivity of the S. haematobium×S. intercalatum hybrid miracidia to B. forskalii is unexpected as usually the hybrid will inherit factors from both its parental species enabling it to be at least equally infective to both the parental intermediate snail hosts. Perhaps the most interesting feature of this hybrid infectivity is the light which it sheds on the host–parasite relationship between trematodes and their molluscan hosts (Wright, 1973). It demonstrates the inheritance of factors, which enable the hybrid schistosome larvae to develop in both species of intermediate host of the parental schistosomes. It was seen that the hybrid parasites develop more readily and are more successful in the snail hosts of S. haematobium, B. truncatus compared with B. forskalii, the intermediate host of S. intercalatum. This suggests that the hybrids are genetically close to S. haematobium perhaps increasing the chances of backcrossing with S. haematobium. Other observations, such as the aggregation of S. haematobium [male ]×S. intercalatum [female] F1 hybrid cercariae at the surface film of the water, similar to S. intercalatum cercariae, whereas the reverse cross behaves like S. haematobium cercariae, that is, more evenly distributed throughout the water, indicated that this trait may be maternally inherited. All these observations contribute towards a further understanding of the genetic inheritance of hybrids from their parental species.
S. haematobium and S. intercalatum are known to occur sympatrically in many areas of Africa, and so able to infect the same human host resulting in hybridization and production of hybrid schistosomes. In Loum, Cameroon the field evidence over a 10-year period (1968–1978) shows clearly that S. intercalatum is being replaced by S. haematobium and the hybrid (Southgate & Rollinson, 1980; Rollinson & Southgate, 1985). Although the intermediate and definitive hosts of S. intercalatum have a much wider distribution than the parasite, it has been argued that introgressive hybridization between S. intercalatum and S. haematobium could be a factor influencing the restricted distribution of S. intercalatum in Africa (Tchuem Tchuenté et al. 1997).
There is evidence of numerous occurrences of natural hybridization between S. intercalatum and S. haematobium in Cameroon, and other parts of Africa (Southgate et al. 1976; Ratard & Greer, 1991; Burchard & Kern, 1985; Zwingenberger et al. 1990). Loum, Cameroon has been a place of much interest due to its dynamics in the prevalence of schistosomiasis and more recent data based on molecular studies clearly suggest a shift from S. intercalatum to S. haematobium over the last 30 years with S. haematobium and the recombinants still present today (Webster et al. 2003).
Suggestions to find an explanation for this dramatic change in prevalence of the two species have been examined from both environmental and behavioural stand-points, in addition to genetic dynamics within populations (Southgate et al. 1976, 1982; Southgate, 1978). The parental species and the hybrids have been studied in the laboratory, and environmental changes in Loum have been reported over the last 30 years (Wright et al. 1974; Wright & Southgate, 1976; Tchuem Tchuenté et al. 1997). There are several traits that place S. haematobium with reproductive, behavioural and genetic advantages over S. intercalatum and these could afford some explanation, together with environmental factors, as to why S. haematobium is replacing S. intercalatum, leaving only S. haematobium and the recombinants in Loum, Cameroon today (Morand et al. 2002).
This paper shows that the S. haematobium [male ]×S. intercalatum [female] F1 hybrid hybrids may have many enhanced characteristics in phases of the life-cycle, such as increased infectivity of the miracidia and cercariae and increased cercarial production. A further advantage of the hybrid parasite is that it is able to develop in both the intermediate hosts of the parental species B. truncatus and B. forskalii, and the pre-patent periods are shorter than seen in the parental species (hence a potentially faster reproduction rate). Also S. haematobium and the hybrids will have some advantage over S. intercalatum due to the fact that the B. truncatus intermediate host is a considerably larger snail, hence capable of producing more cercariae than the smaller B. forskalii.
Also, in the natural situation where hybridization exists, the advantage of S. haematobium will be further emphasized because, in succeeding generations, where segregation and back-crossing will occur, there will be greater numbers of S. haematobium cercariae together with those of the hybrid and this will result in a shift in favour of S. haematobium.
In describing the focus of hybridization between S. haematobium and S. intercalatum at Loum, Cameroon, Southgate et al. (1976) drew attention to the way in which S. haematobium and the hybrid appeared to be replacing the endemic S. intercalatum since S. haematobium first appeared in Loum. It was suggested that the hybrid, in addition to its ability to use either snail host, shared with S. haematobium the advantage of urinary discharge of its eggs, thus more easily evading the basic level of sanitation in the town. To these advantages can now be added the possible greater infectivity of the hybrid for B. truncatus, the increased infectivity of the hybrid cercariae relative to either of the parental species, the rapid maturation time of the hybrid parasites both in the snails and in the definitive hamsters and potentially greater egg-production by the hybrid adults. So far the only deleterious character of the hybrid which we have been able to identify is its reduced infectivity to B. forskalii relative to that of S. intercalatum, but this is probably more than off-set by its advantages and will only serve to shift the balance between the parental species further in favour of S. haematobium. The probable outcome of this situation will be a new strain of S. haematobium in which the advantageous characteristics of the hybrid may be retained. The combination of environmental and behavioural factors could be effecting the replacement, hence restricted distribution of S. intercalatum by S. haematobium. Therefore environmental and in vivo interactions should be considered when relating laboratory observations to events occurring in the field.
The intensity and prevalence of urinary and intestinal schistosomiasis are increasing in many areas of Africa (Picquet et al. 1996). It is therefore important, to have a greater awareness of behavioural interactions between schistosome species and with their intermediate hosts through laboratory models, as they could have important consequences on the epidemiology of schistosomiasis in nature.
B. L. Webster was in receipt of a Medical Research Council Studentship. V. R. Southgate thanks The Wellcome Trust (056278/Z/98/Z/MWE/KO) for financial support. We thank Mr Mike Anderson and Miss Vivian Tuffney for their assistance throughout the studies, and Mr Clive Moncrieff for assistance with the statistical analysis of the data.

Table 1. Snail infections

Table 2. Hamster infections

Table 3. Patencies and infectivity of Schistosoma haematobium in B. truncatus, S. intercalatum in B. forskalii and the S. haematobium [male ]×S. intercalatum [female] (S. h [male ]×S. i [female]), and S. haematobium [female]×S. intercalatum [male ] (S. h [female]×S. i [male ]), F1 and F2 hybrids in Bulinus truncatus and B. forskalii

Table 4. Survial of the snails infected with the different parasites

Table 5. Mean daily cercarial output per snail, from days 35 to 59 post-infection with, Schistosoma haematobium, S. intercalatum and the S. haematobium [male ]×S. intercalatum [female] (S. h [male ]×S. i [female]) and S. haematobium [female]×S. intercalatum [male ] (S. h [female]×S. i [male ]), F1 hybrids and the duration of cercarial production

Table 6. Worm return from hamsters infected with 200 cercariae of Schistosoma haematobium, S. intercalatum, and the S. haematobium [male ]×S. intercalatum [female] (S. h [male ]×S. i [female]) and S. haematobium [female]×S. intercalatum [male ] (S. h [female]×S. i [male ]) F1 hybrids, immediately post-shedding and 24 h post-shedding from the intermediate snail hosts