Introduction
Extant plants enter into multiple types of associations with other organisms, including bacteria, viruses, algae, fungi and animals (Chapman & Good Reference Chapman and Good1983, Palukaitis et al. Reference Palukaitis, Carr and Schoelz2008, Herrera & Pellmyr Reference Herrera and Pellmyr2009, Soto et al. Reference Soto, Domínguez-Ferreras, Pérez-Mendoza, Sanjuán and Olivares2009, Southworth Reference Southworth2012). Of these, fungi are capable of profoundly affecting the plants and environments in which they occur through the formation of permanent or temporary interactions that range from mutualistic to parasitic (Redman et al. Reference Redman, Dunigan and Rodriguez2001). Moreover, fungi act as the principal decomposers of lignified plant parts (Kirk & Farrell Reference Kirk and Farrell1987). Numerous types of fungal interactions that occur with plants also exist with animals (e.g. Lawrence & Milner Reference Lawrence and Milner1996). Moreover, fungi serve as the primary food source for certain animals (Fogel & Trappe Reference Fogel and Trappe1978), and many animals, especially arthropods, are effective as vectors in the distribution of fungal spores (e.g. Bultman & Mathews Reference Bultman and Mathews1996).
Since fungal interactions are prevalent in modern ecosystems, it would be reasonable to assume that these interactions also existed in ancient ecosystems and, consequently, can be documented from the fossil record. Although fossil evidence of fungal associations and interactions is generally rather rare, the examples reported to date suggest that fungi in fact played important roles in ancient ecosystems and in the evolutionary history of life (Pirozynski & Malloch Reference Pirozynski and Malloch1975, Hawksworth Reference Hawksworth1991, Taylor et al. Reference Taylor, Krings and Taylor2015). Until relatively recently, fossil fungi found in association with other organisms have mostly been described cursorily and rarely been placed into a palaeoecological context. Today, however, the importance of fungal interactions as a driving force in modern ecosystems is widely acknowledged and, consequently, palaeobiologists take increased effort to document fossil fungal associations and interpret the roles these organisms may have played in the ecosystems in which they lived.
Plants may become preserved as fossils in a variety of modes (Schopf Reference Schopf1975), each demonstrating a different complement of information on the organism. However, only three of these preservation types can provide details of the internal organization (anatomy, histology) of a plant, i.e. petrifactions, permineralizations and, to a lesser extent, amber (Taylor et al. Reference Taylor, Taylor and Krings2009). On the other hand, compression fossils may yield information on the epidermal anatomy if preserved in fine-grained sediment. Petrifaction occurs when the cell lumina and walls are completely replaced by minerals, possibly as a weathering effect. In contrast, permineralization is a form of preservation in which minerals have replaced the internal contents of the cells, but the cell walls are not completely replaced by minerals (Williams & Crerar Reference Williams and Crerar1985, Williams et al. Reference Williams, Parks and Crerar1985). The anatomical details provided by these two modes of preservation provide an unparalleled level of resolution to study fungal interactions in the fossil record.
The co-occurrence of permineralized deposits from multiple periods of geologic time in one area is exceptionally rare. Some of the best-known examples come from the Permian, Triassic and Jurassic of the Transantarctic Mountains (Taylor et al. Reference Taylor, Taylor and Collinson1989, Taylor & Taylor Reference Taylor and Taylor1990, Bomfleur et al. Reference Bomfleur, Schöner, Schneider, Viereck, Kerp and McKellar2014b). In contrast to the harsh environment of Antarctica today, favourable climatic conditions existed during warmer periods of Earth history supporting a rich forest vegetation in South Polar latitudes (e.g. Cantrill & Poole Reference Cantrill and Poole2013). Middle Permian (Wordian) permineralized peat (i.e. a chert that formed within a peat-forming environment) occur in the Prince Charles Mountains, East Antarctica (Bennett & Taylor Reference Bennett and Taylor1972, McLoughlin et al. Reference McLoughlin, Lindström and Drinnan1997), whereas Late Permian and Middle Triassic (Anisian) permineralized peats have been reported from the central Transantarctic Mountains (Barrett Reference Barrett1969, Schopf Reference Schopf1970, Taylor et al. Reference Taylor, Taylor and Collinson1989). The Transantarctic Mountain deposits have been suggested as peat mire rafts that eroded into river channels, and once entombed in sand, silica-rich water initiated the permineralization process (Taylor et al. Reference Taylor, Taylor and Collinson1989, Collinson et al. Reference Collinson, Isbell, Elliot, Miller, Miller and Veevers1994), whereas the peats from the Prince Charles Mountains come from outcrops extending for>2 km (e.g. McLoughlin & Drinnan Reference McLoughlin and Drinnan1996). The Permian–Triassic transition marks the shift from the palaeophytic (e.g. Glossopteris-dominated) to more modern and highly diversified vegetation types resulting from the drastic change in physical environment and global temperature (Taylor et al. Reference Taylor, Taylor, Collinson and Elliot1986, Iglesias et al. Reference Iglesias, Artabe and Morel2011). Southern Gondwana experienced a generally warmer and less seasonal climate after the end-Permian event that marked the passage from a global icehouse condition in the Early Permian to the hothouse state of the Early Triassic, and eventually the greenhouse climate, which persisted throughout the Triassic (Kidder & Worsley Reference Kidder and Worsley2004, Lindström & McLoughlin Reference Lindström and McLoughlin2007, Preto et al. Reference Preto, Kustatscher and Wagnall2010, Escapa et al. Reference Escapa, Taylor, Cúneo, Bomfleur, Bergene, Serbet and Taylor2011). The vast majority of what is known about Triassic fungi and plant–fungal interactions is based on Middle Triassic permineralized peat deposits of Fremouw Peak. The initial fragmentation of Gondwana occurred during the transition from the Triassic to Jurassic, and is characterized by increased volcanic activity reflected in the geologic record (Hergt & Brauns Reference Hergt and Brauns2001, Riley et al. Reference Riley, Curtis, Leat, Watkeys, Duncan, Millar and Owens2006, Bromfield et al. Reference Bromfield, Burrett, Leslie and Meffre2007, Elliot & Fleming Reference Elliot and Fleming2008). Jurassic chert containing plant remains and fungi, as well as silicified wood, come from the Gair Mesa Range, northern Victoria Land, and the Carapace Nunatak, southern Victoria Land (Gunn & Warren Reference Gunn and Warren1962, Gair et al. Reference Gair, Norris and Ricker1965, Ballance & Watters Reference Ballance and Watters1971, Bomfleur et al. Reference Bomfleur, Schneider, Schöner, Viereck and Kerp2011, Hieger et al. Reference Hieger, Serbet, Harper, Taylor, Taylor and Gulbranson2015). During the Cretaceous, Antarctica reached the approximate geographical position that it occupies today (Lawver et al. Reference Lawver, Gahagan and Coffin1992), but was still considered a climatic greenhouse. Several sites on the Antarctic Peninsula, including Alexander Island, James Ross Island, Livingston Island, Table Nunatak and Seymour Island have yielded silicified wood (including specimens that contain fungi), as well as foliage fossils, spores and pollen grains, and flowers (Francis et al. Reference Francis, Ashworth, Cantrill, Crame, Howe, Stephens, Tosolini and Thorn2008).
Since the initial review of Antarctic fossil fungi by Taylor (Reference Taylor1990) a considerable body of new information has been amassed on the fungi occurring in the plant-bearing cherts and silicified wood from Antarctica. However, this evidence is scattered and often included in works focusing on other aspects of ancient life in Antarctica. This review critically surveys the evidence of fungi extending from the Permian to Cretaceous in chert deposits and silicified wood from Antarctica, as well as reports on certain fungal palynomorphs, dispersed remains and compression-impression fungal fossils, and is intended primarily as a tool to access the primary literature, but may also be used to define future research perspectives. It focuses on the evidence of mutualistic and parasitic plant–fungal interactions, as well as saprotrophism, but also addresses dispersed fungal remains from the matrix and examples of fungi as components of food webs (see Table I).
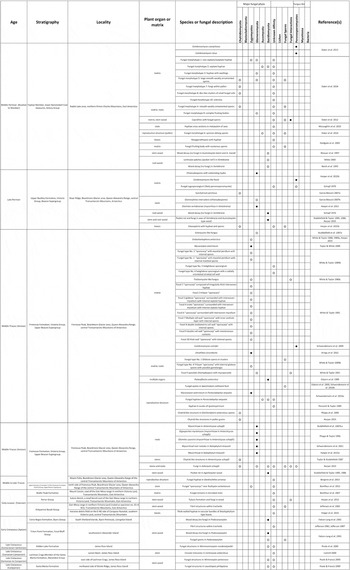
Table I Fungi and fungus-like organisms in structurally preserved plant remains and chert from the late Palaeozoic and Mesozoic of Antarctica. Solid black circles (●) indicate definitive evidence for affinities; open white circles (○) indicate putative or speculative affinity. Fungal palynomorphs and adpression records are excluded.
Mutualistic fungal interactions (mycorrhizas)
Plant growth depends on the availability of a variety of macro- and micronutrients in the rhizosphere, including phosphorous, nitrogen, potassium, copper and zinc (Marschner & Dell Reference Marschner and Dell1994, Talbot et al. Reference Talbot, Allison and Treseder2008). To increase and facilitate the uptake of some of these nutrients, most extant plants enter into one or several types of mycorrhizal associations with fungi. These associations are generally mutualistic (commonly interpreted to be beneficial for both partners), but in some cases may become weakly parasitic (Johnson et al. Reference Johnson, Graham and Smith1997, Kirk et al. Reference Kirk, Cannon, Minter and Stalpers2008). Mycorrhizal associations occur in an estimated 90% of extant plants, including bryophytes, lycophytes, pteridophytes, gymnosperms and angiosperms (Wang & Qiu Reference Wang and Qiu2006). Despite the prevalence of mycorrhizal associations today, documented fossil evidence of mycorrhizas is scarce. Mycorrhizal associations have been described in three seed plant taxa from Antarctica.
Vertebraria spp.
The Glossopteridales, an extinct group of Palaeozoic arborescent seed ferns that dominated the forest ecosystems of Antarctica during the Permian, are characterized by tongue-shaped leaves with a unique pattern of reticulate venation, stems with secondary xylem exhibiting mixed pitting (Australoxylon spp.) and an interesting type of rooting structure known as Vertebraria (Schopf Reference Schopf1970, Rigby Reference Rigby1972, Mussa Reference Mussa1978). The most distinctive features of older (woody) Vertebraria roots are radiating, wedge-shaped lacunae (Neish et al. Reference Neish, Drinnan and Cantrill1993, Decombeix et al. Reference Decombeix, Taylor and Taylor2009). In contrast, young rootlets lack lacunae. They consist of a central vascular strand surrounded by a parenchymatous cortex and the rhizodermis. Evidence of a mycorrhiza involving a glomeromycotan fungus (Glomites vertebrariae C.J. Harper, T.N. Taylor, M. Krings et E.L. Taylor) occurs in small Vertebraria rootlets (0.3–1 mm in diameter) from Skaar Ridge in the central Transantarctic Mountains (Fig. 1a). The fungus is characterized by intracellular septate hyphae that extend through a discrete zone of the cortex 2–3 cell layers below the rhizoepidermis. Arbuscules range from serpentine to coiled and are morphologically similar to extant Paris-type mycorrhizas (Harper et al. Reference Harper, Taylor, Krings and Taylor2013). Additional structural features of the fungal partner occur in the form of globose–ellipsoid vesicles that lack a septum at the hyphal attachment. The Vertebraria–G. vertebrariae association represents the only example to date of a mycorrhiza in seed ferns, and the oldest fossil evidence of Paris-type morphology in mycorrhizal fungi.
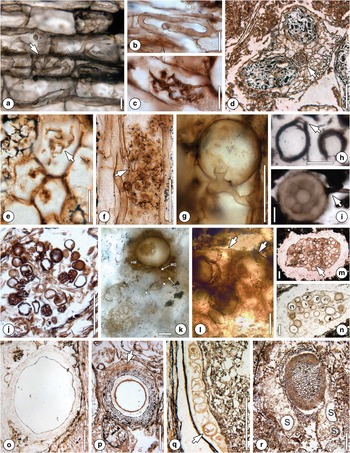
Fig. 1 Mycorrhizal associations, complementary evidence of mycorrhizal fungi (additional information in the text). a. Cortical cells of Vertebraria sp. containing Paris-type morphology of mycorrhizal hyphae with hyphal knob (arrow). Scale bar=25 µm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2013: Pl. II, 2. b. Coiled Gigasporites myriamyces hyphae in Antarcticycas schopfii. Scale bar=25 µm. Originally illustrated in Phipps & Taylor Reference Phipps and Taylor1996: fig. 3. c. Glomites cycestris arbuscule. Scale bar=25 µm. Originally illustrated in Phipps & Taylor Reference Phipps and Taylor1996: fig. 15. d. Two mature root nodules (N) with small vascular cylinder (arrow) of Notophytum krauselii. University of Kansas Division of Paleobotany (KUPB) specimen 11277 B2 side bot #18. Scale bar=200 µm. e. Putative arbuscules (arrow) in cortex of N. krauselii root nodule. Scale bar=30 µm. Originally illustrated in Schwendemann et al. Reference Schwendemann, Decombeix, Taylor, Taylor and Krings2011: fig. 1F. f. Arbuscule in cortical cell of non-nodule forming N. krauselii rootlet showing attachment to trunk hypha (arrow). Scale bar=25 μm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2015a: Pl. II, 9. g. Spherical vesicle (V) in non-nodule forming N. krauselii rootlet. Scale bar=25 µm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2015a: Pl. II, 12. h. Terminal multi-layered (arrow) chlamydospore in peat matrix. Scale bar=25 µm. Originally illustrated in García Massini Reference Garcia Massini2007a: fig. 1G. i. Asexual spore with subtending hypha (arrow); note internal contents. Scale bar=25 µm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2015b: Pl. 1, 13. j. Sclerocystis-like fungal fossil. Scale bar=15 µm. Originally illustrated in Stubblefield et al. Reference Stubblefield, Taylor and Seymour1987a: fig. 1. k. Jimwhitea circumtecta (Endogonaceae) holotype. Zygosporangium (Z) surrounded by hyphal mantle (HM). Sporangium arises from megagametangium (MG) subtended by megasuspensor (MS). Laterally attached to megagametangium is microgametangium (mG) subtended by microsuspensor (mS). The entire complex appears to have developed from loose network of hyphae (G). Scale bar=20 µm. Originally illustrated in Krings et al. Reference Krings, Taylor, Dotzler and Persichini2012: figs 1A & 3. l. Portion of sporocarp attributed to J. circumtecta. Arrows indicate narrow peridium. Scale bar=50 µm. Originally illustrated in Krings et al. Reference Krings, Taylor, Dotzler and Persichini2012: fig. 2A. m. ‘Fungus no. 2’. Cluster of spores enveloped in massive peridium. Scale bar=100 µm. Originally illustrated in White & Taylor 1989: Pl. I, 3. n. ‘Fungus no. 3’. Mantled spores surrounded by mycelial peridium. Scale bar=100 µm. Originally illustrated in White & Taylor 1989: Pl. II, 1. o. Endochaetophora antarctica showing interwoven hyphal meshwork surrounding central cavity. Scale bar=250 µm. Originally illustrated in White & Taylor Reference White and Taylor1989a: Pl. I, 4. p. Mycocarpon asterineum spheroidal ‘sporocarp’ with empty cavity, acellular investment layer, surrounded by loosely interwoven mycelial investment (arrow). Scale bar=100 µm. Originally illustrated in White & Taylor Reference White and Taylor1991: Pl. I, 3. q. Mycocarpon asterineum (arrow) in Parasciadopitys aequata. Scale bar=100 μm. Originally illustrated in Schwendemann et al. Reference Schwendemann, Taylor, Taylor and Krings2010a: fig. 4D. r. Hyphal network containing what appear to be ‘sporocarps’ (S) enveloping a petriellalean stem (P). Scale bar=250 µm. Originally illustrated in Bomfleur et al. Reference Bomfleur, Decombeix, Schwendemann, Escapa, Taylor, Taylor and McLoughlin2014a: fig. 4J.
Antarcticycas schopfii
Cycads are an ancient group of seed plants that are interpreted to have originated during the late Palaeozoic (Norstog & Nicholls Reference Norstog and Nicholls1997, Pant Reference Pant2002). From the Middle Triassic Fremouw Peak locality, the cycad Antarcticycas schopfii E.L. Smoot, T.N. Taylor et T. Delevoryas has been reconstructed based on structurally preserved stems (Smoot et al. Reference Smoot, Taylor and Delevoryas1985), cataphylls, petiole bases, leaves (Yelchophyllum omegapetiolaris E. Hermsen, T.N. Taylor, E.L. Taylor et D.W. Stevenson), stems, microstrobili (Delemaya spinulosa S.D. Klavins, E.L. Taylor, M Krings et T.N. Taylor) and roots (Stubblefield et al. Reference Stubblefield, Taylor and Trappe1987b, Reference Stubblefield, Taylor and Trappe1987c, Phipps & Taylor Reference Phipps and Taylor1996, Klavins et al. Reference Klavins, Taylor, Krings and Taylor2003, Hermsen et al. Reference Hermsen, Taylor and Taylor2009). Small rootlets of A. schopfii are typically radial and diarch; surrounding the vascular cylinder is a band of cells filled with a dark ergastic substance bounded on the outside by the endodermis and at the periphery a parenchymatous cortex (Smoot et al. Reference Smoot, Taylor and Delevoryas1985). Many rootlets are colonized by two distinct types of glomeromycotan fungi, Gigasporites myriamyces C.J. Phipps et T.N. Taylor and Glomites cycestris C.J. Phipps et T.N. Taylor, that form mycorrhizal associations with A. schopfii (Phipps & Taylor Reference Phipps and Taylor1996). Both fungi produce inter- and intracellular hyphae, arbuscules (one per host cell), and ovoid to ellipsoidal vesicles. However, G. myriamyces is distinguished from G. cycestris by hyphal loops and coils that commonly branch dichotomously (Fig. 1b), in some cases trichotomously, and coarse, robust arbuscules that branch twice. Conversely, G. cycestris hyphae are typically straight (sporadically sinuous), branch dichotomously and produce delicate arbuscules that terminate in segments < 1 µm in diameter (Fig. 1c). These two distinct mycorrhizas in A. schopfii indicate that vesicular arbuscular mycorrhizal associations represent an ancient relationship in the cycad lineage.
Notophytum krauselii
A second example of the co-occurrence of two mycorrhizal fungi in a gymnosperm from the Triassic of Antarctica is Notophytum krauselii B. Meyer-Berthaud et T.N. Taylor, a voltzialean conifer (Meyer-Berthaud & Taylor Reference Meyer-Berthaud and Taylor1991, Axsmith et al. Reference Axsmith, Taylor and Taylor1998, Bomfleur et al. Reference Bomfleur, Decombeix, Escapa, Schwendemann and Axsmith2013). The Voltziales are a morphologically heterogeneous group of conifers considered to be transitional between the Palaeozoic Cordaitales and the modern conifers (Florin Reference Florin1951, Hernández-Castillo et al. Reference Hernández-Castillo, Rothwell and Mapes2001, Rothwell et al. Reference Rothwell, Mapes and Hernández-Castillo2005). Schwendemann et al. (Reference Schwendemann, Decombeix, Taylor, Taylor and Krings2011) describe rootlets of N. krauselii that are characterized by paired prolate spheroidal structures interpreted as root nodules (Fig. 1d). In the cortex of the nodules are mycorrhizal fungi comprised of hyphae, vesicles, intracellular hyphal coils and arbuscules (Fig. 1e). Fungal hyphae are also present on the surface of the nodules, and dispersed glomoid spores occur scattered throughout the matrix surrounding the nodules. More recently, Harper et al. (Reference Harper, Taylor, Krings and Taylor2015a) reported a different mycorrhiza that also occurs in young N. krauselii rootlets. These latter rootlets resemble the nodule-bearing rootlets regarding internal organization but lack nodules. The mycorrhizal fungus is restricted to the outer root cortex and characterized by septate hyphae, multi-branched arbuscules (Fig. 1f) and vesicles (Fig. 1g). The occurrence of mycorrhizal fungi in two different types of N. krauselii roots may suggest that this plant entered into several types of mycorrhizal associations simultaneously, perhaps at different soil levels or in different regions of the rooting system, or was able to switch between different mycorrhizal associations, a condition that also occurs in some extant plants (e.g. Demchenko et al. Reference Demchenko, Winzer, Stougaard, Parniske and Pawlowski2004). Another report of putative root nodules in fossil gymnosperms from Antarctica comes from the Upper Cretaceous (Albian) of Alexander Island. Compression fossils of small conifer rootlets show numerous swellings that are morphologically similar to extant podocarp root nodules (see Pl. 7, fig. b of Cantrill & Falcon-Lang Reference Cantrill and Falcon-Lang2001). Mycorrhizal fungi may have helped the Mesozoic gymnosperms of Antarctica to function in adverse environmental conditions, and it will be interesting to see if other groups of plants growing in the polar forest palaeoecosystems also entered into complex mutualistic associations with fungi.
Complementary evidence of mycorrhizal fungi
Fossil mycorrhizas are ideally documented from evidence showing both partners together. However, most evidence of fossil Glomeromycota and other possibly mycorrhizal fungi from Antarctica does not occur in situ, but rather dispersed within litter or the peat matrix. For example, a cluster of terminal and intercalary chlamydospores, formally described as Glomorphites intercalaris García Mass. (Fig. 1h), occurs within highly degraded plant material from the Permian Skaar Ridge locality (García Massini Reference Garcia Massini2007a). The affinities of these spores with the Glomeromycota were suggested based on morphological similarities to extant Glomus. Dispersed (chlamydo-)spores have also been reported (Fig. 1i) from Skaar Ridge (Harper et al. Reference Harper, Taylor, Krings and Taylor2015b). Many of these spores are close to Vertebraria rootlets in the matrix, thus suggesting that they might belong to G. vertebrariae (see above). Still other (chlamydo-)spores, some containing evidence of intrusive microfungi, with possible affinities to the Glomeromycota come from the Triassic peat at the Fremouw Peak site (White & Taylor Reference White and Taylor1989b, Reference White and Taylor1991). Some of these scattered fungal remains are similar to extant forms and have been directly compared to modern genera. Stubblefield et al. (Reference Stubblefield, Taylor and Seymour1987a) report one example of a peculiar fossil interpreted as a Sclerocystis-like spore cluster (Fig. 1j). However, modern Sclerocystis forms spores in sporocarps bounded by a massive peridium (Yao et al. Reference Yao, Pegler and Young1996). The absence of a peridium argues against affinities of the fossil to Sclerocystis. Rather, several extant glomoid species are known to produce spore clusters not enveloped in a peridium, e.g. Funneliformis badium (Oehl, D. Redecker et Sieverd.) C. Walker et A. Schüßler, Glomus fuegianum (Speg.) Trappe & Gerd. and Glomus rubiforme (Gerd. & Trappe) R.T. Almeida et N.C. Schenck (Godfrey Reference Godfrey1957, Gerdemann & Trappe Reference Gerdemann and Trappe1974, Almeida & Schenck Reference Almeida and Schenck1990, Oehl et al. Reference Oehl, Redecker and Sieverding2005), that closely resemble the fossil. An alternative hypothesis is that early in the evolution of Sclerocystis, spores were produced in clusters that lacked a peridium.
Besides Glomeromycota, it is known that several members of the Endogonales (Mucoromycotina) also enter into mycorrhizal associations with plants (Morton Reference Morton1990, Walker & Trappe Reference Walker and Trappe1993, Read et al. Reference Read, Duckett, Francis, Ligrone and Russell2000). However, compelling evidence of fossil endogonalean mycorrhizas has not been discovered to date (but see Strullu-Derrien et al. Reference Strullu-Derrien, Kenrick, Pressel, Duckett, Rioult and Strullu2014). Several (putative) endogonalean fungi have been reported from the Triassic peats of Antarctica but none are closely associated with a plant. The most significant of these fossils is perhaps Jimwhitea circumtecta M. Krings, T.N. Taylor, N. Dotzler et G. Persichini (Krings et al. Reference Krings, Taylor, Dotzler and Persichini2012), a structure interpreted as a zygosporangium-apposed gametangia complex resembling extant Endogone (Fig. 1k). Moreover, a sporocarp containing J. circumtecta-like structures suggests that the zygosporangia were produced within a sporocarp (Fig. 1l). There are two other examples of sporocarps, informally named fungus no. 2 and fungus no. 3, in the Triassic peats (White & Taylor Reference White and Taylor1989b). These sporocarps are bounded by a massive mycelial peridium and contain numerous ‘spores’. The spores of fungus no. 2 (Fig. 1m) are thin-walled and some show what appear to be inflated subtending hyphae or gametangia. The spores of fungus no. 3 are characterized by a hyphal mantle and contain opaque matter (Fig. 1n).
Two types of enigmatic fungal fossils, Endochaetophora antarctica J.F. White et T.N. Taylor (White & Taylor Reference White and Taylor1988, Reference White and Taylor1989a) and Mycocarpon asterineum T.N. Taylor et J.F. White (Taylor & White Reference Taylor and White1989), have also been described from the Triassic of Antarctica. Both structures consist of a central cavity enveloped in a hyphal investment, but differ in the organization of the investment. Together with similar structures from the Devonian and Carboniferous (e.g. Krings et al. Reference Krings, Taylor, Taylor, Kerp and Dotzler2014), these two Triassic fossils have collectively been termed fungal ‘sporocarps’ (see Krings et al. Reference Krings, Taylor and White2011). ‘Sporocarps’ have variously been hypothesized as representing ascomycete cleistothecia, mucoromycotinan zygosporangia or glomeromycotan spores, but structural features that could be used to determine their affinities have not yet been documented (Taylor et al. Reference Taylor, Krings and Taylor2015).
Endochaetophora antarctica is characterized by a three-layered investment, with a non-hyphal middle layer that developed secondarily after the outer and inner layers had been established (White & Taylor Reference White and Taylor1988). In addition, numerous appendages extend from the inner layer to the outside of the structure, and a distinct opening is present in some of the specimens (Fig. 1o). Taylor & White (Reference Taylor and White1989) reconstructed several developmental stages in E. antarctica, and discussed the ecological role of this fungus as a saprotrophic organism in the peat. Mycocarpon asterineum is morphologically similar to several Carboniferous representatives of Mycocarpon (Hutchinson Reference Hutchinson1955), but differs from the latter regarding the investment, which is composed of an outer hyphal and inner non-hyphal component in M. asterineum (Fig. 1p). Other specimens of M. asterineum have been described as endophytes in a Parasciadopitys aequata X. Yao, T.N. Taylor et E.L. Taylor (voltzialean conifer) seed (Fig. 1q; Schwendemann et al. Reference Schwendemann, Taylor, Taylor and Krings2010a).
In another comprehensive study, White & Taylor (Reference White and Taylor1991) describe several examples of what appear to be fungal ‘sporocarps’ of uncertain systematic affinities, some of which are enveloped in hyphal investments and contain varying numbers of spores. Finally, several specimens of an interesting ‘sporocarp’ occur embedded in a confluent mycelial meshwork surrounding a specimen of the petriellalean stem Rudixylon serbetianum B. Bomfleur, A.-L. Decombeix, A.B. Schwendemann, I.H. Escapa, E.L. Taylor, T.N. Taylor et S. McLoughlin (see fig. 4J of Bomfleur et al. Reference Bomfleur, Decombeix, Schwendemann, Escapa, Taylor, Taylor and McLoughlin2014a). Although the authors did not describe the fungus in their study, it appears to be morphologically similar to other Triassic ‘sporocarps’, especially Endochaetophora (Fig. 1r).
Parasitism
Parasitism is a nutritional mode in which one organism derives nutrients from another organism, typically at the other organism’s expense (Zelmer Reference Zelmer1998). Parasitism in fossils can be recognized through the presence of disease symptoms, some type of host response such as cell and tissue alteration, or local necroses (e.g. Mendgen et al. Reference Mendgen, Hahn and Deising1996, Pearce Reference Pearce1996). However, not all parasites elicit host responses, and thus it may be difficult to determine the nutritional modes of asymptomatic fossil fungi associated with intact host tissue. Moreover, many of the host responses known in extant plants (e.g. chemical responses; Swain Reference Swain1977) are not identifiable in fossils or are easily mistaken for natural degradation activities (e.g. necroses; Van Loon et al. Reference Van Loon, Rep and Pieterse2006).
A possible parasitic fungus and host response in the form of tyloses occurs in silicified conifer wood from the Jurassic of Antarctica (Harper et al. Reference Harper, Bomfleur, Decombeix, Taylor, Taylor and Krings2012). Extensive hyphal proliferation is visible throughout the wood, with numerous tyloses that extend into the tracheids (Fig. 2a). Harper et al. (Reference Harper, Bomfleur, Decombeix, Taylor, Taylor and Krings2012) demonstrate that the fungus penetrates the tyloses to form coils, and subsequently exits the tyloses to spread out within the tracheid lumen; however, other hyphae do not penetrate but rather grow around the tyloses. Tyloses, some of which are associated with fungi, have also been documented in Permian Australoxylon mondii L. Weaver, S. McLoughlin et A.N. Drinnan wood from the Prince Charles Mountains (see figs 7a–f of Weaver et al. Reference Weaver, McLoughlin and Drinnan1997). These authors comment that it is not possible to generalize about the cause of tylosis formation, but it is possible to suggest that tylosis formation and fungal decay are related because of the highly degraded nature and reported fungal decay in the wood.
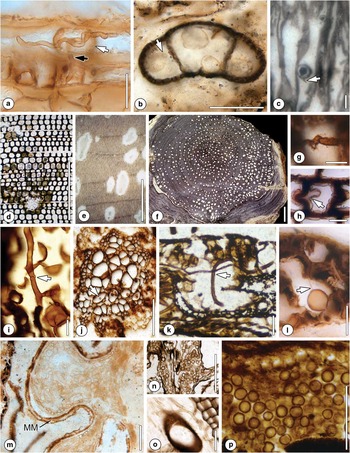
Fig. 2 Parasitic and saprotrophic fungi (additional information in the text). a. Hypha (black arrow) penetrating tylosis wall (white arrow). University of Kansas Division of Paleobotany (KUPB) slide TS-GIX-SB-036-01. Scale bar=25 μm. b. Chytrid-like organisms with pore (arrow) in pollen grain corpus and sacci. KUPB slide 26590. Scale bar=25 µm. c. Putative endoparasitic chytrid Synchytrium permicus. Arrow indicates possible discharge papilla. Scale bar=10 μm. Originally illustrated in García Massini Reference Garcia Massini2007b: figs 1–4. d. Transverse section of Australoxylon wood showing decay cavity surrounded by damaged tracheids. Scale bar=500 µm. Originally illustrated in Weaver et al. Reference Weaver, McLoughlin and Drinnan1997: fig. 9A. Courtesy of S. McLoughlin. e. Diffuse decay cavities in Australoxylon. Scale bar=5 mm. Originally illustrated in Weaver et al. Reference Weaver, McLoughlin and Drinnan1997: fig. 11A. Courtesy of S. McLoughlin. f. Cross section of Araucarioxylon axis showing pockets of decay. Arrow indicates pocket within a single growth ring. Scale bar=2 cm. Originally illustrated in Stubblefield & Taylor Reference Stubblefield and Taylor1986: fig. 1. g. Simple-intermediate medallion clamp connection in Vertebraria root wood. Scale bar=10 μm. h. Swollen cell wall pinching into cell lumen (arrow) in Vertebraria root wood. Scale bar=10 μm. i. Vegetative and reproductive features of Palaeofibulus antarctica with partially developed clamp connection (arrow). Scale bar=20 µm. Originally illustrated in Osborn et al. Reference Osborn, Taylor and White1989: fig. 5. j. Fungal hyphae (arrow) penetrating Paurodendron tracheids. Scale bar=100 µm. Originally illustrated in McLoughlin et al. Reference McLoughlin, Drinnan, Slater and Hilton2015: Pl. II, 7. Courtesy of S. McLoughlin. k. Hyphae (arrow) ramifying through mesophyll of Noeggerathiopsis leaf. Scale bar=100 µm. Originally illustrated in Holdgate et al. Reference Holdgate, McLoughlin, Drinnan, Finkelman, Willett and Chiehowsky2005: fig. 14i. Courtesy of S. McLoughlin. l. Degraded mesophyll containing chytrid-like organism showing minute hyphal attachment (arrow). Scale bar=10 μm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2015b: Pl. 1, 3. m. Fungus in megaspore membrane (MM) of Dordrechtites arcanus; note hyphae in multiple orientations. Scale bar=50 µm. Originally illustrated in Bergene et al. Reference Bergene, Taylor and Taylor2013: fig. 6E. n. Small fungal spores. Scale bar=500 μm. Originally illustrated in Slater et al. Reference Slater, McLoughlin and Hilton2015: fig. 6D. Courtesy of S. McLoughlin and B.J. Slater. o. Septate fungal hyphae penetrating Vertebraria root cells, Scale bar=100 μm. Originally illustrated in Slater et al. Reference Slater, McLoughlin and Hilton2015: fig. 6F. Courtesy of S. McLoughlin and B.J. Slater. p. Fungal fruiting body containing smooth-walled spores. Scale bar=100 µm. Originally illustrated in Holdgate et al. Reference Holdgate, McLoughlin, Drinnan, Finkelman, Willett and Chiehowsky2005: fig. 14i. Courtesy of S. McLoughlin.
Resting spores, sporangia and rhizoids of what appear to be parasitic chytrid-like organisms have been documented in the stem parenchyma of the Triassic cycad A. schopfii (see figs 3 & 4 of Taylor & Stubblefield Reference Taylor and Stubblefield1987). Moreover, many sporangia of the fern Gleichenipteris antarcticus C.J. Phipps, B.J. Axsmith, T.N. Taylor et E.L. Taylor from Fremouw Peak contain spherical bodies up to 30 µm in diameter, some of which are characterized by tubular projections extending from the surface, while others show a single orifice or pore in the wall (see Pl. 2, figs 6 & 7 of Phipps et al. Reference Phipps, Axsmith, Taylor and Taylor2000). These authors suggest that the bodies are morphologically similar to certain extant members in the Chytridiomycota that parasite spores and pollen grains. In another study, Slater et al. (Reference Slater, McLoughlin and Hilton2015) distinguish ten different fungal morphotypes from the Prince Charles Mountains. Among these morphotypes is one that occurs in association with pollen grains and is interpreted as evidence of a parasitic or saprotrophic chytrid. Similar associations of chytrid-like fungi in pollen grains have been documented from the Triassic Fremouw Peak locality (Fig. 2b; Harper Reference Harper2015).
Finally, several developmental stages of Synchytrium permicus García Mass., interpreted as a Synchytrium-like fossil member of the Chytridiomycota, have been reported in plant remains from the Skaar Ridge locality (García Massini Reference Garcia Massini2007b; Fig. 2c). Synchytrium permicus is interpreted as a parasite based on what appear to be hypertrophic host responses in plant roots, leaves and stems where the fungus occurs.
Saprotrophism
The most important ecological role of fungi today is the decomposition of organic material (Cooke & Rayner Reference Cooke and Rayner1984). Saprotrophic fungi are difficult to recognize as fossils, however, because there are no consistent structural features characteristic of this nutritional mode. Silicified wood is probably the only type of plant fossil in which the activities of fungi can be preserved faithfully enough to reveal stages in the degradation process (Stubblefield & Taylor Reference Stubblefield and Taylor1985, Reference Stubblefield and Taylor1986, Stubblefield et al. Reference Stubblefield, Taylor and Beck1985). Historically, there are three principal types of fungal decay or wood rot: white, brown and soft rot. The delineating structural characteristics of these rot types include the type of wood being degraded, pattern of degradation, presence or absence of certain cell wall components in the degraded wood and the systematic affinities of the fungus involved (Schwarze et al. Reference Schwarze, Engels, Mattheck and Linnard2000, Stokland et al. Reference Stokland, Siitonen and Jonsson2012).
A specimen of Australoxylon bainii L. Weaver, S. McLoughlin et A.N. Drinnan, a wood type attributed to the Glossopteridales (Merlotti & Kurzawe Reference Merlotti and Kurzawe2006), from the Permian Prince Charles Mountains exhibits irregular cavities that lack definitive margins (Fig. 2d), as well as gradational decay patterns extending into the surrounding xylem, tracheids with small holes in the cell walls near the edges of the cavities, and characteristic cell wall appositions (Weaver et al. Reference Weaver, McLoughlin and Drinnan1997). The causative agent for these alterations was not determined, but fungal decay was suggested. However, filamentous structures do occur within tracheid lumina and may represent simple hyphae (see fig. 11g & h of Weaver et al. Reference Weaver, McLoughlin and Drinnan1997). Another specimen of Australoxylon from the same locality exhibits decay symptoms in the form of spindle-shaped cavities that occur both within individual growth rings and across growth ring boundaries. The cavities are restricted to specific regions of the wood or randomly dispersed throughout the wood (Fig. 2e; Weaver et al. Reference Weaver, McLoughlin and Drinnan1997). As in A. bainii, appositions may be present. Finally, White (Reference White1969) reports axes of silicified Permian wood with lenticular patches (see figs 5 & 6 of White Reference White1969) that superficially resemble the spindle-shaped cavities produced by certain white pocket rot fungi.
White pocket rot and white rot fungi also occur in Triassic Agathoxylon (formerly Araucarioxylon; see Rössler et al. Reference Rössler, Philippe and van Konijnenburg-van Cittert2014), a conifer-type stem or branch wood (see Seward & Ford Reference Seward and Ford1906), and Permian Vertebraria (Glossopteridales; see above) root wood from Antarctica (Stubblefield & Taylor Reference Stubblefield and Taylor1985, Reference Stubblefield and Taylor1986). Specimens of Agathoxylon display a range of different decay pocket distribution patterns when viewed in transverse section, including pockets restricted to individual growth rings or specific areas of the wood, and others in which the pockets cross growth ring boundaries (Fig. 2f). These distribution patterns can be used to infer details about the types of fungi involved and the timing of fungal colonization (Ander & Eriksson Reference Ander and Eriksson1977, Blanchette Reference Blanchette1984). In contrast to the Australoxylon material described by Weaver et al. (Reference Weaver, McLoughlin and Drinnan1997), several of the Agathoxylon specimens display not only decay symptoms but also contain remains of the fungi involved in the decay process, including branched septate hyphae in the pockets, tracheids and ray parenchyma (Eriksson et al. Reference Eriksson, Blanchette and Ander1990). Some hyphae possess clamp connections, which are diagnostic of fungi included in the Basidiomycota. Detailed analyses of cell wall layer degradation (Stubblefield & Taylor Reference Stubblefield and Taylor1986, Harper Reference Harper2015) indicate that the secondary walls of the tracheids are first degraded along the long axis resulting in a collenchyma-like pattern, with intact cell walls only remaining in the corner areas between adjacent cells. Subsequently, the cell wall material from the corner areas is also degraded, resulting in only the middle lamella remaining. White pocket rot in woody Vertebraria roots has initially been documented based on poorly preserved hyphae that show little detail, and irregularly shaped pockets (Schopf Reference Schopf1970, Stubblefield & Taylor Reference Stubblefield and Taylor1985, Reference Stubblefield and Taylor1986, Neish et al. Reference Neish, Drinnan and Cantrill1993). However, more recent studies indicate that the hyphae are septate and form multiple types of clamp connections (Fig. 2g); host responses (Fig. 2h) and arthropod coprolites were also documented in this wood (Harper Reference Harper2015).
Fungal decay has also been reported in Early Jurassic wood from the Kirkpatrick Basalt Group in the Mesa Range area of northern Victoria Land (Jefferson et al. Reference Jefferson, Siders and Haban1983). Silica was deposited onto helical fibrillar structures within the cell walls (see fig. 2d of Jefferson et al. Reference Jefferson, Siders and Haban1983) and these were interpreted as a result of early fungal delignification. Moreover, Jefferson (Reference Jefferson1982) reports fungal colonization in a wood specimen from the Lower Cretaceous Fossil Bluff Formation on Alexander Island (see Pl. 67, fig. 6; Pl. 68 all figs of Jefferson Reference Jefferson1982). Other examples of fungi in Cretaceous wood from Alexander Island include hyphae and structures interpreted as fungal spores (see Pl. 28, figs 12–13 of Jefferson Reference Jefferson1987). In addition, Falcon-Lang et al. (Reference Falcon-Lang, Cantrill and Nichols2001) found large, spindle-shaped cavities that are restricted to the latewood in several wood specimens from Alexander Island (see fig. 6d of Falcon-Lang et al. Reference Falcon-Lang, Cantrill and Nichols2001) and abundant hyphae in tracheids (Falcon-Lang & Cantrill Reference Falcon-Lang and Cantrill2001). The specimens also contain oval fungal structures (9–12 by 3–15 µm), which the authors compare to modern basidiospores or teliospores. They hypothesize that the restricted occurrence of the rot symptoms in latewood might be related to the trees’ defence mechanisms being particularly vulnerable during the low light levels of late autumn or dark winter. Another example of fungal rot restricted within tree rings is seen in a Podocarpoxylon specimen from the Lower Cretaceous (Aptian) Cerro Negro Formation of Livingston Island (see fig. 5h & i of Falcon-Lang & Cantrill Reference Falcon-Lang and Cantrill2002). The wood axial parenchyma is reported to contain abundant fungal hyphae. Similar to the woods from Alexander Island, the presence of rots within growth increments, followed by distorted tissue zones, has been used to imply that rotting occurred during the growing season.
Several specimens of Maastrichtian (Late Cretaceous) silicified sapwood from Vega and Seymour islands in the Antarctic Peninsula area contain abundant fungal hyphae associated with tracheids, vessels and ray parenchyma (Poole & Cantrill Reference Poole and Cantrill2006). These authors speculate that the prevailing moist conditions may have encouraged fungal growth within the wood debris on the forest floor. Finally, there are several reports of angiosperm wood containing spherical inclusions and filaments that possibly represent fungi, including the xylotaxa Laureliopsis philippiana (Looser) Schodde (southern sassafras; see Pl. I, figs 2 & 3 of Poole & Francis Reference Poole and Francis1999), Winteroxylon jamesrossii I. Poole et J. Francis (see fig. 2 of Poole & Francis Reference Poole and Francis2000) and Weinmannioxylon nordenskjoeldii I. Poole, D.J. Cantrill, P. Hayes et J. Francis (see Pl. 1, fig. 1 of Poole et al. Reference Poole, Cantrill, Hayes and Francis2000).
An example of another possible saprotrophic fungus occurs in Spaciinodum collinsonii J.M. Osborn et T.N. Taylor, a Triassic sphenophyte from Fremouw Peak (Osborn et al. Reference Osborn, Taylor and White1989, Reference Osborn, Phipps, Taylor and Taylor2000). Apices of this plant may contain numerous minute structures that were initially interpreted as spores of S. collinsonii (Osborn et al. Reference Osborn, Phipps, Taylor and Taylor2000), but later reinterpreted as the spores produced by a saprotrophic or parasitic fungus (Schwendemann et al. Reference Schwendemann, Taylor, Taylor, Krings and Osborn2010b). Co-occurring with S. collinsonii in some peat blocks is Palaeofibulus antarctica J.M. Osborn, T.N. Taylor et J.F. White, a fungus with probable affinities to the Basidiomycota that produced large spherical spores (Fig. 2i; Osborn et al. Reference Osborn, Taylor and White1989). However, a physical connection between P. antarctica and the fungal spores in S. collinsonii has not been demonstrated to date.
Fungal remains of uncertain affinities and nutritional mode
The vast majority of fungal remains in the fossil record occur as dispersed units and fragments lacking definitive features that could be used to determine their systematic affinities and nutritional mode (Taylor et al. Reference Taylor, Krings and Taylor2015). For example, axes of the lycophyte Paurodendron stellatum S. McLoughlin, A.N. Drinnan, B.J. Slater et J. Hilton from the Permian Toploje Member peat of the Prince Charles Mountains contain fragments of hyphae of an unidentified fungus in the metaxylem tracheids (Fig. 2j; McLoughlin et al. Reference McLoughlin, Drinnan, Slater and Hilton2015). These hyphae, together with the widespread absence of thin-walled tissues in the axes, are interpreted as signifying moderate aerobic decay before fossilization. What appear to be thick-walled fungal hyphae also occur in the vascular bundles of Jurassic Brachyphyllum-type foliage occurring cheirolepidiaceous pollen cones from Carapace Nunatak (see Pl. I, fig. 10 of Hieger et al. Reference Hieger, Serbet, Harper, Taylor, Taylor and Gulbranson2015).
Leaves constitute a harsh habitat for fungi due to temporary nutrient availability and extreme fluctuations in humidity, temperature, gas-exchange gradients and ultraviolet radiation (Goodman & Weisz Reference Goodman and Weisz2002). Nevertheless, leaf fungi constitute a major component of fungal associations with plants today (Leben Reference Leben1965, Carroll Reference Carroll1988, Arnold Reference Arnold2007, Rodriguez et al. Reference Rodriguez, White, Arnold and Redman2009). Fungi associated with Permian leaves from Antarctica have been reported from the Prince Charles Mountains in the form of narrow hyphae ramifying through the mesophyll of a Noeggerathiopsis leaf (Fig. 2k; Holdgate et al. Reference Holdgate, McLoughlin, Drinnan, Finkelman, Willett and Chiehowsky2005). There was no discussion of the nature of this fungal association, but it probably represents a saprotroph based on the partially degraded condition of the leaf. Partially degraded Glossopteris leaves from Skaar Ridge also contain ramifying hyphae, mycelia, remains suggestive of chytrid-like organisms, mantled spores and globose structures (Fig. 2l), probably belonging to a community of saprotrophs involved in the decomposition of the leaves (Harper et al. Reference Harper, Taylor, Krings and Taylor2015b). The paucity of documented evidence of leaf fungi in the permineralized peats from Antarctica may be due in part to the mechanical destruction of most leaves prior to fossilization. The possibility also exists that the prevailing climatic conditions during these periods of time and the physiology of the high-palaeolatitude plants did not support extensive growth of fungi on/in leaves.
We are aware of only a single report of fungi associated with adpression foliage fossils from Antarctica. Several hyphae have been documented on a leaf of Heidiphyllum elongatum (Morris) Retallack from the Upper Triassic flora of the Allan Hills in southern Victoria Land (see fig. 6c of Bomfleur et al. Reference Bomfleur, Decombeix, Escapa, Schwendemann and Axsmith2013). Reports on Permian adpression fossils suggesting indirect evidence of the presence of fungi include damaged areas on leaves in the form of spots on Gangamopteris sp. cf. G. obovata (Carruthers) White from Milorgfjella, Dronning Maud Land (see Pl. IXc of Plumstead Reference Plumstead1975) and leaves with fungal damage from the Whichaway Nunataks, Coats Land (Plumstead Reference Plumstead1962).
Equally rare are reports of fossil fungi associated with plant reproductive structures. Several specimens of the enigmatic gymnosperm ovulate structure Dordrechtites arcanus J.A. Bergene, E.L. Taylor et T.N. Taylor from the Middle Triassic of Mount Falla in the central Transantarctic Mountains contain abundant fungal hyphae in the megaspore membrane and transfusion parenchyma (Fig. 2m; Bergene et al. Reference Bergene, Taylor and Taylor2013). A similar pattern of fungal colonization has also been reported in the conifer ovule Parasciadopitys aequata (Schwendemann et al. Reference Schwendemann, Taylor, Taylor and Krings2010a) from Fremouw Peak. These fungi probably represent saprotrophs based on the poor preservation of most of the specimens and the lack of recognizable host responses. Finally, Perovich & Taylor (Reference Perovich and Taylor1989) report on hyphae of an unidentified fungus that occur in Middle Triassic Ignotospermum ovules from the Fremouw Peak permineralized peat.
Fungi constitute a large portion of the total biodiversity within modern soil communities (Baldrian et al. Reference Baldrian, Větrovský, Cajthaml, Dobiášová, Petránková, Šnajdr and Eichlerová2013, Wardle & Lindahl Reference Wardle and Lindahl2014). It is, therefore, not surprising that the vast majority of fungal remains in the Antarctic permineralized peats occur in the peat matrix. Slater et al. (Reference Slater, McLoughlin and Hilton2015) distinguish ten fungal morphotypes, including septate and aseptate (Fig. 2n) hyphae, hyphae with swellings, spores (Fig. 2o), ornamented spores, disc-like structures, possible sclerotia and complex fruiting bodies (Fig. 2p). A fossil closely resembling one of the putative fungal fruiting bodies illustrated by Slater et al. (Reference Slater, McLoughlin and Hilton2015) has also been described by Holdgate et al. (Reference Holdgate, McLoughlin, Drinnan, Finkelman, Willett and Chiehowsky2005). The systematic affinities and ecological roles of these fungi currently remain unknown.
Reports of fungi from the Cretaceous of Antarctica are primarily from dispersed remains and silicified wood. Some exceptions include circular inclusions (15–20 µm in diameter) within cells of the cycad Centricycas antarcticus from James Ross Island (see fig. 3a & b of Cantrill Reference Cantrill2000). Cantrill (Reference Cantrill2000) discusses that the inclusions might represent original cell contents, but it is also possible that they are fungal. Moreover, globular bodies on the surface of the Late Cretaceous lycophyte megaspore Cabochonicus from the Table Nunatak at the end of Kenyon Peninsula on the eastern margin of the Antarctic Peninsula have been interpreted as a result of fungal attack (see fig. 2a–c of Eklund et al. Reference Eklund, Cantrill and Francis2004). Cantrill & Drinnan (Reference Cantrill and Drinnan1994) note that some Antarctic Triassic lycopsid megaspores were previously misinterpreted as fungi.
Fungus-like organisms
One group of fungus-like organisms that appears to have been quite abundant in several peat-forming palaeoenvironments of Antarctica is the Peronosporomycetes (Oomycota). In modern ecosystems, Peronosporomycetes function as saprotrophs and facultative or obligate parasites of plants, animals and other fungi (Padgett et al. Reference Padgett, Kendrick, Hearth and Webster1988, Dick Reference Dick1992, Reference Dick2001). The fossil record of Peronosporomycetes from Antarctica consists entirely of dispersed microfossils assigned to the fossil genus Combresomyces (Schwendemann et al. Reference Schwendemann, Taylor, Taylor, Krings and Dotzler2009, Slater et al. Reference Slater, McLoughlin and Hilton2013, Harper et al. Reference Harper, Taylor, Krings and Taylor2015b). Combresomyces, first reported from the Carboniferous of France and Great Britain (Dotzler et al. Reference Dotzler, Krings, Agerer, Galtier and Taylor2008, Strullu-Derrien et al. Reference Strullu-Derrien, Kenrick, Rioult and Strullu2010), consists of spheroidal to pyriform reproductive units located at the tip of broad hyphae. The reproductive units are characterized by a complex surface ornament comprised of ‘antler-like’ extensions positioned on hollow papillations of the wall. Clavate antheridia attached to some of the specimens from France indicate that Combresomyces represents a peronosporomycete oogonium (Dotzler et al. Reference Dotzler, Krings, Agerer, Galtier and Taylor2008).
Two species of Combresomyces have been described by Slater et al. (Reference Slater, McLoughlin and Hilton2013) from the Permian of the Prince Charles Mountains, including C. caespitosus B.J. Slater, S. McLoughlin et J. Hilton, characterized by long, hollow, slender, conical papillae with at least two orders of apical branches (Fig. 3a). It was noted that C. caespitosus occurs close to Vertebraria roots and Glossopteris and Noeggerathiopsis leaves. On the other hand, C. rarus B.J. Slater, S. McLoughlin et J. Hilton is characterized by broad conical papillae that terminate in at least one bifurcation producing a pair of generally acutely divergent and sharply pointed branches (Fig. 3b). Slater et al. (Reference Slater, McLoughlin and Hilton2013) suggest that Combresomyces played a significant role in the decomposition of organic matter or perhaps thrived as a parasite of plants and/or animals. A third Combresomyces species comes from the Triassic Fremouw Peak locality (Fig. 3d). This form is morphologically similar to the type species C. cornifer from the Carboniferous of France, but is considerably larger (Schwendemann et al. Reference Schwendemann, Taylor, Taylor, Krings and Dotzler2009, Reference Schwendemann, Taylor, Taylor and Krings2010a). Combresomyces-like fossils have recently also been discovered in Skaar Ridge peat (Fig. 3c; Harper et al. Reference Harper, Taylor, Krings and Taylor2015b). Moreover, an enigmatic fossil from Skaar Ridge (Upper Permian) illustrated by Schopf (see fig. J of Schopf Reference Schopf1970) superficially resembles an ornamented oogonium attached to a fragment of a wide parental hypha. The occurrence of Combresomyces in the Permian and Triassic of Antarctica has been used to suggest that these organisms were able to recover during times of global climate change and floral turnover, and perhaps were effective as generalists in the high latitude peat-forming environments (Schwendemann et al. Reference Schwendemann, Taylor, Taylor, Krings and Dotzler2009).

Fig. 3 Fungus-like organisms, animal interactions (additional information in the text). a. Combresomyces caespitosus oogonium containing indeterminate spherules. Scale bar=25 µm. Originally illustrated in Slater et al. Reference Slater, McLoughlin and Hilton2013: fig. 1J. Courtesy of S. McLoughlin and B.J. Slater. b. Combresomyces rarus oogium with attached subtending hypha. Scale bar=50 µm. Originally illustrated in Slater et al. Reference Slater, McLoughlin and Hilton2013: fig. 2F. Courtesy of S. McLoughlin and B.J. Slater. c. Combresomyces-like oogonium attached to wide hypha. Scale bar=25 μm. Originally illustrated in Harper et al. Reference Harper, Taylor, Krings and Taylor2015a: Pl. 1, fig.17. d. Combresomyces cornifer. Oogonium with subtending hypha. Scale bar=20 µm. Originally illustrated in Schwendemann et al. Reference Schwendemann, Taylor, Taylor, Krings and Dotzler2009: Pl. I, 1. e. Coprolite (C) composed of fragmented fungal spores (arrow). Scale bar=200 μm. Originally illustrated in Slater et al. Reference Slater, McLoughlin and Hilton2012: Pl. 6, 4. Courtesy of S. McLoughlin and B.J. Slater. f. Arthropod borings in Australoxylon, some containing coprolites (arrow). Scale bar=5 mm. Originally illustrated in Weaver et al. Reference Weaver, McLoughlin and Drinnan1997: fig. 10A. Courtesy of S. McLoughlin and B.J. Slater. g. Galleries in decayed Vertebraria wood filled with coprolites (arrow). Scale bar=100 μm. Originally illustrated in Harper 2015; pl. 12, fig. 156. h. Putative trichomycete. Palisade of thalli (arrow) on possible arthropod cuticle. Scale bar=100 µm. Originally illustrated in White & Taylor Reference White and Taylor1989c: fig. 1.
Fungi in food webs
Co-occurring with the plants and fungal fossils in the Permian and Triassic peats from Antarctica are various animal remains, e.g. arthropod cuticles and coprolites. Slater et al. (Reference Slater, McLoughlin and Hilton2012) describe coprolites from the Permian Toploje Member peat of the Prince Charles Mountains. These frass specimens are composed almost exclusively of broken fungal spores and crushed hyphae (Fig. 3e), some within arthropod-excavated galleries of Australoxylon or Vertebraria. Coprolites of this type attest to the occurrence of fungivory in the palaeoecosystems as represented by these peats. Arthropod borings, typically restricted to the latewood, with coprolites in some of the cavities (Fig. 3f) also occur in Australoxylon mondii from the Prince Charles Mountains (Weaver et al. Reference Weaver, McLoughlin and Drinnan1997). Similar evidence of arthropod–plant interactions has been discovered in white rot-affected Vertebraria wood from Skaar Ridge (Fig. 3g).
Direct evidence of an interaction of a fungus-like organism and an animal from the Triassic of Antarctica occurs in the form of hyphal thalli with possible affinities to the Eccrinales (Trichomycetes) that are attached to what was interpreted to represent an insect cuticle (Fig. 3h; White & Taylor Reference White and Taylor1989c). However, Cafaro (Reference Cafaro2005) challenged the affinities of the fossil with the Eccrinales since septal plugs are not known in modern members of this group. Moreover, there is no conclusive evidence to suggest that the fossil is in fact attached to the cuticle of an arthropod.
Palynomorphs and the dispersed record
Extensive palynological sampling and analysis have been conducted in several Cretaceous sequences from Antarctica (see Truswell Reference Truswell1989), primarily to reconstruct palaeovegetation and assess palaeoenvironmental and palaeoclimatic conditions. Fungal palynomorphs (e.g. spores, conidia, mycelial fragments) frequently occur in the samples, and some have been used as proxy indicators of several climate parameters. For example, certain fungal palynomorphs have been used to document warm and cold episodes and the occurrence of seasonal sea ice during the Late Cretaceous of Antarctica (Bowman et al. Reference Bowman, Francis and Riding2013).
Among the most abundant fungal palynomorphs during the Cretaceous of Antarctica are representatives of Pluricellaesporites sp. from Lower Cretaceous deposits on Byers Peninsula, Livingston Island, and the Upper Cretaceous of Seymour Island, Antarctic Peninsula (see Pl. 8, fig. 7 of Duane Reference Duane1996, see fig. 4–30 of Bowman et al. Reference Bowman, Francis, Askin, Riding and Swindles2014). Several palynomorph assemblages that include fungal remains have also been reported from Seymour Island (Cranwell Reference Cranwell1959, Askin Reference Askin1989, Bowman et al. Reference Bowman, Francis, Askin, Riding and Swindles2014). The fungal remains have been interpreted as reflecting saprotrophic degradation of terrestrial biomass in a humid palaeoenvironment. Reports of remains of more complex fungal structures such as microthyriaceous fruiting bodies from the Lower Cretaceous Byers Group on Livingston Island (see Pl. 8, figs 9 & 10 of Duane Reference Duane1996) and Asterothyrites (see fig. 8Y of Di Pasquo & Martin Reference Di Pasquo and Martin2013) from James Ross Island are interpreted to reflect low lying, coastal regions that experienced a moist, temperate to tropical climate. Abundant fungal spores in Late Cretaceous (Campanian–Maastrichtian) palynological debris have also been reported from King George Island, Antarctic Peninsula (Song & Cao Reference Song and Cao1994, Dutra & Batten Reference Dutra and Batten2000). Finally, several authors have described Reticulatisporites pudens Balme as a fungal spore, suggesting that this taxon could be used to infer that volcanically perturbed post-eruption riparian systems occurred in the upper Cerro Negro Formation from Walker Bay erratics (Lower Cretaceous) on Livingston Island (Chen et al. Reference Chen, Stilwell and May2015). Although documented evidence of fungi from the Antarctic Cenozoic is beyond the scope of this review, one report of the Palaeocene (Danian) epiphyllous fungus Trichopeltinites on cuticles from Seymour Island is particularly interesting because this fungus has been interpreted as becoming at the Cretaceous–Palaeogene boundary in the Western Interior of North America (see fig. 2e of Upchurch & Askin Reference Upchurch and Askin1989).
The palynomorph Reduviasporonites is the focal point of several influential studies suggesting a major accumulation (a so-called ‘fungal spike’, ‘fungal abundance event’ or ‘fungal disaster event’) of this fossil at the end of the Permian. Such evidence has been used to suggest that this accumulation is indicative of the destruction of terrestrial vegetation by fungal pathogens that led to the end-Permian collapse of terrestrial ecosystems (Visscher et al. Reference Visscher, Brinkhuis, Dilcher, Elsik, Eshet, Looy, Rampino and Traverse1996, Reference Visscher, Sephton and Looy2011, Steiner et al. Reference Steiner, Eshet, Rampino and Schwindt2003, Vajda & McLoughlin Reference Vajda and McLoughlin2007). Visscher et al. (Reference Visscher, Sephton and Looy2011) hypothesize that fungal disease was an essential accessory in the destabilization of the vegetation that accelerated widespread tree mortality during the end-Permian crisis. Moreover, they dismiss results from a study by Foster et al. (Reference Foster, Stephenson, Marshall, Logan and Greenwood2002), who, based on geochemical evidence, concluded that Reduviasporonites might be of algal origin. Reduviasporonites chalastus (Foster) Elsik has been reported from the Prince Charles Mountains and constitutes 24% of the latest Permian palynomorph assemblage at this locality. The taxon is also present but less common (4–10%) in the earliest Triassic (Lindström & McLoughlin Reference Lindström and McLoughlin2007). These authors note that several typically Permian taxa have their last occurrences c. 19 and 24 m below the Reduviasporonites zone, possibly corresponding to an initial extinction level. Overall, the occurrence of Reduviasporonites in Antarctica demonstrates the worldwide distribution of this taxon near the P-T boundary.
Discussion
Although the Permian, Triassic, Jurassic and Cretaceous permineralized peat and wood from Antarctica are well known as sources of new information on the morphology and internal organization of the plants that occurred on this continent during the late Palaeozoic and Mesozoic, relatively little is known to date about the associations and interactions that these plants formed with other ecosystem constituents in order to exist in the extreme habitats. Future research with Antarctic palaeobiological systems will be directed towards screening the permineralized peats and silicified wood for organisms associated with the plants, especially fungi. It comes as no surprise that a large number of fungi and fungal interactions have been discovered in recent years, some of which are represented by exceptional examples that in turn provide the opportunity for detailed comparisons with modern analogues. The presence of major lineages of fungi, such as the Glomeromycota, is documented by dispersed remains in the matrix, but also by direct evidence of vesicular arbuscular mycorrhizas in three gymnosperm taxa. We are confident that further studies of the wide array of permineralized plants from Antarctica will yield additional examples of mycorrhizal associations in these polar palaeoecosystems. On the other hand, several major fungal lineages have not been recorded from Antarctica to date, including Blastocladiomycota and Ascomycota. One possible reason for the apparent absence of Blastocladiomycota may be the compacted nature and usually highly degraded content of the permineralized peats that may not allow preservation of very delicate fungal structures. Additionally, the environments during the Permian through Cretaceous in Antarctica were perhaps not conducive for members of the Blastocladiomycota. On the other hand, ascomycete fruiting bodies (e.g. cleistothecia and perithecia) are structures that should readily lend themselves to preservation in recognizable form. We speculate that these structures either have not yet been recognized or have simply been neglected in studies focusing on Antarctic plants. Some of the ‘sporocarps’ from the Triassic (e.g. Endochaetophora) were initially considered as members of the Ascomycota. However, new data suggest that these structures may be more closely related to the Mucoromycotina (see Krings et al. Reference Krings, Taylor and Dotzler2013, Reference Krings, Taylor, Taylor, Kerp and Dotzler2014).
The vast majority of fungal remains reported from Antarctica to date come from Permian and Triassic peats, as well as from Cretaceous silicified woods. Evidence of fungi in Jurassic sediments are presently limited to a brief note by Bomfleur et al. (Reference Bomfleur, Schneider, Schöner, Viereck-Götte and Kerp2007) on fungal remains that appear to occur within a microbial mat from Mount Carson (northern Victoria Land), they associated with a tylosis-forming conifer wood (Harper et al. Reference Harper, Bomfleur, Decombeix, Taylor, Taylor and Krings2012), and putative hyphae within leaf vascular bundles (Hieger et al. Reference Hieger, Serbet, Harper, Taylor, Taylor and Gulbranson2015). These studies indicate that the Jurassic provides a largely untapped source of information on fungal diversity and plant–fungal interactions in the Mesozoic of Antarctica. There are also several reports of fossil fungi in Cenozoic wood from Antarctica (e.g. Pujana et al. Reference Pujana, Marenssi and Santillana2015), but no systematic studies of these fungi have been conducted. Finally, there is an informative palynological and dispersed record that may be used in palaeoecosystem and palaeoclimatology reconstructions.
Future perspective
This review surveys and briefly characterizes the numerous fungi that have been described from deposits in the Permian to Cretaceous of Antarctica, including well-preserved examples of fungal associations and interactions with land plants. Although the record indicates that fungi were important constituents of the Antarctic polar terrestrial palaeoecosystems, the roles that fungi played in these environments remain incompletely understood. We contend that a concerted research effort that brings together different types of data can eventually ask and potentially answer a series of interesting questions regarding the polar palaeoecosystems. For example, what adaptations were used by plants, especially large trees, to live in environments that, based on environmental considerations today, would be regarded as largely adverse? Together with detailed information on the anatomy and physiology of the host plants, as well as data on the sedimentology and palaeoclimatology, the study of fungi from Antarctica may make it possible to provide a more comprehensive palaeoecological analysis of how fungal relationships may have contributed to the success of plant growth in Gondwanan polar ecosystems.
Acknowledgements
We extend our appreciation to Steve McLoughlin and Ben J. Slater for help, assistance and permission to use images from their research in this review. Thanks to Benjamin Bomfleur for discussing Jurassic material, Rudolph Serbet for fruitful discussion and technical assistance, and Erik L. Gulbranson for helpful discussion on geologic settings. Financial support was provided by the Alexander von Humboldt-Foundation (3.1-USA/1160852 STP to C.J.H.), the National Science Foundation (EAR-0949947 to T.N.T. and M.K.; OPP-0943934 to E.L.T. and T.N.T.), and a University of Kansas Department of Ecology and Evolutionary Biology Donald J. Obee Botany Dissertation Fellowship to C.J.H. This manuscript greatly benefited from the comments and suggestions of two anonymous reviewers.
Author contributions
All authors contributed equally and declare no conflict of interest.







