Introduction
Plant seeds are excellent food for different groups of animals as they are a source of protein, sugar, fat and minerals. Opportunities for seed predation depend on seed availability and vary according to the size and structure of fruits and seeds, the length of time that seeds remain on the host plant, and the degree of morphological and chemical plant protection. Loss of seeds due to seed predation can be very high and, in consequence, seed predation is one of the major factors influencing plant fitness, plant abundance, and the distribution and dynamics of plant populations (e.g. Janzen, Reference Janzen1971; Fröborg and Eriksson, Reference Fröborg and Eriksson2003; Maron and Crone, Reference Maron and Crone2006). Seed predation has been suggested to play a crucial role in plant population dynamics, acting as a strong selective force in the evolution of traits (Hulme and Benkman, Reference Hulme, Benkman, Herrera and Pellmyr2002).
Seeds are the most accessible to predators while they remain on the mother plant. Pre-dispersal seed predation may have strong effects on plant population dynamics by reducing recruitment and lowering population growth (reviewed by Kolb et al., Reference Kolb, Ehrlen and Eriksson2007). However, the strength of this influence varies highly between species both spatially and temporally. Some studies have reported that pre-dispersal seed predation by insects could lead to losses greater than 90% of the total seed crop (Fenner and Thompson, Reference Fenner and Thompson2005). This kind of predation could also affect the selection of several plant traits, such as flowering phenology and flower number, which is usually interpreted mainly in the context of plant–pollinator interactions (Cariveau et al., Reference Cariveau2004; Kolb et al., Reference Kolb, Ehrlen and Eriksson2007).
Plant species from the Asteraceae family are an excellent food source for many groups of insects because they usually produce a large number of seeds in many capitula. Larvae of Diptera (e.g. Tephritidae), Coleoptera (e.g. Curculionidae) and Lepidoptera were reported from capitula of different Asteraceae species (e.g. Campagna and Rapparini, Reference Campagna and Rapparini2002; Honek and Martinkova, Reference Honek and Martinkova2005; Koprdova et al., Reference Koprdova2015). In many cases, they are inconspicuous specialized species, of which the life cycle is connected with one or a few closely related plant species (e.g. Holt and Zwölfer, Reference Holt and Zwölfer2007). The proportion of damaged seeds is reported to be usually high, an effect of fruits remaining for long time intervals on the mother plant (e.g. Briese, Reference Briese2000).
Rare plants with limited ranges and isolated plant populations are more susceptible to the events that could decrease plant fitness, e.g. a change of habitat conditions, herbivory, a lack of pollinators, etc. In this respect, the loss of seeds as a result of predation may be a potential threat for maintenance of rare plant populations. Various examples, but still insufficient, have highlighted how insect seed predation affects rare plant population dynamics. In the case of the narrow endemic Ebenus armataigei, seed predation by bruchid beetles was considered a major threat to its existence (Hegazy and Eesa, Reference Hegazy and Eesa1991). In some cases, the loss of seeds could be significant – up to 78% of seeds of the rare species Sidalcea oregana var. calva (Zimmerman and Reichard, Reference Zimmerman and Reichard2005) or up to 82% in the narrow endemic Astragalus sinuatus (Combs et al., Reference Combs2011, Reference Combs, Lambert and Reichard2013). The impact of seed predators on the reproductive output of rare plants may represent a mechanism that might help explain plant rarity (e.g. Lavergne et al., Reference Lavergne, Debussche and Thompson2005) and may provide valuable information for the management of endangered species. Combs et al. (Reference Combs, Lambert and Reichard2013) proposed that conservation managers should consider periodically reducing pre-dispersal seed predators to increase seed production and seedling recruitment to support the long-term survival of narrow endemic A. sinuatus.
Cirsium decussatum Janka (Asteraceae family) is a rare species in Central and Eastern Europe. It is sparsely distributed in Ukraine, Romania, Hungary, Slovakia and Poland (Werner, Reference Werner, Tutin, Heywood, Burges, Moore, Valentine, Walters and Webb1976). In Poland, it has only a few locations in the Carpathian Foothills: Przemyśl Foothills (Piórecki and Zarzycki, Reference Piórecki, Zarzycki and Kaźmierczakowa2014). It grows in tall oatgrass meadows on formerly cultivated lands, and in pastures (Barabasz-Krasny and Sołtys-Lelek, Reference Barabasz-Krasny and Sołtys-Lelek2010). The species is listed in the national red list and red book with vulnerable category of threat (Piórecki and Zarzycki, Reference Piórecki, Zarzycki, Mirek and Piękoś-Mirkowa2008, Reference Piórecki, Zarzycki and Kaźmierczakowa2014; Kaźmierczakowa et al., Reference Kaźmierczakowa2016). The plant belongs to the herbaceous biennial (monocarpic) species. It forms a basal rosette of prickly leaves during the first year and flowers during the second year. Plant height can reach 250 cm. The upper parts of the stems are branched with alternate large pinnate leaves with long spines on the edges, floccose beneath. Deep purple disc flowers create large spherical inflorescences (anthodia) up to 8 cm in diameter. The involucre consists of several rows of cottony-hairy bracts, and the outer ones have prominent spines. The number of capitula in one individual may reach 30 and the flowering season spans from July to September. Its fruits are achenes covered by a feathery-branched pappus of the calyx (Szafer et al., Reference Szafer, Kulczyński and Pawłowski1986). Our observations indicate that inflorescences are visited by bumblebees (Bombus spp.) and different species of butterflies. The species does not reproduce vegetatively. Some taxonomists regarded C. decussatum as a subspecies of C. eriophorum (C. eriophorum subsp. decussatum (Janka) Petrak; Dostal Reference Dostal1958).
The aim of this study was to investigate the influence of seed predators on plant fitness of the rare monocarpic thistle C. decussatum. We examined which insect species laid eggs and developed in the inflorescences of the thistle. We tested several hypotheses: (1) pre-dispersal seed predators reduce the number of dispersed propagules of C. decussatum; (2) seed predators select larger inflorescences as oviposition sites; (3) the size of capitulum is correlated with seed productivity; and (4) there is competition between seed predators in the oviposition.
Materials and methods
Materials
The capitula of Cirsium decussatum were collected at the end of the fruiting season (September 2013) in one locality: Cisowa on the Przemyśl Foothills, south-eastern Poland (49°42′14″ N, 22°35′52″ E). From 20 randomly selected individuals, all capitula were picked up. In total, 331 capitula were gathered. The number of individuals included in the study was the consequence of the plant's rarity in the region. As the fruits of the thistle are released after dying of above-ground parts of the plant at the end of the annual growing season, we were certain that all potential seeds had developed, they did not leave the capitulum, and all potential insects had laid eggs. The collected capitula were subjected to stratification for a period of 5 months. Each capitulum was separately stored in an unheated, relatively dry room to experience a temperature similar to natural conditions. In mid-March, when the larvae pupated, every pupa was removed from each capitulum and kept separately. This procedure allowed us to estimate the degree of infestation of each capitulum and to determine the species of insects. After all insects and their larvae had been removed from the capitula the traits of each capitulum and fruits were recorded. The diameter of each capitulum was measured with a digital caliper. Each capitulum was thoroughly examined to check if it had fruits inside. All capitula, both unfertilized (without any fruits) and fertilized (at least one fruit inside) were taken for analyses. The fruits (each fruit has one seed) were counted and classified by whether or not they were fertilized. Next, the fruits were examined and classified into the following categories: (1) healthy seeds (fertilized, developed fruit without any sign of insect feeding); (2) damaged seeds (signs of insect feeding; Fig. 1A); (3) aborted seeds (flat, empty fruit). We used the term seed production for the number of fruits (healthy + damaged + aborted). We calculated the level of seed predation in each capitulum: damaged seeds/(healthy seeds + damaged seeds). We also categorized the capitula: (1) fertilized capitulum containing healthy and/or damaged seeds, i.e. healthy, damaged, aborted; (2) unfertilized capitulum not containing any seeds. In this study, the term ‘seeds’ is used for achenes (fruit) and ‘capitulum’ or ‘head’ for anthodium of C. decussatum.

Figure 1. (A) Seeds of Cirsium decussatum: a, healthy seed; b, seed damaged by Terellia longicauda larvae; c, pupa of Terellia longicauda. (B) Terellia longicauda cleans the aculeus with its sensitive receptors on C. decussatum inflorescence (photo: A. Palaczyk).
Data analysis
We tested data sets for normality using the Shapiro–Wilk normality test. We used a log10 (x + 1) transformation to address normality assumptions and equal variance requirements. The data of capitulum diameter and damaged seeds were transformed by square root as the raw data were left-skewed.
To test whether plant traits differed among individuals we used one-way analyses of variance (ANOVAs). We compared capitulum diameter, seed production, number of seed categories as well as seed predation level and number of Terellia longicauda larvae. A non-parametric test of variance (Kruskal–Wallis test) was used for proportion of seed categories and number of other insects due to the low abundance of data. We used all capitula as independent experimental objects as there were no differences between individuals in terms of the diameter of capitula (ANOVA, F 19,330 = 1.19, P = 0.26). To examine the relationship between measured traits of capitula, we used a Pearson correlation for log-transformed data. In case of a high number of zeros in our data set, a non-parametric approach (Spearman correlation) was used. We also tested the correlation between the number of insect larvae and the number of damaged seeds using a Pearson or Spearman correlation. In order to check if T. longicauda prefers larger inflorescences to lay eggs, a difference in head diameter between infested and non-infested capitula was tested using a Mann–Whitney non-parametric test. Infested and non-infested heads were also compared in terms of characteristics related to seed production using the Mann–Whitney test. The occurrence of other insect larvae was tested using the chi-square test. We also compared the diameter of unfertilized capitula and capitula with at least one fertilized seed to check if the size of the capitulum is a good predictor of fertile seed production. All analyses were performed using Statistica software.
Results
Plants
Individuals of C. decussatum produced heads ranging in diameter from 0.7 to 6.5 cm, with a mean value of 3.18 cm (Table 1). On average, there were 45 seeds per capitulum with a maximum of 200 seeds. In total, we counted 14,713 seeds in our analyses. Seed production was positively correlated with capitulum diameter (n = 331, y = 0.07x + 0.44, R 2 = 0.33, P < 0.001). Healthy seeds were found in 65.6% of all capitula and in 83.5% of fertilized capitula. Seed production and number of healthy seeds per capitulum differed between individuals (Table 1, F 19,330 = 1.92, P = 0.01, F 19,330 = 2.62, P < 0.001, respectively). In our sample, 21.5% of the capitula did not contain any seeds.
Table 1. Traits of Cirsium decussatum capitula

F, P, results of ANOVA for 20 individuals of C. decussatum; F A, Kruskal–Wallis results for non-normally distributed variables; a number of capitula with Cecidomyiidae larvae.
We observed signs of insect feeding, measured by the number of damaged seeds, in 89.2% of fertilized capitula. On average, 17 seeds per capitulum were damaged and the mean level of seed predation per capitulum was 41.3%. There were 7.3% of capitula (8.6% of fertilized capitula) with all seeds damaged by insects. In addition, there were 10.9 aborted seeds per capitulum on average (23.4%). They occurred in 89.6% of fertilized heads and 19 heads (5.7%) contained only aborted seeds. There were no differences between plant individuals in the proportion of aborted seeds per head in our sample (H 20 = 29.4, P > 0.05).
The size of the capitulum was a good predictor of fertile seed production. The relationship between capitulum diameter and a number of fertile seeds was positive (n = 331, y = 0.08x + 0.18, R 2 = 0.39, P < 0.001). The mean value of fertilized capitula diameter was 3.5 cm against that of unfertilized: 2.2 cm (H 260,71 = 98.7, P < 0.001).
Insects
Several insect taxa that belong to different taxonomic groups were identified in the heads of C. decussatum: flies, butterflies, beetles, bugs and wasps. They inhabited the heads from late autumn to the next spring. These were: larvae of Terellia longicauda (Meigen 1838) (Diptera: Tephritidae) and its parasitoid Crataepus marbis (Walker 1839) (Hymenoptera: Eulophidae), the larvae of Larinus sturnus (Schaller 1783) (Coleoptera: Curculionidae) and its parasitoid Exeristes roborator (Fabricius 1793) (Hymenoptera: Ichneumonidae), the larvae of Toxoneura modesta (Meigen 1930) (Diptera: Pallopteridae), the larvae of Cecidomyiidae (Diptera), butterfly caterpillars from Pyralidae and Noctuidae, and imago of Corticaria obscura Brisout 1863 (Coleoptera: Lathridiidae) and Tingis cardui (Linnaeus 1758) (Hemiptera: Tingidae).
Larvae of Terellia longicauda were observed in 44.7% of capitula (56.5% of fertilized capitula and only in one unfertilized capitulum; Table 1). A total of 1216 larvae were counted in our sample. On average, eight larvae inhabited one capitulum with a maximum of 35 (the group of infested capitula). In 71% of capitula in which the larvae were present, their number did not exceed 10. Some of the larvae (13.6%) were infected by the parasitoid Crataepus marbis. Parasitized larvae were observed in 47.9% of capitula. There was no significant difference of capitulum infestation by T. longicauda between thistle individuals (Table 1).
The weevil Larinus sturnus, the second insect taxon by attendance in the thistle heads, inhabited 31.1% of sampled heads. In total, we recorded 188 individuals as an imago or larvae. The species occurred only one or, less frequently, two per capitulum and it was the only seed predator in 7.3% of capitula. Forty-two per cent of seeds were damaged in these capitula. Parasitoid of the weevil Exeristes roborator occurred in four heads. There was no relationship between the capitulum diameter and weevil infestation (z 84,247 = −3.6, P > 0.05).
Cecidomyiidae larvae were observed in 20.5% of capitula with no relationship with other insects (Pearson correlations, P > 0.05). Only one up to a few larvae were counted per head. They were the only settled insects in the 7.9% of sampled heads and on average, 28% of seeds per capitulum were damaged by these larvae.
Toxoneura modesta larvae were recorded in 5.4% of the capitula. There was no relationship between the number of larvae and number of fertile seeds, capitulum diameter, or other insects (Pearson correlations, P > 0.05).
Butterfly caterpillars were found in 4.5% of heads. They represented mainly the Noctuidae family, and there was only one case of the Pyralidae family. Most frequently, one caterpillar was found per head. The presence of the butterfly caterpillars was independent of other insects, and they were the only insects in six cases. In this small sample, the proportion of damaged seeds ranged from 8 to 32% (average 16%). There was no relationship between capitulum diameter and butterfly infestation (z 15,316 = −5.8, P > 0.05).
Only one imago of Tingis cardui was found in the heads of C. decussatum.
The effect of insect larvae on plant fitness
The percentage of infested capitula of C. decussatum was high in our sample and amounted to 75.5%. When considering only fertilized capitula, this percentage was even higher and reached 81.9%.
There was a positive relationship between the number of damaged seeds as well as seed predation level per capitulum and number of T. longicauda larvae. A number of damaged seeds was higher when more T. longicauda larvae were observed per capitulum (Fig. 2A). Similarly, the seed predation level was positively correlated with the number of T. longicauda larvae per capitulum (Fig. 2B). There was no such relationship between other insects identified in sampled thistle heads (Fig. 2C,D). We did not detect a correlation between T. longicauda larvae and other insects inside heads (Fig. 3C,D), although almost half of the heads infested by T. longicauda also harboured other insect taxa. There was also no correlation between L. sturnus and other insects (Spearman correlation, P > 0.05). However, we found that L. sturnus occurred more frequently when the fly was not inside the capitulum (Table 2).
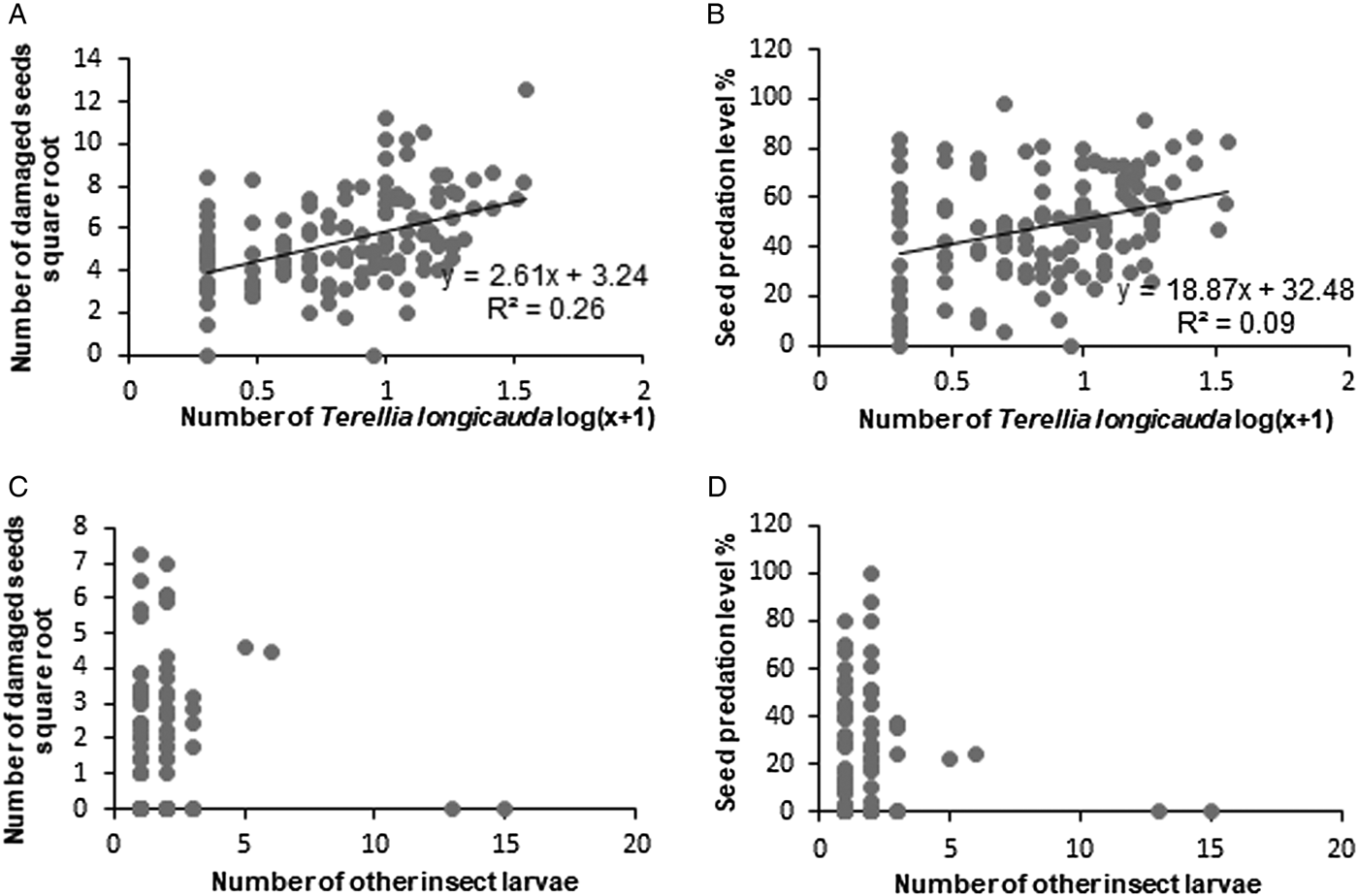
Figure 2. Number of damaged seeds (DS) and seed predation level (SP) per capitulum of Cirsium decussatum as a function of the number of insect larvae: (A) DS in capitula infested only by Terellia longicauda larvae (n = 85 capitula), Pearson coefficient, P < 0.001; (B) SP in capitula infested only by Terellia longicauda larvae (n = 85); Pearson coefficient, P < 0.001; (C) DS in capitula infested by other insect larvae (n = 102); (D) SP in capitula infested by other insect larvae (n = 102).
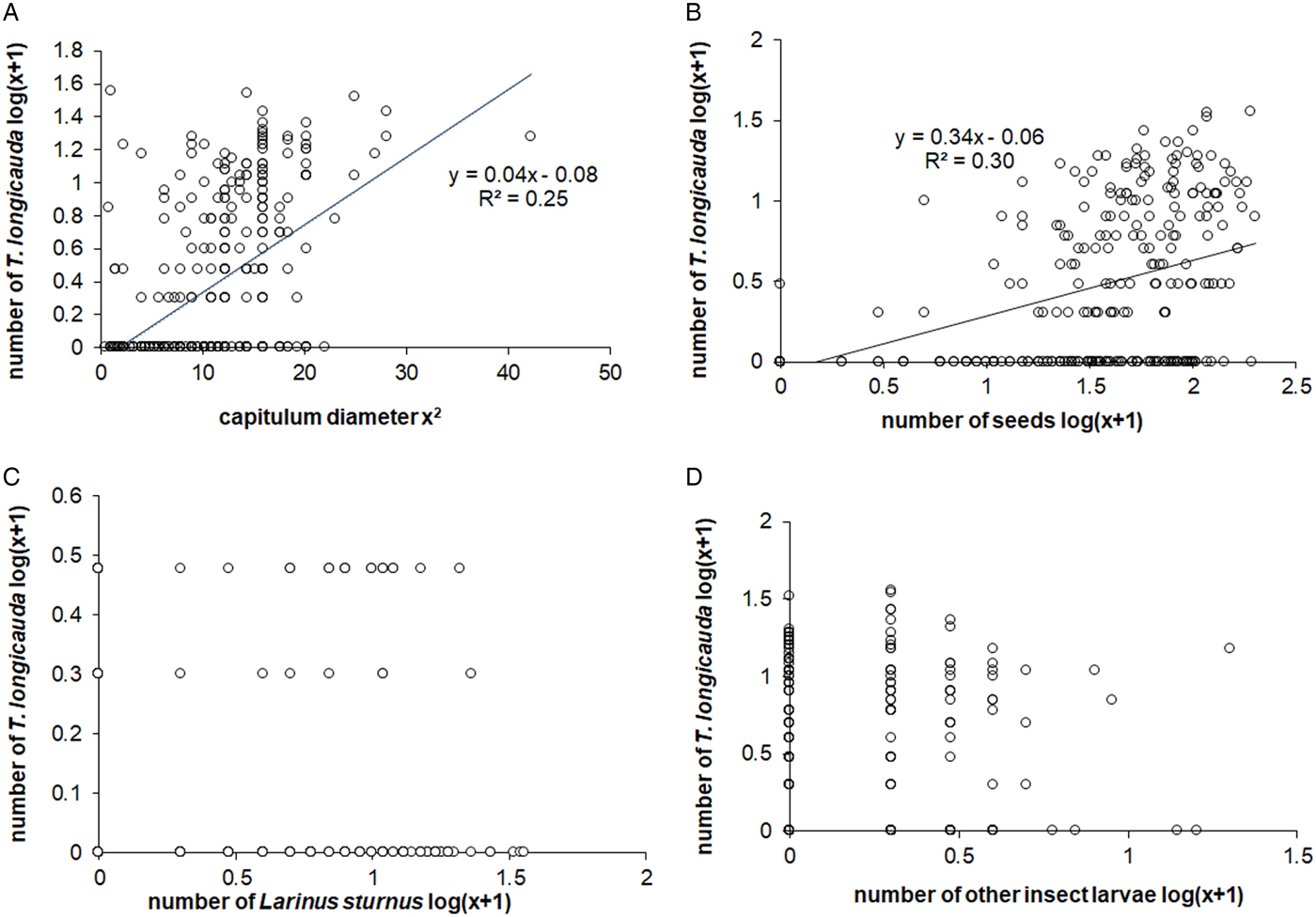
Figure 3. Relationship between traits of the Cirsium decussatum capitulum and number of Terellia longicauda larvae: (A) capitulum diameter, Pearson coefficient, P < 0.001; (B) total seeds number, Pearson coefficient, P < 0.05; (C) number of Larinus sturnus, Spearman coefficient, P > 0.05; (D) number of other insect larvae, Spearman coefficient, P > 0.05.
Table 2. Mean values (±SE) of Cirsium decussatum capitula with or without Terellia longicauda larvae

z, P, Mann–Whitney pairwise test results’ z A, chi-square test results.
The number of T. longicauda larvae correlated positively with the head diameter (Fig. 3A). Furthermore, the mean value of capitulum diameter with the larvae inside was 3.73 cm versus 2.74 cm mean diameter of capitulum without larvae (Table 2). The smallest inhabited capitulum had a diameter of 2 cm in comparison with 0.7 cm in our sample, and the largest was 6.5 cm. It is worth noting that of all sampled heads, six were larger than 5 cm, and five were settled by fly larvae. The diameter of unfertilized capitula or capitula with all aborted seeds was smaller than the diameter of fertilized capitula, both with and without T. longicauda (Fig. 4). The correlation between the number of T. longicauda larvae and seed number was positive (Fig. 3B). Seed production in the heads infested and non-infested by the fly differed significantly with mean values 68.7 and 24.9, respectively (Table 2). These groups also differed with the proportion of damaged seeds and seed predation level. When we considered the fertilized heads inhabited only by T. longicauda, there was a negative relationship between the sum of fertile seeds and the proportion of damaged seeds (n = 146, log-linear model a = −0.05, b = 4.4, P < 0.001).

Figure 4. Mean values of capitulum diameter of Cirsium decussatum in groups: (A) fertilized capitula with Terellia longicauda larvae (n = 146); (B) fertilized capitula without Terellia longicauda larvae (n = 113); (C) capitula with only aborted seeds (n = 19); (D) unfertilized capitula (n = 71). Mann–Whitney pairwise test results with P < 0.001 for A-B, A-C, A-D, B-D, and with P < 0.05 for B-C.
It is worth noting that insect larvae also occupied unfertilized capitula (37 heads, 52.1% of unfertilized heads). These heads were infested by L. sturnus, Cecidomyiidae larvae, Palloptera modesta larvae, and in two cases by T. longicauda.
Discussion
Our studies strongly support the hypothesis that seed predators reduce the number of dispersed propagules of C. decussatum. In our dataset, only 37% of seeds were without signs of feeding and 75.5% of the heads were infested by insects. In fact, the percentage of seed loss is probably higher, as some portion of the seeds could be consumed whole and, in consequence, not detected in our study. Insect identity and feeding mode play a critical role in influencing predation rates (e.g. Honek and Martinkova, Reference Honek and Martinkova2005; Combs et al., Reference Combs, Lambert and Reichard2013). The reproduction success in monocarpic biennial plants is estimated to be low. For other common Asteraceae, Cirsium palustre and Senecio jacobea, only 0.13 and 0.17% of the seed bank, respectively, had a chance to reproduce as they created individuals with flowers (van der Meijden, Reference van der Meijden, den Boer and Gradwell1971; Falińska, Reference Falińska1997). Cirsium decussatum has a limited distribution range in Poland, and is a plant potentially threatened with extinction. There is no vegetative spread and reproduction depends entirely upon seed output for establishment and persistence. According to our results, pre-dispersal seed predation probably acts as one of the limiting factors of the plant fitness reducing seed output as has been pointed for other plants with narrow distribution (e.g. Hegazy and Eesa, Reference Hegazy and Eesa1991; Zimmerman and Reichard, Reference Zimmerman and Reichard2005; Combs et al., Reference Combs2011, Reference Combs, Lambert and Reichard2013; Kurkjian et al., Reference Kurkjian, Carothers and Jules2017). Münzbergová (Reference Münzbergová2005) studied rare and common Cirsium species (C. pannonicum and C. acaule subsp. acaule) and pointed out that seed predation is the key factor causing a lower population growth rate in the rare thistle. According to Louda and Potvin (Reference Louda and Potvin1995), insect seed predation reduced recruitment and subsequent adult density in the population of monocarpic Cirsium canescens. The experimental study of Maron et al. (Reference Maron, Combs and Louda2002) showed that the reduction of insect predators in Cirsium occidentale increased seed production by 144–316% and led to a 130–196% increase in cumulative seedling recruitment in the next generation.
Our study has shown that capitula of C. decussatum are inhabited by insects from a wide range of insect families. Detailed observations of seed and head damage indicate that the seeds of thistle are an important food base for the larvae of flies – T. longicauda (Fig. 1), beetle larvae – Larinus sturnus, and caterpillars of butterflies of the families Pyralidae and Noctuidae. It is most likely that other insect taxa identified in the heads are not responsible for the reduction of seeds. It was found that Toxoneura modesta larvae feed on pappus of Cirsium heterophyllum and C. vulgare seeds (Rotheray, Reference Rotheray2014). So far, there has been no information in the literature on the feeding of Cecidomyiidae larvae on thistle seeds (Batra et al., Reference Batra1981; Tofts, Reference Tofts1999). In addition, we assume that the imago of Tingis cardui found in one capitulum probably wintered there.
The most important role in the reduction of the C. decussatum seeds is played by T. longicauda, which was the most frequent insect observed in the heads. T erellia longicauda is one of the relatively large fruit fly species with a length of up to 8.5 mm. Females have an exceptionally long ovipositor, enabling them to reach deeply situated seeds (Fig. 1B). The species occurs in the area spanning from Spain, France and southern England, across to the Ukraine and Russia to the east, and to Serbia and Greece in the south. It is a monofagous species (ologophagous) and it preys only on two closely related species of Cirsium: C. eriophorum and C. decussatum. The fly locations are insular and associated with the occurrence of these two thistle species. In Poland, T. longicauda was reported only from the Pieniny Mountains, and the Przemyśl Foothills (south-eastern Poland) (Klasa and Palaczyk, Reference Klasa and Palaczyk2004). The biology of the fly has been studied on C. eriophorum (Holt and Zwölfer, Reference Holt and Zwölfer2007). The flight of adult insects takes place from the first half of July until the beginning of August. The females display strong territorial behaviour, fighting off other females trying to access capitula of the thistle. During the long hours of observation, we never recorded T. longicauda on the blooming inflorescences of C. decussatum. It is most likely that they do not participate in the pollination of the thistle. Flies were sitting on stems, leaves or flower buds of the thistle. The adaptive colour of flies (grey-blue) makes them almost invisible on the pubescent green-grey plant parts. According to our observation, it could be stated that T. longicauda belongs to the group of phytophagous species: it uses its host, and the host (plant) did not derive any benefit. The fly benefits from ovipositing in the heads of C. decussatum in many ways. The seeds in capitula provide an abundant food source. The capitulum involucre formed of several rows of cottony-hairy bracts and bracts with relatively long spines makes it difficult to get inside. There are no monophagous insect species besides T. longicauda on C. decussatum. The fly adapts to inhabiting heads of the thistle by synchronizing its life cycle to the life cycle of the host – a short period of egg laying and a relatively high fertility. The disadvantage of monophagy, in this case, is the limited range of the host plant. Any threat to the plant's occurrence also constitutes the threat to its specialized herbivore.
Our studies suggest that the females of T. longicauda choose larger heads for oviposition. ANOVA results indicate that the insects treat each inflorescence as a separate entity, whether on the same or another plant. The choice of larger heads for egg laying is also visible in the case of other insects identified on C. decussatum. For T. longicauda, the choice of larger heads provides a larger food source, as larger heads contain a higher number of seeds. It is known that T. longicauda lays 17 eggs (±10.3) in a single capitulum, but there was no evidence that dragging the ovipositor is accompanied by the deposition of a host marking pheromone (Holt and Zwölfer, Reference Holt and Zwölfer2007). In the laboratory, while we took photos of T. longicauda on C. decussatum (Fig. 1B), we noticed that, when the female put forward her ovipositor, an aromatic odour was clearly perceptible. If we assume that the female marks the head with the pheromone and indeed leaves a trail of scent, subsequent females will avoid laying eggs in marked heads. As a consequence, similar egg numbers should occur in the capitulum independently of capitulum size or seed number, if seed capacity is sufficient to feed the larvae. This hypothesis seems to be confirmed since we found a negative relationship between the sum of fertile seeds and the proportion of damaged seeds in fertile capitula inhabited only by the fly. Furthermore, the number of larvae per capitulum could prove this hypothesis: in 85% of heads inhabited by flies, the number of larvae did not exceed 15. Only in 4% of heads was the number of larvae greater than 21 (22–35), which may (but does not have to) indicate that two females deposited eggs into one capitulum. Further research is required to verify whether flies leave a trace of pheromone. The phenomenon of prenatal care in the form of pheromone signals is known for phytophagous insects (e.g. Aluja and Norrbom, Reference Aluja and Norrbom1999; Steiger and Stökl, Reference Steiger and Stökl2017).
The evolutionary trajectory for flower (inflorescence) size is driven by conflicting selection pressures (Celedon-Neghme et al., Reference Celedon-Neghme, Gonzales and Gianoli2007). On the one hand, a larger floral display attracts more pollinators, which is especially important when a number of pollinators declines (e.g. Thomann et al., Reference Thomann2015). On the other hand, various insect species consume pollen grains or flowers (Larson et al., Reference Larson, Kevan and Inouye2001), and seeds before they are released (e.g. Kolb et al., Reference Kolb, Ehrlen and Eriksson2007). In most of the Asteraceae, it has been shown that pre-dispersal seed predation is positively correlated with flower head size both within and among species (Fenner et al., Reference Fenner2002). Our results confirm these studies. They fit also in the evolutionary debate related to the impact of biotic factors on plant traits, in this case the size of inflorescences. When taking into consideration the range of the thistle head diameter and the pressure of seed predators on larger heads, it can be assumed that, in this case, seed predators act as a strong selective force in the evolution of C. decussatum inflorescence size. Larger capitulum contains more seeds and more seeds could avoid predators as we documented in our study by the negative correlation between fertile seed number and seed predation level in the pool of heads infested by flies.
An interesting question is the presence of sterile seeds. The selective abortion of flowers as a response to excessive seed predation is known, as in the case of plants Glochidion acuminatum and its pollinator moth Epicephala sp. (Goto et al., Reference Goto2010). Koprdova et al. (Reference Koprdova2015) describe the significant impact of gall midge larvae on ovule fertilization, seed abortion and the viability of fully developed flowers of Centaurea cyanus. The abortion of seeds could also be the effect of the competition for resources between developing seeds. Other hypotheses are suggested as to why plants abort their seeds, e.g. poor pollination or investment in the male function (Fenner and Thompson, Reference Fenner and Thompson2005). In the light of our research, it is difficult to verify these hypotheses. We did not confirm the correlation between infertile seeds and insect larvae. The infertile seeds were present in 70.4% of all capitula, including those who did not have any insects (13% of all capitula). However, it should be noted that 91% of capitula inhabited by T. longicauda contained infertile seeds. To test the hypothesis of abortion of seeds in C. decussatum under the influence of feeding insects, further studies are needed.
The larvae of the beetle and butterfly caterpillars may play a significant role in reducing the seed number, but they were not numerous in our sample. It is worth noting that the number of Larinus sturnus is limited by its parasitoids (like in the case of T. longicauda) that develop on their hosts, completely eliminating them. The reduction of plant productivity by the insects’ parasitoids is important in the next generation as parasitoids feed on larvae, which had already damaged seeds.
Limited access of other insects to the thistle heads may be due to its morphology (involucre of several rows of hairy bracts with spines) and chemical composition. The extracts of inflorescences and the leaves of C. decussatum revealed the presence of phenolic acids (chlorogenic acid) and flavonoids (e.g. apigenin, apigenin 7-glucoside, kaempferol 3- rhamnoglucoside) (Kozyra and Skalicka-Woźniak, Reference Kozyra and Skalicka-Woźniak2014). Plant chemical defence against seed-eating pollinators was documented in Trollius europaeus, which accumulates C-glycosyl-flavone in the carpel walls (Ibanez et al., Reference Ibanez2009).
Our study demonstrates that pre-dispersal seed predation can be an important factor reducing seed availability that is of particular significance in the case of rare plants. Although seed production is usually high in Asteraceae species, together with other factors which could influence reproduction success and maintenance of plant population (e.g. seedling recruitment, habitat availability), seed predators could decrease plant fitness. In addition, our results prove that seed predators may act as a strong selective force in the evolution of plant traits, here inflorescence size. We also provide new information on the adaptive behaviour of phytophagous dipterans, a group of insects with relatively few studies on the relationship between specialized seminophagous species and threatened plants.
Acknowledgements
We would like to thank Dr A. Kostro-Ambroziak (University of Białystok) for identification of Ichneumonidae and Dr Ł. Przybyłowicz (ISEA PAS Kraków) for identification of Lepidoptera caterpillars. We would like to thank Professor P. Olejniczak (INC PAS Kraków) for constructive discussion. We are also indebted to the reviewers for helpful comments on the manuscript.









