Bloodstream infection (BSI) is the seventh leading cause of death in the United States, costing 75,000 lives annually; gram-negative bacilli account for ~50% of these cases.Reference Goto and Al-Hasan 1 , Reference Uslan, Crane and Steckelberg 2 Extended-spectrum β-lactamase (ESBL) production is among the most clinically concerning antimicrobial resistance mechanisms of gram-negative bacilli.Reference Jacoby 3 , Reference Paterson and Bonomo 4
The incidence of infections due to ESBL-producing Enterobacteriaceae (ESBLE) in the United States has increased recently in both community and tertiary-care hospitals.Reference Thaden, Fowler, Sexton and Anderson 5 , Reference Kassakian and Mermel 6 More recently, the site of acquisition for most ESBLE infections has been community-onset rather than nosocomial, making these infections less predictable.Reference Thaden, Fowler, Sexton and Anderson 5
Carbapenems are considered the treatment of choice for BSI due to ESBLE.Reference Tamma, Han and Rock 7 There is concern that the increase in incidence of ESBLE infections in the community may prompt an increase in carbapenem utilization, which may in turn contribute to further development of antimicrobial resistance and the spread of carbapenem-resistant Enterobacteriaceae (CRE).Reference Orsi, Bencardino and Vena 8
Stratification of patients based on predicted risk of ESBLE BSI provides a valuable tool for clinical providers to guide empirical antimicrobial therapy. Identification of patients at high risk of ESBLE BSI may improve adequacy of empirical antimicrobial therapy and, subsequently, patient outcomes.Reference Retamar, Portillo and López-Prieto 9 , Reference Cain, Kohn, Bookstaver, Albrecht and Al-Hasan 10 In addition, such stratification may spare patients with low risk of ESBLE BSI from unnecessary carbapenems or other broad-spectrum agents.
In this case-control study, we aimed to develop a clinical score that predicts the risk of ESBLE BSI using prior antibiotic exposure and other clinical variables that are available at the time of initial presentation.
METHODS
Settings
The study was conducted at Palmetto Health Richland and Baptist Hospitals in Columbia, South Carolina. The 2 hospitals provide care to local residents of Richland County and receive referrals from nearby counties. The Institutional Review Board at Palmetto Health approved the study and waived informed consent.
Definitions
The site of infection acquisition was classified as community-acquired, healthcare-associated or hospital-acquired as previously defined.Reference Friedman, Kaye and Stout 11 Outpatient procedures included both invasive procedures (bladder, colon, and prostate biopsies, etc.) and noninvasive procedures (colonoscopy, cystoscopy, duodenoscopy, etc.) within 30 days of index BSI.Reference Dan, Shah and Justo 12 Prior infections or colonization with ESBLE was defined as documented growth of ESBLE in any clinical culture site in both inpatient and ambulatory settings within 12 months prior to index BSI. Prior β-lactam and fluoroquinolone exposure was defined as receiving >24 hours of the respective class of antimicrobial agents within 3–90 days prior to collection of index blood culture. Multiple courses of antibiotics had to be at least 72 hours apart. Concurrent therapy with a β-lactam and a fluoroquinolone or sequential therapy with these agents within 72 hours was considered 1 course of antibiotics. β-lactams included penicillins, cephalosporins, carbapenems, monobactam, and β-lactam/β-lactamase inhibitors. Receipt of prior antibiotics was ascertained from medication administration records from current and prior hospitalizations, clinical notes from current or prior visits (including emergency department, hospital admission, discharge summary, and consultation notes), and electronic prescriptions in medical records from prior visits to affiliated hospitals or ambulatory clinics.
Case Ascertainment
All adult patients with monomicrobial BSIs due to Enterobacteriaceae from January 1, 2010, to June 30, 2015, were identified through microbiology laboratory databases at Palmetto Health (n=1,102). Recurrent episodes of BSI due to same or different Enterobacteriaceae during the study period were excluded (n=38) to ensure that cases and controls were independent of each other, as assumed by the statistical hypothesis. Chromosomally mediated AmpC-producing Enterobacteriaceae (n=153), including Enterobacter, Citrobacter, Serratia, Providencia and Morganella spp., and CRE (n=1) were also excluded due to potential overlap between risk factors for BSI due to these pathogens and ESBLE.Reference Hammer, Stoessel and Justo 13 The remaining 910 unique patients with first episodes of BSI due to Escherichia coli, Klebsiella spp., Proteus mirabilis, and Salmonella spp. were included in the study. Screening for ESBL production using the disk diffusion method with cefotaxime/clavulanate combination disks was performed in bloodstream isolates that were determined to be nonsusceptible in vitro to any tested third-generation cephalosporin according to Clinical and Laboratory Standards Institute criteria. Surveillance for CRE was available at both hospitals. In vitro antimicrobial susceptibility testing for carbapenems was routinely performed on all Enterobacteriaceae bloodstream isolates throughout the study period. In addition, PCR for KPC gene was performed on all bloodstream isolates from October 1, 2014, until the end of the study.
Statistical Analysis
Logistic regression was used to identify risk factors for ESBL production among Enterobacteriaceae bloodstream isolates in this case-control study. Demographics and clinical variables were collected for patients with BSI due to ESBLE (cases) and Enterobacteriaceae without ESBL production (controls). Prior antibiotic use was analyzed as a categorical variable: none, 1, or ≥2 courses of β-lactams and/or fluoroquinolones. Variables that were associated with ESBLE in the univariate analysis with P<.10 were included in multivariate logistic regression. Risk factors were retained in the final model if they were independently associated with ESBLE, with P<.05 in multivariate logistic regression using likelihood ratio test and individually if they were retained in ≥95% of 200 bootstrap samples. Each bootstrap sample was derived by applying the same model entry and retention criteria.
The ESBL prediction score (ESBL-PS) was derived from the final model. Points were assigned for each independent risk factor and weighted approximately by the corresponding regression coefficients. Area under receiver operating characteristic curve (AUC) was used to quantify the discriminative ability of ESBL-PS; a value of 0.5 denoted random predictions and a value of 1.0 denoted perfect predictions.
In addition to bootstrap resampling, model calibration was examined to internally validate the model. Deciles of predicted risk of ESBLE BSI were plotted from the ESBL-PS model by the actual fraction of patients who had BSI due to ESBLE to visually access calibration. The performance characteristics of the ESBL-PS as a binary classification test were examined. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated from the receiver operating characteristic curve for the best cutoff points.
JMP Pro version 12.0 (SAS Institute, Cary, NC) was used for statistical analyses. The level of significance for statistical testing was P<.05 (2-sided) unless otherwise specified.
RESULTS
Demographics, Clinical Characteristics, and Microbiology
During the study period, 910 patients with Enterobacteriaceae BSI were included in the study. Overall, their median age was 66 years, and 401 patients (44%) were men. Escherichia coli (n=597; 66%) was the most common bloodstream isolate overall, followed by K. pneumoniae (n=211; 23%), P. mirabilis (n=78; 9%), K. oxytoca (n=14; 2%), and Salmonella species (n=10; 1%).
Among Enterobacteriaceae bloodstream isolates, 42 (4.6%) demonstrated ESBL production. Escherichia coli was also the most common ESBLE (n=25; 60%), followed by K. pneumoniae (n=15; 36%) and K. oxytoca (2; 5%). Most ESBLE BSIs were acquired outside the hospital (n=33; 79%), including 12 community-acquired BSIs (29%) and 21 healthcare-associated BSIs (50%). The baseline demographics and clinical characteristics of patients in the ESBLE and control groups are shown in Table 1.
TABLE 1 Demographics and Clinical Characteristics of patients With Bloodstream Infections Due to Enterobacteriaceae
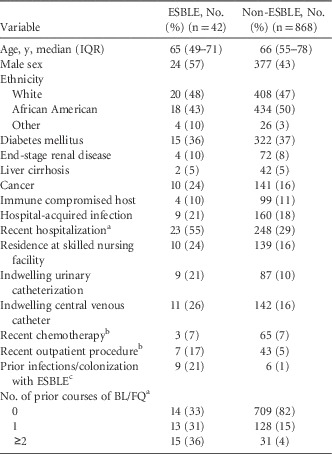
NOTE. ESBLE, extended-spectrum β-lactamase–producing Enterobacteriaceae; IQR, interquartile range; BL, β-lactams; FQ, fluoroquinolones.
Data shown as number (%) unless otherwise specified.
a Within 90 days of bloodstream infection.
b Within 30 days of bloodstream infection.
c Within 365 days of bloodstream infection.
Risk Factors for BSI Due to ESBLE
In univariate logistic regression, male sex, recent hospitalization, indwelling urinary catheters, recent outpatient procedures, prior infections or colonization with ESBLE, and prior antibiotics were associated with ESBLE. Notably, nosocomial acquisition was not associated with ESBLE (Table 2).
TABLE 2 Univariate Logistic Regression Results for Risk Factors of Bloodstream Infection Due to Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae (ESBLE)

NOTE. OR, odds ratio; CI, confidence interval; BL, β-lactams; FQ, fluoroquinolones.
a Within 90 days of bloodstream infection.
b Within 30 days of bloodstream infection.
c Within 365 days of bloodstream infection.
After adjustments in the multivariate model, male sex (adjusted odds ratio [aOR], 1.6; 95% confidence intervals [CI], 0.8–3.4; P=.18), recent hospitalization (aOR, 1.0; 95% CI, 0.4–2.3; P=.98), and indwelling urinary catheters (aOR, 1.5; 95% CI, 0.6–3.5; P=.40) were not independently associated with ESBLE BSI. Recent outpatient procedures, prior infections or colonization with ESBLE, and prior exposure to antibiotics were independently associated with ESBLE BSI (Table 3).
TABLE 3 Independent Risk Factors of Bloodstream Infection Due to Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae (ESBLE) and Point Allocation in ESBL Prediction Score

NOTE. aOR: adjusted odds ratio; CI: confidence interval; GI: gastrointestinal; GU: genitourinary; BL: β-lactams; FQ: fluoroquinolones.
a Within 30 days of bloodstream infection.
b Within 3 to 90 days of bloodstream infection.
c Multiple courses of antibiotics are given at least 3 days apart.
d Within 365 days of bloodstream infection.
Development and Internal Validation of ESBL Prediction Score
All 3 variables that were independently associated with ESBLE BSI were retained in ≥95% of bootstrap samples and were included in the final model. The AUC for the final logistic regression model was 0.86. The ESBL prediction score (ESBL-PS) was developed by assigning points for each risk factor in the final model, weighted approximately by the corresponding regression coefficients (Table 3). The ESBL-PS is the cumulative number of points for each individual from their risk factors, and ranges from 0 to 8. The AUC for the ESBL-PS was 0.86 (Supplementary Figure 1). Model calibration was satisfactory; the observed outcomes were fairly close to the predictions (Supplementary Figure 2).
The ESBL-PS equips clinicians with a tool with which to predict the risk of ESBLE BSI (Figure 1). A higher score corresponds to an increased risk of ESBLE BSI. For example, patients with an ESBL-PS of 0 have <1% predicted probability of ESBLE BSI. The estimated risk increases to >20% and >50% in patients with ESBL-PSs of 3 and 5, respectively.

FIGURE 1 Estimated probability of bloodstream infection due to extended-spectrum β-lactamase Enterobacteriaceae (ESBLE) based on ESBL prediction score. Size of marker is approximately proportional to number of subjects with that particular score. BSI, bloodstream infection.
The performance characteristics of the ESBL-PS as a binary classification test for selected cutoff points are shown in Table 4. Using a cutoff score ≥3 to indicate high risk of ESBLE BSI provided the best performance in the statistical model with a NPV of 97%. A more conservative approach using a cutoff score ≥1 improved sensitivity and NPV to 88% and 99%, respectively, at the expense of specificity and PPV.
TABLE 4 Performance of Extended-Spectrum β-Lactamase (ESBL) Prediction Score as a Binary Classification Test

NOTE: ESBL-PS, ESBL prediction score; PPV, positive predictive value; NPV, negative predictive value.
a Using an ESBL-PS ≥3 to indicate high predicted risk of bloodstream infection due to ESBL-producing Enterobacteriaceae provides the best performance in statistical model.
DISCUSSION
Independent Risk Factors for ESBLE BSI
The results of this study demonstrate that the risk of BSI due to ESBLE can be estimated with high discrimination based on prior antibiotic exposure, prior infections or colonization with ESBLE, and recent outpatient procedures.
The association between recent antibiotic use and ESBLE infections has been well established in previous studies.Reference Jacoby 3 , Reference Paterson and Bonomo 4 , Reference Tumbarello, Trecarichi and Bassetti 14 – Reference Ben-Ami, Rodríguez-Baño and Arslan 16 In the current study, we used a novel approach of categorizing this variable by the number of antibiotic courses received within the prior 90 days. This method improved model discrimination due to considerably higher risk of ESBLE BSI within a smaller proportion of patients who received multiple courses of antibiotics compared to those who received only 1 prior course. Identification of prior infections or colonization with ESBLE within the prior 12 months as the strongest risk factor for ESBLE BSI was consistent with the results of a large investigation in Netherlands.Reference Rottier, Bamberg, Dorigo-Zetsma, van der Linden, Ammerlaan and Bonten 15 Recent outpatient genitourinary and gastrointestinal procedures have been associated with increased risk of acquisition of bacteria that harbor antimicrobial resistance.Reference Dan, Shah and Justo 12 , Reference Williamson, Roberts and Paterson 17 , Reference Epstein, Hunter and Arwady 18 The increased risk of ESBLE BSI following these procedures calls for improvements in infection control practices in these settings.
Clinical Applications of ESBL-PS
The implementation of the ESBL-PS into workflow practices of healthcare providers is feasible due to the small number and accessibility of these variables. Some risk factors may be displayed in real time in electronic medical records (ie, prior infections or colonization with ESBLE); other data (ie, prior antibiotics or outpatient procedures) may be collected in a patient interview or quickly obtained from the electronic medical record if the patient is unable to communicate.
Application of the ESBL-PS provides clinicians with opportunities to improve both empirical antimicrobial therapy and carbapenem utilization. The ESBL-PS categorizes patients based on predicted risk of ESBLE BSI into 3 risk groups: low, moderate, and high (Figure 2). Low-risk patients have no risk factors; moderate-risk patients are those with minor risk factors such as an outpatient procedure or 1 prior course of antibiotics; and high-risk patients are those with major risk factors such as prior infections or colonization with ESBLE or multiple courses of antibiotics. The decision to withhold carbapenem therapy was straightforward in nearly 75% of patients in the cohort who had low predicted probability of ESBLE BSI. Similarly, empirical carbapenem therapy appears to have been appropriate in the high-risk group, which represented 6% of the study cohort; however, prescription of carbapenems to the remaining 20% of patients in the moderate-risk group does not appear to have been a high-yield intervention. To improve the benefit-to-risk ratio of antimicrobial therapy, the stratification of the moderate-risk group further is suggested, according to the acute severity of illness using the Pitt bacteremia scoreReference Patterson, Ko, Von Gottberg, Mohapatra and Casellas 19 or the predicted prognosis using the BSI mortality risk score (BSIMRS).Reference Al-Hasan, Lahr, Eckel-Passow and Baddour 20 , Reference Al-Hasan, Juhn, Bang, Yang and Baddour 21 Because survival benefit from adequate empirical antimicrobial therapy has been clearly demonstrated in critically ill patients (Pitt bacteremia score ≥4) and those with guarded prognosis at initial presentation (ie, BSIMRS ≥5),Reference Cain, Kohn, Bookstaver, Albrecht and Al-Hasan 10 carbapenem therapy may be justified in those patients in the moderate-risk group. Application of the proposed algorithm results in the exposure of only 10% of patients to carbapenems (which is nearly twice the overall incidence of ESBLE among bloodstream isolates in the current study) while providing adequate empirical therapy to ~98% of overall cohort and >99% of critically ill patients. It is reassuring that this improvement in the adequacy of empirical therapy will not result in an increase in overall carbapenem utilization, even in institutions with existing carbapenem restrictions (like the 2 hospitals included in this study). In the current cohort of 910 patients, 92 (10.1%) received empirical carbapenem therapy, including 9 of 42 (21.4%) in the ESBLE and 83 of 868 (9.6%) in the control group. Modification of institutional criteria for empirical carbapenem use according to the proposed algorithm is justified because it allows for earlier appropriate therapy in high-risk patients without an increase in overall carbapenem use.

FIGURE 2 Proposed algorithm for application of extended-spectrum β-lactamase (ESBL) prediction score in management of bloodstream infections. BSI, bloodstream infection; ESBL-PS, extended-spectrum β-lactamase prediction score.* Patterson DL, et al. Ann Intern Med 2004;140:26–32.
Changing Epidemiology of ESBLE BSI
Since ESBLEs were first reported in 1983,Reference Knothe, Shah, Krcmery, Antal and Mitsuhashi 22 prolonged hospitalization has been considered among the most important risk factors for harboring these organisms.Reference Jacoby 3 , Reference Paterson and Bonomo 4 However, ESBLEs have emerged as community-onset pathogens since the beginning of this century,Reference Rodríguez-Baño, Navarro and Romero 23 , Reference Pitout, Hanson, Church and Laupland 24 and they have recently been reported with increasing frequency in community-onset infections in North America and Europe.Reference Thaden, Fowler, Sexton and Anderson 5 , Reference Kassakian and Mermel 6 , Reference Courpon-Claudinon, Lefort, Panhard, Clermont, Dornic and Fantin 25 The current study confirms that most ESBLE BSIs are acquired outside the hospital and that nosocomial acquisition is not a risk factor for ESBLE in this setting. We suspect that the widespread use of broad-spectrum antibiotics in the community (both orally and intravenously) and improvements in infection control practices in hospitals have shifted most ESBLE infections to the community-onset setting. In addition, E. coli has surpassed Klebsiella spp. to become the most common ESBLE.Reference Thaden, Fowler, Sexton and Anderson 5 , Reference Kassakian and Mermel 6 The ability of E. coli, particularly sequence type 131, to adapt to local environments and spread in the community poses serious challenges to the healthcare system.Reference Nicolas-Chanoine, Bertrand and Madec 26 , Reference Banerjee, Johnston and Lohse 27 Moreover, the widespread dissemination of the CTX-M-15 genotype among E. coli sequence type 131 in the southeastern United States requires an urgent call to change antibiotic prescription practices in the community.Reference Hayakawa, Gattu and Marchaim 28 , Reference Chen, Freeman and Nicholson 29 Efforts to minimize unnecessary prescription of antibiotics, particularly β-lactams and fluoroquinolones, in outpatient settings should be emphasized.Reference Meeker, Linder and Fox 30
The main strength of this study is the inclusion of patients from 2 community hospitals in addition to data from inpatient and outpatient procedure records, prior culture results, and antibiotic prescriptions from several affiliated hospitals, emergency rooms, urgent treatment centers, and outpatient clinics in the area.Reference Kutob, Justo, Bookstaver, Kohn, Albrecht and Al-Hasan 31 Despite the limitations of retrospective design, only 5 of 42 patients with ESBLE BSIs did not have identifiable risk factors. Of these 5 patients, 2 were temporary visitors with recent international travel and no documentation of prior culture results, antibiotic exposures, or procedures in medical records. Another 2 were transferred from nearby medical facilities with incomplete transfer records of administered medications. Transfers from other healthcare facilities within the same country and receiving healthcare internationally have been associated with increased risk of harboring ESBLE in prior studies,Reference Tumbarello, Trecarichi and Bassetti 14 , Reference Goodman, Lessler and Cosgrove 32 likely due to uncaptured prior antibiotic exposure, procedures, and culture results. Antimicrobial stewardship programs should explore educating healthcare providers regarding the value of inquiring about prior antibiotic use upon selection of empirical therapy for potentially life-threatening infections. Requesting records of medications, procedures, and prior culture results of patients transferred to tertiary care hospitals is equally important. Second, due to the relatively small number of ESBLE BSI, the study may have lacked power to identify other minor risk factors with equal or less weight to outpatient procedures or 1 course of antibiotics. Third, the model was derived from 2 hospitals within the same healthcare system and geographical location. Data from multiple settings may provide external validity and generalizability. Finally, molecular testing was not performed on bloodstream isolates to identify CTX-M or other ESBL genotypes.
In the era of increasing antimicrobial resistance among community-onset bacterial isolates, efforts to improve utilization of currently available antibiotics and to prevent further induction of antimicrobial resistance are of vital importance. Prediction of ESBLE based on patient-specific risk factors provides an alternative solution to the nonstratified use of carbapenems for the general fear of ESBLE infections. The ESBL-PS allows healthcare providers to stratify patients based on estimated risk of BSI due to ESBLE with high discrimination. Application of the ESBLE-PS based on acute severity of illness may improve the adequacy of empirical antimicrobial therapy without increasing carbapenem utilization.
ACKNOWLEDGMENTS
The authors thank the Palmetto Health Antimicrobial Stewardship and Support Team in Columbia, South Carolina, for their help in conducting this study.
Financial support: M.R.A. received funding from the Research Program for Medical Students at the University of South Carolina School of Medicine in Columbia, South Carolina. The funding source had no role in study design.
Potential conflicts of interest: J.A.J. is an advisory board member for Cempra Pharmaceuticals. P.B.B. is a research advisory board for and receives research funding from Actavis Pharmaceuticals. He also serves on the continuous medical education steering committee and is a speaker for Rockpointe Corporation, Columbia, Maryland.
M.N.A. is on the continuous medical education steering committee for Rockpointe Corporation, Columbia, Maryland. All other authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2016.292








