Introduction
Obsessive-compulsive disorder (OCD) is a severe and relatively common mental disorder characterized by obsessions and/or compulsions that significantly interfere with daily functioning. It is currently classified as an anxiety disorder in DSM-IV-TR (APA, 2000). In ICD-10 (WHO, 1992), OCD is classified under the broad umbrella of ‘neurotic, stress-related and somatoform disorders’, consistent with the historical and phenomenological links between OCD and other anxiety and somatoform disorders. However, the status of OCD as an anxiety disorder has been a matter of debate for years (Stein et al. Reference Stein, Fineberg, Bienvenu, Denys, Lochner, Nestadt, Leckman, Rauch and Phillips2010). This was clearly illustrated in a recent survey among 187 international OCD experts, who were asked whether they would support the exclusion of OCD from the current supra-ordinate category of anxiety disorders in DSM-5; approximately 60% agreed and the remaining 40% disagreed (Mataix-Cols et al. Reference Mataix-Cols, Pertusa and Leckman2007). Another contentious issue for DSM-5 is the suggestion that OCD should be classified alongside a ‘spectrum’ of other disorders that are characterized by repetitive thoughts and/or behaviours and are presumed to be related to OCD (Greeven et al. Reference Greeven, van Balkom, van Rood, van Oppen and Spinhoven2006; Fineberg et al. Reference Fineberg, Saxena, Zohar and Craig2007; Hollander et al. Reference Hollander, Braun and Simeon2008). Earlier conceptualizations of the OCD spectrum included a broad range of potential members but these have been recently reduced to a smaller number of candidate disorders including body dysmorphic disorder, hoarding disorder, hypochondriasis, Tourette's disorder, trichotillomania and obsessive-compulsive personality disorder (Hollander et al. Reference Hollander, Kim, Braun, Simeon and Zohar2009; Phillips et al. Reference Phillips, Stein, Rauch, Hollander, Fineberg, Saxena, Mataix-Cols, Wilhelm, Leckman, Kelly, Fallonm and Barsky2010).
The research agenda for the DSM-5 emphasizes the importance of applying the findings from basic and clinical neurosciences to guide psychiatric classification (APA, 2002). Surprisingly, direct comparisons of brain function and structure between OCD and other anxiety or OCD spectrum disorders have been very scarce (Rauch et al. Reference Rauch, Savage, Alpert, Fischman and Jenike1997; van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005b). Rauch et al. (Reference Rauch, Savage, Alpert, Fischman and Jenike1997) measured relative regional cerebral blood flow using positron emission tomography in the context of symptom provocation paradigms in OCD, specific phobia and post-traumatic stress disorder and reported that hyperactivation in several paralimbic brain regions, the inferior prefrontal cortex, the basal ganglia and the brain stem were common across these anxiety disorders. An functional magnetic resonance imaging (fMRI) study by our group found both overlapping and unique neural correlates during a disease-specific emotional Stroop task in OCD, panic disorder (PD) and hypochondriasis, involving both limbic and frontal-striatal regions (van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005b). A recent meta-analysis of voxel-based morphometry studies in OCD and other anxiety disorders revealed common as well as distinct neural substrates in OCD and other anxiety disorders (Radua et al. Reference Radua, van den Heuvel, Surguladze and Mataix-Cols2010). This paucity of data might be partly explained by different traditions in OCD and anxiety research and the use of different experimental paradigms, thus hampering direct comparisons (Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006).
In the context of the current deliberations regarding whether or not OCD should be classified as an anxiety disorder and whether there should be a new grouping of obsessive-compulsive spectrum disorders in DSM-5 (Greeven et al. Reference Greeven, van Balkom, van Rood, van Oppen and Spinhoven2006; Fineberg et al. Reference Fineberg, Saxena, Zohar and Craig2007; Mataix-Cols et al. Reference Mataix-Cols, Pertusa and Leckman2007; Hollander et al. Reference Hollander, Braun and Simeon2008, Reference Hollander, Kim, Braun, Simeon and Zohar2009; Phillips et al. Reference Phillips, Stein, Rauch, Hollander, Fineberg, Saxena, Mataix-Cols, Wilhelm, Leckman, Kelly, Fallonm and Barsky2010; Stein et al. Reference Stein, Fineberg, Bienvenu, Denys, Lochner, Nestadt, Leckman, Rauch and Phillips2010), we set out to investigate the specificity of altered frontal-striatal and limbic activations during planning in OCD, a prototypical anxiety disorder (PD) and a putative OCD spectrum disorder (hypochondriasis). Using the Tower of London paradigm (Shallice, Reference Shallice1982; van den Heuvel et al. Reference van den Heuvel, Groenewegen, Barkhof, Lazeron, van Dyck and Veltman2003), a task frequently used to probe planning processes, we have previously reported impaired recruitment of dorsal frontal-striatal brain regions in unmedicated patients with OCD compared with healthy controls (van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom, van Hartskamp, Barkhof and van Dyck2005a). Since most fMRI paradigms that have been developed to study anxiety disorders use disorder-specific stimuli, these paradigms are less well suited to investigate shared neural substrates across diagnoses. Symptom-independent tasks, such as the Tower of London, can potentially be used to make useful comparisons across disorders. Since the frontal-striatal circuits seem to be involved in the pathophysiology of OCD, other anxiety disorders, as well as in other repetitive behaviours within the obsessive-compulsive spectrum (van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005b; Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006), this task is potentially useful to investigate the overlapping and differentiating neural correlates across these disorders. Here, we tested the null hypothesis that OCD, PD and hypochondriasis would show similar functional alterations in frontal-striatal circuits during planning, compared with healthy controls. In OCD, other anxiety disorders as well as in obsessive-compulsive spectrum disorders, the frontal-striatal failure is accompanied by limbic disturbances (Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006). Therefore, we also predicted similar interactions between frontal-striatal and limbic regions across these disorders.
Method
Participants
In total, 50 unmedicated patients (mean age=34.0, range=18–64 years) meeting diagnostic criteria for OCD (n=22), PD (n=14), or hypochondriasis (n=14) and 22 healthy individuals (mean age=29.8, range=21–49 years) participated in the study. None of the patients met criteria for any of the other two disorders. The patients and the healthy controls all participated in previous studies (van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom, van Hartskamp, Barkhof and van Dyck2005a, Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyckb). Diagnoses were established using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (First et al. Reference First, Spitzer, Gibbon and Williams1996). Patients were recruited from the out-patient department for anxiety disorders of GGZ in Geest in Amsterdam, the Netherlands Anxiety, OCD and Phobia Foundation and by advertisement. Exclusion criteria were the presence of major somatic disorders, other major psychiatric disorders (except co-morbid depression) and the use of psychotropic medication during the 4 weeks prior to scanning. Healthy controls were recruited from hospital and university staff and by advertisement. They were interviewed similarly to exclude any somatic and psychiatric disorder. The ethical board of the VU University Medical Center approved the study and all participants provided written informed consent.
fMRI paradigm
A pseudo-randomized self-paced version of the Tower of London was used, discussed in detail previously (van den Heuvel et al. Reference van den Heuvel, Groenewegen, Barkhof, Lazeron, van Dyck and Veltman2003). In summary, the task consisted of one baseline condition and five planning conditions ranging from one to five moves. In the planning conditions, subjects were presented with a starting configuration and a target configuration with the instruction to ‘count the number of steps’. Two possible answers were shown at the bottom of the screen, from which the correct one had to be selected. In both configurations, three coloured beads were placed on three vertical rods, which could accommodate one, two and three beads each. One bead could be moved at a time and only when there was no other bead on top. Subjects were requested to determine the minimal number of moves necessary to reach the target configuration and to press the button corresponding to the side (right or left) of the screen where the correct answer was presented. In the baseline condition, subjects simply had to count the number of yellow and blue beads. A pseudo-randomized design was adopted, with a baseline condition after each planning trial of three or more moves, to control for carryover effects due to perseveration of task-related cognitive processes after a difficult trial. A maximum of 30 s for each trial was allowed. No feedback on performance was provided during the task. Directly after task completion, while still in the magnetic resonance (MR) magnet, each subject rated his/her subjective general distress using a 100-point visual analogue scale, answering the question, ‘how distressed do you feel at this moment?’. To ensure that participants were familiar with the task, the task was explained and practised outside the scanner 1 week prior to data collection and shortly repeated while lying in the scanner prior to scanning.
Data acquisition and processing
Imaging was performed on a 1.5 Tesla Sonata system (Siemens Medical Solutions, Germany) with a standard circularly polarized head coil. Stimuli were generated by a Pentium personal computer (Dell Inc., USA) and projected on a screen at the end of the scanner table, which was seen through a mirror mounted above the subject's head. Two magnet-compatible four-key response boxes (Lumitouch; Photon Control, Canada) were used to record the subject's performance and response latencies. To reduce motion artefacts, foam pads were used to immobilize the participants' heads.
The anatomical scans included 160 coronal slices (slice thickness=1.5 mm) acquired with a 3D gradient-echo T1-weighted sequence [flip angle=8°; repetition time (TR)=2700 ms; echo time (TE)=4 ms; inversion time=950 ms; bandwidth=190 Hz/pixel], with an in-plane resolution of 256×192 pixels (pixel size 1 mm2). For fMRI, an echo-planar imaging (EPI) sequence (flip angle=90°; TR=3045 ms; TE=45 ms; matrix 64×64 pixels; field of view 192×192 mm) was used, creating, in total, 433 transversal whole brain acquisitions (35 slices, slice thickness=2.5 mm; interslice gap=0.5 mm; in-plane resolution=3×3 mm).
Images were processed and analysed using SPM5 (Wellcome Department of Cognitive Neurology, UK). The origin of each MR volume was aligned on the anterior commissure. After discarding the first four volumes, the series was corrected for differences in slice acquisition times and realigned. Spatial normalization into approximate Talairach and Tournoux space was performed using a standard statistical parametric mapping EPI template. Data were resliced to 3×3×3 mm voxels and spatially smoothed using an 8 mm isotropic Gaussian kernel.
Data analyses
After high-pass filtering (cut-off 128 s), functional scans were analysed in the context of the general linear model using δ functions convolved with a canonical haemodynamic response function to model responses of varying lengths. Error trials were modelled separately as a regressor of no interest. The number of EPIs for each condition did not significantly differ between groups. For each subject, weighted contrasts were computed for both main effect (i.e. planning versus baseline) and task load effects (van den Heuvel et al. Reference van den Heuvel, Groenewegen, Barkhof, Lazeron, van Dyck and Veltman2003). The contrast images derived from these first-level analyses, containing parameter estimates of main effects (planning versus baseline and task load effects), were entered into second-level analyses, using one-way analyses of variance (ANOVA) for each contrast. In addition, the associations between task-related activations and state anxiety and performance within the patient group were examined using whole-brain multiple regression analyses, with the individual scores for latency, accuracy and state anxiety as separate regressors. Effects for groups are reported at p<0.05 corrected for multiple comparisons (false discovery rate). Group by task interaction effects, masked with the appropriate main effect (in this way restricting the search volume and reducing the need for correction) are reported at an uncorrected threshold of p<0.001, enabling a directed search with high sensitivity to small but meaningful effects. The regression analyses were thresholded similarly.
Demographic and behavioural data were analysed using SPSS software (version 15.0; SPSS Inc., USA).
Results
Sample characteristics
Demographic and clinical characteristics of the patient and control groups are listed in Supplementary Table 1 (available online). The four groups were comparable in terms of mean age and handedness. There was a significantly greater proportion of females in the OCD group and of males in the hypochondriasis group. The prevalence of co-morbid depression also differed across the diagnostic groups (9.1%, 25.0% and 41.7% in the OCD, PD and hypochondriasis groups, respectively). Therefore, both gender and presence of co-morbid depression were controlled for in subsequent analyses.
Behavioural data
Response latencies showed a significant main effect for task load [Huynh–Feldt corrected: F=351.59, degrees of freedom (df)=1.802, p<0.001] but no main effect of group (F=2.651, df=1, p=0.110) or group by task interaction effect (F=0.653, df=1.802, p=0.507) (see Supplementary Fig. 1 a, available online). Accuracy of performance (see Supplementary Fig. 1 b, online) showed a significant main effect for task load (Huynh-Feldt corrected: F=20.48, df=2.804, p<0.001) and a significant main effect for group (F=6861.14, df=1, p=0.013), but no task×group interaction effect (F=1.458, df=2.804, p=0.229). Post-hoc independent sample t tests comparing patients (combined) and controls showed that patients were less accurate on all levels of task load, except for the highest difficulty level (five moves). Further post-hoc tests showed that the main effect of group was driven by a significant difference between OCD patients and controls (F=8.082, df=1, p=0.007).
Subjective anxiety data
The patients (combined) had significantly higher state anxiety scores than controls (Student's t test for independent samples: F=10.844; df=68.109; p=0.001). Post-hoc tests revealed that this difference was primarily driven by the OCD group (ANOVA with Hochberg's post-hoc test: F 3.65=4.75; p=0.010), whereas no significant differences were found between the PD, hypochondriasis and control groups. Within the pooled patient group there was a significant negative correlation between state anxiety and performance accuracy for the highest planning condition (five moves) (Pearson's r=−0.356, p=0.011). A similar pattern of correlation was seen in each of the three patient groups: OCD (Pearson's r=−0.558, p=0.007); PD (Pearson's r=−0.464, p=0.11); hypochondriasis (Pearson's r=−0.356, p=0.012).
Neural correlates
Main effects of task and group by task interaction effects
The main effects of task are presented in Supplementary Table 2 a, b (planning versus baseline) and Supplementary Table 3 a, b (task load effects). In controls, planning (compared with baseline) was related to increased activation of the premotor cortex, striatum, thalamus and visuospatial brain regions (precuneus, inferior parietal and superior occipital cortices). Unlike the healthy controls, all diagnostic groups (both combined and individually) showed unique activations in dorsolateral prefrontal regions and a lack of striatal activations (Supplementary Table 2 a, b).
Compared with healthy controls, patients (combined) showed decreased recruitment of task-related brain regions, i.e. the precuneus, caudate nucleus, globus pallidus and thalamus (Table 1 and Fig. 1). Although the comparisons between healthy controls and each diagnostic group separately showed subtle differences across the groups, there were no statistically significant differences in activation between the three diagnostic groups (absence of an effect of group). A conjunction analysis showed a shared decreased recruitment of the precuneus, caudate nucleus, globus pallidus and thalamus across the three disease groups.

Fig. 1. Decreased activation in subcortical brain regions, such as left caudate nucleus (plot A) and left thalamus (plot B), in patients with obsessive-compulsive disorder (OCD), hypochondriasis and panic disorder compared with healthy controls during planning versus baseline.
Table 1. Group×task interactions

BA, Brodmann's area; T, T value; k E, cluster size; OCD, obsessive-compulsive disorder; PFC, prefrontal cortex; PD, panic disorder.
* At lower significance level (p=0.001, T=3.16): L caudate nucleus (x, y, z=−6, 12, 0).
† No significant results for comparison between each individual diagnostic group versus healthy controls.
In both controls and patients, task load correlated with activation in the same brain regions and additionally with activation of the more anterior parts of the dorsal prefrontal cortex and the anterior cingulate cortex (Supplementary Table 3 a, b). Compared with healthy controls, patients (combined) showed decreased recruitment of the precuneus (Table 1). Although the comparisons between healthy controls and each diagnostic group separately showed that this effect was significant only in patients with PD, conjunction analysis showed shared decreased recruitment of the precuneus across the three disease groups and there were no statistically significant differences in activation between the three diagnostic groups.
All analyses for main effects of task and task by group interaction effects remained unchanged when gender was modelled as regressor of no interest. The results of the comparisons across diagnostic groups also remained when controlling for co-morbid depression and state anxiety. Moreover, task-related differences between patients and healthy controls remained after the exclusion of those patients with co-morbid depression (n=14). Direct comparison between patients with and without co-morbid depression showed no significant results for planning versus baseline and increased recruitment of the left ventrolateral prefrontal cortex (x, y, z=−48, 18, 15; T=4.79. k E=15, p<0.001 uncorrected) in patients with co-morbid depression with increased task load.
Effect of performance
Investigating the within-group effect of task performance (n=50 patients), using both response latency and accuracy, we found a positive correlation between task performance (increased accuracy and decreased response latency) and activation in dorsolateral and anterior prefrontal cortex, premotor cortex, supplementary motor area (SMA) and visuospatial brain regions (Table 2 and Fig. 2). In contrast, task performance was negatively correlated with activation in the ventrolateral prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex, medial prefrontal cortex, amygdala, insula and temporal cortex (Table 2 and Fig. 2). Regression analyses with performance scores in healthy controls only showed a positive correlation between accuracy and activation in the anterior cingulate cortex (x, y, z=0, 42, 9; T=4.34, k E=4, ρ=0.602, p=0.003).
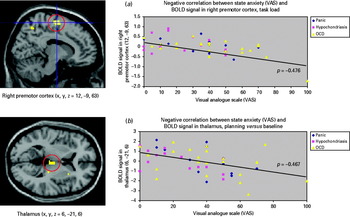
Fig. 2. Activation in the dorsolateral prefrontal cortex (a) correlates positively with performance (planning versus baseline; in all patients ρ=0.630, p<0.001; in obsessive-compulsive disorder (OCD) ρ=0.623, p=0.002; in hypochondriasis ρ=0.521, p=0.056; in panic disorder: ρ=0.881, p<0.001), whereas activation in the right amygdala (b) correlates negatively with performance (task load effects; in all patients ρ=−0.317, p=0.025; in OCD ρ=−0.423, p=0.05; in hypochondriasis ρ=−0.046, p=0.876; in panic disorder ρ=−0.481, p=0.081). BOLD, blood oxygen level-dependent; DLPFC, dorsolateral prefrontal cortex.
Table 2. Regressions with performance (n=50)

BA, Brodmann's Area; k E, cluster size; ρ, Spearman's rho; VLPFC, ventrolateral prefrontal cortex; ACC, anterior cingulate cortex; OFC, orbitofrontal cortex; PFC, prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; SMA, supplementary motor cortex.
* p<0.05, ** p<0.01, *** p<0.001.
Effect of state anxiety
Within-group regression analyses (n=50 patients) showed that state anxiety negatively correlated with activation in subcortical (thalamus and caudate nucleus) and cortical (precuneus, premotor cortex, SMA and anterior cingulate cortex) brain regions (see Supplementary Table 4 and Fig. 3). The healthy control participants did not show any significant correlations between state anxiety and task-related blood oxygen level-dependent (BOLD) response.

Fig. 3. Negative correlation between state anxiety, measured with the visual analogue scale (VAS) and activation in frontal-striatal brain regions, such as premotor cortex [(a), task load effects; in all patients ρ=−0.476, p<0.001; in obsessive-compulsive disorder (OCD) ρ=−0.528, p=0.012; in hypochondriasis ρ=−0.210, p=0.470; in panic disorder ρ=−0.702, p=0.005] and thalamus [(b), planning versus baseline; in all patients ρ=−0.467, p=0.001; in OCD ρ=−0.363, p=0.097; in hypochondriasis ρ=−0.547, p=0.043; in panic disorder ρ=−0.625, p=0.017]. BOLD, blood oxygen level-dependent.
Association between brain activation, performance and state anxiety
In order to examine which variables were most strongly predictive of task performance, we conducted a hierarchical multiple regression analysis controlling for state anxiety in the first step (Enter method) and then including the % BOLD signal change in the dorsolateral prefrontal cortex (DLPFC) and amygdala as regressors in the second step (stepwise method). We found activation in DLPFC and amygdala were independent predictors of task performance across the three patient groups (adjusted R 2=0.45; DLPFC β=0.48, p<0.001; amygdala β=−0.34, p=0.004), over and above the effects of state anxiety, which was not a significant predictor (β=−0.18, p=0.09). Collinearity statistics were examined and found to be adequate (in all cases, tolerance >0.89 and variance inflation factor >1.12).
Discussion
The present study constitutes one of the few direct comparisons between OCD and other potentially related disorders, using a standardized symptom-independent fMRI paradigm that probes the frontal-striatal circuits, which are known to be dysfunctional in OCD and related disorders. We found that patients with OCD, PD and hypochondriasis show similar alterations in task-related frontal-striatal and visuospatial regions during planning compared with healthy controls. Overall, patients showed decreased recruitment of planning-related brain regions, such as precuneus, caudate nucleus, globus pallidus and thalamus. Pair-wise comparisons between healthy controls and each diagnostic group separately showed subtle differences between the diagnostic groups, with predominant failure of the caudate nucleus in patients with OCD and decreased recruitment of thalamus and anterior prefrontal cortex in patients with PD. Direct comparison across the diagnostic groups, however, showed no statistically significant differences between the three patient groups. Thus, the frontal-striatal dysfunctions commonly described in OCD patients (van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom, van Hartskamp, Barkhof and van Dyck2005a) may not be specific to OCD and rather be shared with these other disorders. The findings cannot be explained by an overlap in co-morbid anxiety diagnoses across the patient groups. The overlap across the diagnoses can only partly be explained by the effect of state anxiety, since similar results remain after controlling for state anxiety. We also controlled for gender and co-morbid depression and found that these variables had no impact on the results.
We found a positive correlation between task performance and activation in dorsolateral and anterior prefrontal cortex, premotor cortex, SMA and visuospatial brain regions in all patients combined (n=50) and in each disorder separately. In contrast, task performance was negatively correlated with activation in ventrolateral prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex, medial prefrontal cortex, amygdala, insula and temporal cortex across disorders. Since in the present study patients with OCD showed the most serious planning impairment at the behavioural level (lowest accuracy scores), these patients also showed the most significant dorsal frontal-striatal impairment. This effect cannot be explained by deviant brain activation during false responses, since in our task only the correct answered events are modelled (van den Heuvel et al. Reference van den Heuvel, Groenewegen, Barkhof, Lazeron, van Dyck and Veltman2003). These findings suggest a similar interaction between brain regions involved in ‘higher order cognitive’ processes (dorsal frontal-striatal regions) and brain regions involved in ‘emotional’ processes (ventral frontal-striatal and limbic regions) in all three disorders, thus supporting the hypothesis that reciprocal influences between cognitive and emotional processes are shared across multiple disorders (Remijnse et al. Reference Remijnse, van den Heuvel and Veltman2005; Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006). The involvement of the dorsal frontal-striatal regions is consistent with a growing literature suggesting that frontal-striatal alterations in OCD are not limited to ventral/limbic circuits, but also extend to dorsal ‘cognitive’ regions (Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006; Menzies et al. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore2008; Harrison et al. Reference Harrison, Soriano-Mas, Pujol, Ortiz, Lopez-Sola, Hernandez-Ribas, Deus, Alonso, Yucel, Pantelis, Menchon and Cardoner2009). Of note, our multiple regression analyses further suggested that this dorsal frontal-striatal hypoactivation and limbic hyperactivation are not directly correlated, but that both processes may contribute independently to executive dysfunction across the anxiety disorders.
We were able to examine the contribution of state anxiety to task performance and neural activation. While state anxiety correlated with task performance (at a difficulty level of five moves only) and activation in subcortical (thalamus and caudate nucleus) and cortical (precuneus, premotor cortex, SMA and anterior cingulate cortex) brain regions, the results of our multiple regression analysis showed that task performance was best predicted by activation in DLPFC (positive correlation) and amygdala (negative correlation), over and above the effect of state anxiety.
Overall, the present results suggest an imbalance within and between the dorsal cognitive-related and ventral, limbic-related frontal-striatal circuits and a dysfunctional relationship of these circuits with the amygdala, underlying the symptoms and specific cognitive deficits across these three disorders (Remijnse et al. Reference Remijnse, van den Heuvel and Veltman2005; Mataix-Cols & van den Heuvel, Reference Mataix-Cols and van den Heuvel2006). Along which pathways the interactions between the prefrontal cortex and the amygdala are precisely organized is as yet not completely understood. Whereas the basal amygdala projects to virtually the entire prefrontal cortex, including its dorsolateral division (Amaral & Price, Reference Amaral and Price1984), direct prefrontal projections to the amygdala appear to be sparser and they stem mainly from the medial and orbitofrontal areas. Yet, an inhibitory influence of the prefrontal cortex on the amygdala may be explained by prefrontal cortical inputs to the so-called intercalated cell groups of the amygdala, which, in turn, provide an inhibitory input to the central, medial and basal amygdaloid nuclei (Pare et al. Reference Pare, Quirk and LeDoux2004). Functional studies in rodents have shown that at least a subset of these intercalated cell groups may play a role in the regulation of anxiety and fear extinction (Barbas & Zikopoulos, Reference Barbas and Zikopoulos2007; Jungling et al. Reference Jungling, Seidenbecher, Sosulina, Lesting, Sangha, Clark, Okamura, Duangdao, Xu, Reinscheid and Pape2008; Likhtik et al. Reference Likhtik, Popa, Pergis-Schoute, Fidacaro and Pare2008). Future studies are necessary to elucidate the pathways along which the DLPFC can exert its presumed top-down control of the amygdala and in which way limbic activation might directly or indirectly influence executive functioning by the dorsal frontal-striatal circuit. Rich networks of cortico-cortical connections (Barbas, Reference Barbas2000) or interconnections between functionally different prefrontal cortical-thalamocortical circuits via thalamocortical and dopaminergic brainstem projections (Haber, Reference Haber2008) may play a role in such interactions between prefrontal cortical areas and associated cortical basal ganglia circuits involved in cognitive and emotional functions. Recent developments in non-invasive neuroimaging techniques, e.g. diffusion tension imaging, might contribute to our understanding of functional connectivity within the human brain and altered connections in patients with psychiatric disorders.
Strengths and limitations
In addition to the direct comparison of three potentially related disorders and its potential relevance to the DSM-5 process, the main strengths of our study are the use of a well-validated fMRI paradigm and the inclusion of unmedicated patients, thus ruling out the potential influence of psychopharmacological treatment on BOLD response.
One limitation of the present study is the uneven rates of co-morbid depression across the patient groups. Patients with PD and hypochondriasis were more likely to have co-morbid major depression than OCD patients. However, despite these differences our results primarily showed no differences in brain activation across the anxiety disorders. We tested the influence of co-morbid depression on our results in three different ways (co-morbid depression as regressor of no interest in the model, the exclusion of the co-morbid depressive patients and the direct comparison between depressed and non-depressed patients) and the results remained largely unchanged. Furthermore, patients with major depressive disorder tend to show increased, rather than decreased, dorsolateral prefrontal activation when they perform executive functioning tasks (Wagner et al. Reference Wagner, Koch, Reichenbach, Sauer and Schlosser2006; Fitzgerald et al. Reference Fitzgerald, Srithiran, Benitez, Daskalakis, Oxley, Kulkarni and Egen2008). Another potential limitation of the study relates to the use of a planning task to address the question of ‘relatedness’ across disorders. As mentioned earlier, this has advantages but it is possible that a broader range of psychiatric disorders, e.g. bipolar disorder, may show similar disruptions in frontal-striatal and limbic structures to those detected here. Therefore, we can only cautiously conclude that OCD, PD and hypochondriasis are similar on this particular task but may differ on other tasks that specifically tap into more relevant psychopathological processes involved in each disorder. A third limitation is the way we measured state anxiety (using a single visual analogue scale score directly after task performance). Perhaps more objective measures of state anxiety and with higher temporal resolution (such as pupil diameter or heart rate) would reveal more robust associations with both task performance and brain activation. Finally, despite being one of the largest studies conducted to date, some of the between group comparisons may have been underpowered to detect subtle differences across the diagnostic groups included in this study.
Conclusions and future directions
With these caveats in mind, we hope that our results will contribute to the current deliberations regarding the classification of OCD and related disorders in DSM-5. Since this and other studies that have directly compared OCD with other anxiety disorders (Rauch et al. Reference Rauch, Savage, Alpert, Fischman and Jenike1997; van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005b; Radua et al. Reference Radua, van den Heuvel, Surguladze and Mataix-Cols2010) suggest that there may be a substantial degree of neurobiological overlap between these disorders, our results would support maintaining the current classification of OCD as an anxiety disorder. This conclusion is consistent with the current recommendations by the DSM-5 Anxiety, Obsessive-Compulsive Spectrum, Post-Traumatic, and Dissociative Disorders Work Group (Stein et al. Reference Stein, Fineberg, Bienvenu, Denys, Lochner, Nestadt, Leckman, Rauch and Phillips2010). Furthermore, the results also support the view that hypochondriasis is not only related to OCD but also to other anxiety disorders (Abramowitz & Braddock, Reference Abramowitz and Braddock2006; Olatunji et al. Reference Olatunji, Deacon and Abramowitz2009; Phillips et al. Reference Phillips, Stein, Rauch, Hollander, Fineberg, Saxena, Mataix-Cols, Wilhelm, Leckman, Kelly, Fallonm and Barsky2010).
A key question, however, remains for the future: to what extent are the observed decreases in frontal-striatal recruitment specific to anxiety disorders? Or are they rather a non-specific feature of a broader range of psychiatric disorders, for instance, a result of chronic stress? A growing body of literature from studies in laboratory animals demonstrates that the prefrontal cortex is highly sensitive to stress and that stress-induced alterations in prefrontal function seem to underlie deficits in executive function observed in stressed rodents and the executive component of many neuropsychiatric disorders, such as anxiety disorders (Holmes & Wellman, Reference Holmes and Wellman2009). This issue of specificity needs further investigation by the comparison between disorders from more diverse diagnostic categories, for instance, schizophrenia and mood disorders.
Finally, it is important to note that these conclusions do not necessarily imply common aetiological mechanisms across these disorders. Rather, it is likely that each of these disorders, and even subtypes within each disorder, may be the result of both shared as well as unique disorder-specific (or subtype-specific) causes.
Note
Supplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org).
Acknowledgements
This work was supported by AGIKO-grant MW 940–37–018 (OAvdH) and VENI-grant ZonMW 016.086.038 (OAvdH) from the Dutch Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO). We thank Patricia van Oppen, PhD, and Julie van Dijk-van Hartskamp, MD, for their contribution to patient selection and inclusion and contribution to data collection.
Declaration of Interest
None.







