Cardiac rhabdomyomas are the most common congenital cardiac tumours and they often occur with Tuberous sclerosis complex. Reference Beghetti, Gow, Haney, Mawson, Williams and Freedom1,Reference Amonkar, Kandalkar and Balasubramanian2 Management is conservative in most patients; however, some cases are unstable and may require surgery. Reference Beghetti, Gow, Haney, Mawson, Williams and Freedom1,Reference Cleary and McMahon3,Reference Dahdah4
Tuberous sclerosis results from mutations in TSC1 and TSC2 genes that encode the TSC1/TSC2 protein complex, which is involved in the control of cell growth and suppressing of tumours through control of the mammalian target of rapamycin. In recent years, mTOR inhibitors were found to be an effective treatment in the manifestation of Tuberous sclerosis probably due to compensation for the lack of mammalian target of rapamycin inhibition. Reference Cleary and McMahon3,Reference Dahdah4 Treatment is now approved in many countries for tumours related to Tuberous sclerosis, Reference Franz, Belousova and Sparagana5,Reference Saffari, Brösse and Wiemer-Kruel6 and there are reports of response in symptomatic rhabdomyoma. Most cases reported had a diagnosis of Tuberous sclerosis. Reference Cleary and McMahon3,Reference Dahdah4,Reference Ninic, Kalaba and Jovicic7
We report a case of rhabdomyoma causing severe right ventricle outflow obstruction in a baby with negative genetics for Tuberous sclerosis, which had a rapid response to Sirolimus, eliminating the need for surgery.
Case presentation
At 39 weeks, 3090 g female neonate was born to healthy parents at our hospital with a prenatal diagnosis of a large cardiac tumour narrowing the outflow tracts of the right ventricle.
After birth, a transthoracic echocardiogram was performed which demonstrated two large masses attached to the right side of the intraventricular septum, and which protruded into the right ventricle outflow tract. There was mild obstruction of the outflow with a peak pressure gradient of 30 mmHg (Fig 1). During follow-up, there was a gradual increase in gradient and severe obstruction was demonstrated at age 4 months with a gradient of 90 mmHg across the right ventricle outflow tract and evidence of systemic right ventricle pressure. The maximal combined area of the tumours before treatment measured 3.8 cm2 and the largest mass diameter measured 1.8 cm. As part of her work up, a genetic test was taken and was negative for TSC-1 and TSC-2 mutations. A cardiac MRI was performed, and the tumour was characterised as rhabdomyoma. Also, there was flattening of the interventricular septum during systole and a moderate decrease in global right ventricular function partly due to the paradoxical movement of the septum at the area of the tumour attachment.
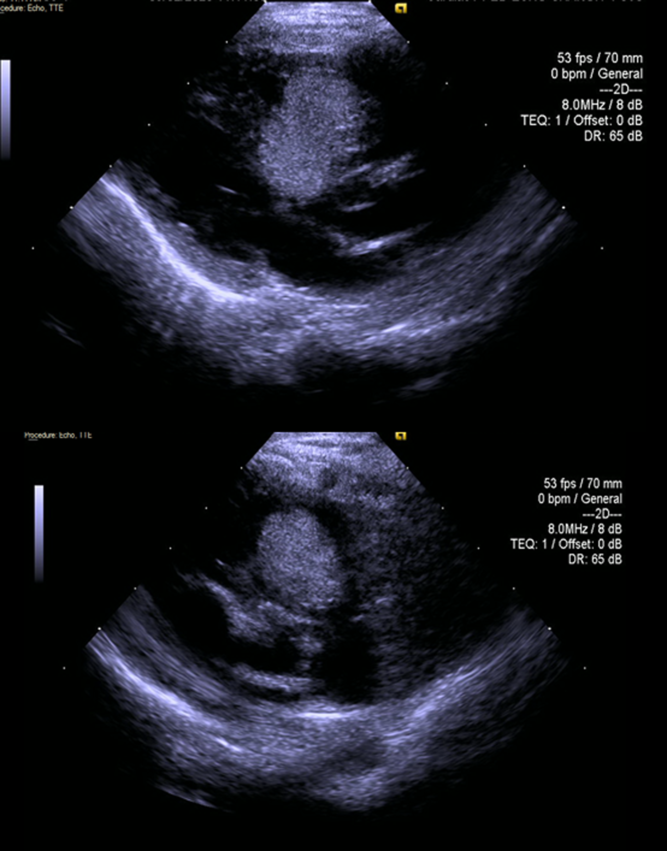
Figure 1. Parasternal long and short axis before treatment.
Due to the progressive worsening of the obstruction, intervention was needed. We decided to start the treatment with Sirolimus before proceeding to surgical resection. The patient was started on 0.4 mg per day (1 mg/m2 body surface once daily), which was reduced to 0.8 mg/m2 aiming for blood levels of 5–15 ng/ml as was previously reported. Reference Cleary and McMahon3
Response was noted within a week, with reduction of the gradient from 90 mmHg to 60 mmHg and decrease in the tricuspid regurgitation jet. During follow-up, there was a significant reduction in gradient eliminating the need for surgery. Within 4 weeks of treatment, the right ventricular outflow gradient was only 15 mmHg and a decrease in tumour circumference to 50% of the original size was achieved. Two weeks after discontinuation of treatment, an echo study revealed normal size and function of the right ventricle with only one tumour attached to the septum with an area of 1.4 cm2 and a maximal diameter of 12 mm, without any gradient across the outflow (Fig 2). Four weeks after discontinuation of therapy, there was a mild enlargement of the tumour and some increase in right ventricular outflow gradient and treatment was restarted again with quick response.
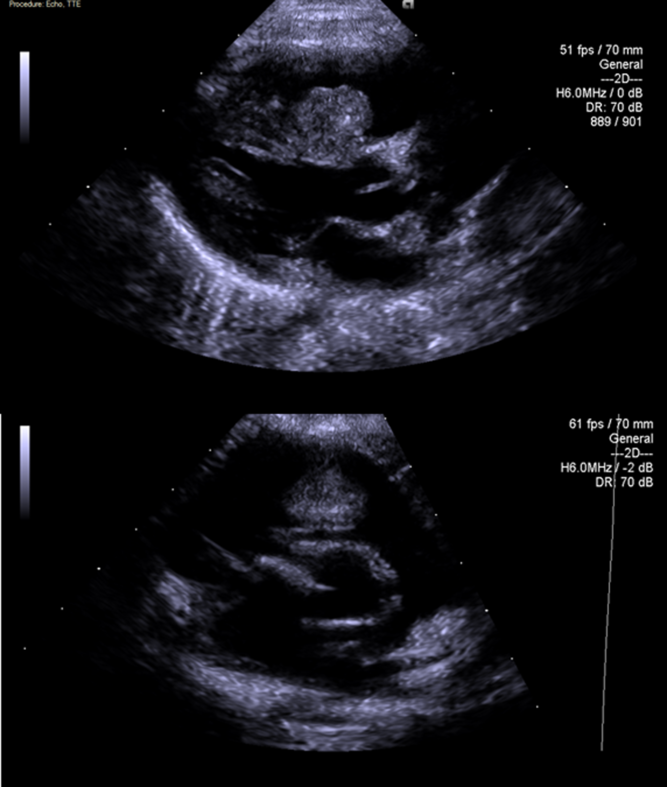
Figure 2. Parasternal long and short axis after treatment.
No significant side effects were noticed during treatment; she grew according to her growth chart and was asymptomatic during the treatment period although Holter study was performed after the beginning of treatment demonstrated a significant amount of atrial premature beats including episodes of short non-sustained supraventricular tachycardia, ventricular premature beats (5% and 2% of the day, respectively), and three short episodes of Mobitz type 2. Holter after the end of treatment was normal without ectopy or conduction delay. After beginning the second course of treatment, there was again a large burden of ventricular premature beats (12% of the day).
Discussion
Mammalian target of rapamycin inhibitors is a novel therapy for Tuberous sclerosis-related tumours including rhabdomyomas. A review of cases that were reported to date was recently published, Reference Cleary and McMahon3 and this review covers 25 articles, of which were case reports or small case series covering a total of 39 patients and also 1 large report from China of 51 patients that were excluded from most analysis, because complete details of each patient were missing. Some of the patients needed a second course of treatment as our patient.
Mammalian target of rapamycin inhibitors was first described as a therapy for Tuberous sclerosis-related tumours and other manifestations of Tuberous sclerosis. Most cases described to date of patients with rhabdomyoma and response to the treatment were also diagnosed with Tuberous sclerosis. Expression of mammalian target of rapamycin pathway and decreased hamartin and tuberin in rhabdomyoma was described in relation to Tuberous sclerosis, explaining the response to treatment in those patients. Reference Amonkar, Kandalkar and Balasubramanian2,Reference Kotulska, Larysz-Brysz and Grajkowska8
The questions, whether treatment should be given to patients without the diagnosis of Tuberous sclerosis and if treatment should be started before the diagnosis of Tuberous sclerosis is established, remains unanswered. Two case reports to date Reference Chang, Chiou, Yao, Chou and Lin9,Reference Davis, Dodeja and Clark10 have reported responses to Everolimus in four babies with negative genetic studies for Tuberous sclerosis.
Many patients with rhabdomyoma present with ectopy, tachyarrhythmia, and bradyarrhythmia, and have been reported to respond to mammalian target of rapamycin inhibitors. Reference Dahdah4,Reference Ninic, Kalaba and Jovicic7 Interestingly, our patient had ectopy and conduction abnormalities presenting during the treatment. These findings adds to another report that described the worsening of ectopy and non-sustained ventricular tachycardia during therapy despite a reduction in tumour size. Reference Davis, Dodeja and Clark10 The fact that rhythm disturbances may also worsen during therapy needs to be considered during the monitoring of treatment in these patients.
Conclusion
Our patient responded rapidly to Sirolimus and adds information regarding the treatment of patients with rhabdomyoma who are not diagnosed with Tuberous sclerosis. We believe, based on our case as well as previous cases presented, that a treatment trial should be given for unstable patients with rhabdomyoma and negative Tuberous sclerosis genetics. An increase in ectopy and conduction abnormalities during treatment was observed and needs a consideration.
Acknowledgements
None.
Financial support
This report received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Rambam Health Campus Committees.





