Introduction
The relationship between humans and mosquitoes (Diptera: Culicidae) has been turbulent since the origin of humanity (Becker et al., Reference Becker, Petric, Zgomba, Boase, Madon, Dahl and Kaiser2010). The World Health Organization (WHO) considers this group of insects as the deadliest animals in the world for their capacity to transmit several pathogens such as malaria, dengue and yellow fever. In addition, mosquitoes can also reduce neighbourhood life-quality and the host-seeking behaviour of anthropophilic species can also have a negative impact on the tourism industry (UNDP, 2017).
Mosquito sub-adult stages can breed in a wide range of aquatic habitats (Clements, Reference Clements1999). These habitats are usually changing environments where the presence and abundance of mosquito larvae are modulated by environmental factors of their breeding sites (Juliano, Reference Juliano2009; Yee et al., Reference Yee, Kneitel and Juliano2010).
Most of the tourist residences in the Spanish Mediterranean coast are located near natural wetlands, where mosquitoes are commonly present. Knowledge of these sites is important to understand the drivers that could increase the risk of nuisance biting by adult mosquitoes or potential outbreaks of vector-borne diseases (Burroni et al., Reference Burroni, Loetti, Marinone, Freire and Schweigmann2013; Cardo et al., Reference Cardo, Vezzani and Carbajo2013; Estallo et al., Reference Estallo, Sangermano, Grech, Ludueña-Almeida, Frías-Cespedes, Ainete, Almirón and Livdahl2018).
From a total of 65 species of mosquitoes recorded in Spain, 17 species are present in the Balearic Islands (Robert et al., Reference Robert, Günay, Le Goff, Boussès, Sulesco, Khalin, Medlock, Kampen, Petrić and Schaffner2019). One of them is the Asian tiger mosquito, Aedes albopictus (Skuse, 1894), an invasive mosquito with strong anthropophilic behaviour (Muñoz et al., Reference Muñoz, Eritja, Alcaide, Montalvo, Soriguer and Figuerola2011). However, other opportunistic species can also feed on humans in areas nearby to their habitats. These species (e.g. Aedes caspius (Pallas, 1771) and Aedes mariae (Sergent and Sergent, 1903)) usually breed in natural areas (e.g. wetlands, salt marshes and rock-pools) affecting human settlements within their flight range (Rioux et al., Reference Rioux, Croset, Corre, Simonneau and Gras1968; Porretta et al., Reference Porretta, Canestrelli, Bellini, Celli and Urbanelli2007).
Currently, Ae. mariae is not a vector of human diseases (Ribeiro et al., Reference Ribeiro, Ramos, Pires and Capela1988) but can play a role in the transmission of avian malaria, Plasmodium relictum (Grassi and Feletti, 1891) (summarized in Schaffner et al. (Reference Schaffner, Angel, Geoffroy, Hervy, Rhaiem and Brunhes2001)). This species was first recorded in the Balearic Islands in 2011 (Bueno-Marí and Jiménez-Peydró, Reference Bueno-Marí and Jiménez-Peydró2011) and belongs to the Ae. mariae complex (Coluzzi and Sabatini, Reference Coluzzi and Sabatini1968). This complex includes other two sibling species: Aedes zammitii (Theobald, 1907) and Aedes phoeniciae (Coluzzi and Sabatini, Reference Coluzzi and Sabatini1968). Aedes mariae is mainly restricted to the Western Mediterranean basin but in Spain can be also recorded in Huelva and Cantabria (Bueno-Marí and Serna-Mompeán, Reference Bueno-Marí and Serna-Mompeán2015). Aedes zammitii and Ae. phoeniciae are distributed within central and Eastern Mediterranean coasts (Coluzzi and Sabatini, Reference Coluzzi and Sabatini1968; Coluzzi et al., Reference Coluzzi, Sabatini, Bullini and Ramsdale1974; Mastrantonio et al., Reference Mastrantonio, Porretta, Bellini, Nascetti and Urbanelli2015). Immature stages of these species complex breed in supralittoral rock-pools of the Mediterranean basin (Schaffner et al., Reference Schaffner, Angel, Geoffroy, Hervy, Rhaiem and Brunhes2001; Mastrantonio et al., Reference Mastrantonio, Porretta, Bellini, Nascetti and Urbanelli2015) tolerating wide ranges of salinity concentration (Rioux, Reference Rioux1958).
Previous research, such as that of Margalef (Reference Margalef1949), studied the biology of Ae. mariae; however, these studies mainly focused on molecular systematics, hybridization, natural selection and evolution of the Ae. mariae complex (Urbanelli et al., Reference Urbanelli, Porretta, Mastrantonio, Bellini, Pieraccini, Romoli, Crasta and Nascetti2014; Mastrantonio et al., Reference Mastrantonio, Porretta, Bellini, Nascetti and Urbanelli2015; Rosenfeld et al., Reference Rosenfeld, Porretta, Rahav, Mastrantonio, Duchet and Blaustein2018; Delgado-Serra et al., Reference Delgado-Serra, Viader, Ruiz-Arrondo, Miranda, Barceló, Bueno-Marí, Hernández-Triana, Miquel, Lester, Jurado and Paredes-Esquivel2021), or related to the larvae bioecology of the sibling species Ae. phoeniciae (Rosenfeld et al., Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019). Hence, there is a lack of information about adult activity and abiotic/biotic requirements for Ae. mariae sub-adult stages.
The aim of this study was to determine the factors affecting the abundance and presence of Ae. mariae focusing on its breeding sites near a hotel area of Majorca island. In addition, its host-seeking activity was also evaluated. This information could be useful for predicting mosquito responses and improve future control strategies.
Materials and methods
Area of study
Majorca is the largest island of the Balearic archipelago (3640 km2) with 896,000 inhabitants in 2019. The island is an important tourist destination, recording 16.5 million visitors in 2019 (IBESTAT, 2020). Its climate is characterized by hot dry summers and wet winters. Mean annual temperatures ranged from 27.7°C during the hottest month (August) to 5.0°C during the coldest month (February). The mean annual precipitation on the island is 411 mm (AEMET, 2020).
The study was conducted in Sa Colònia de Sant Jordi municipality located in the South of Majorca island (39°19′00″N 2°59′34″E) (fig. 1a). This coastal municipality has 2692 inhabitants and an important tourist destination with more than 3500 hotel rooms (FEHM, 2019). In fact, one of the most visited beaches in Majorca, Es trenc, is located in this municipality.
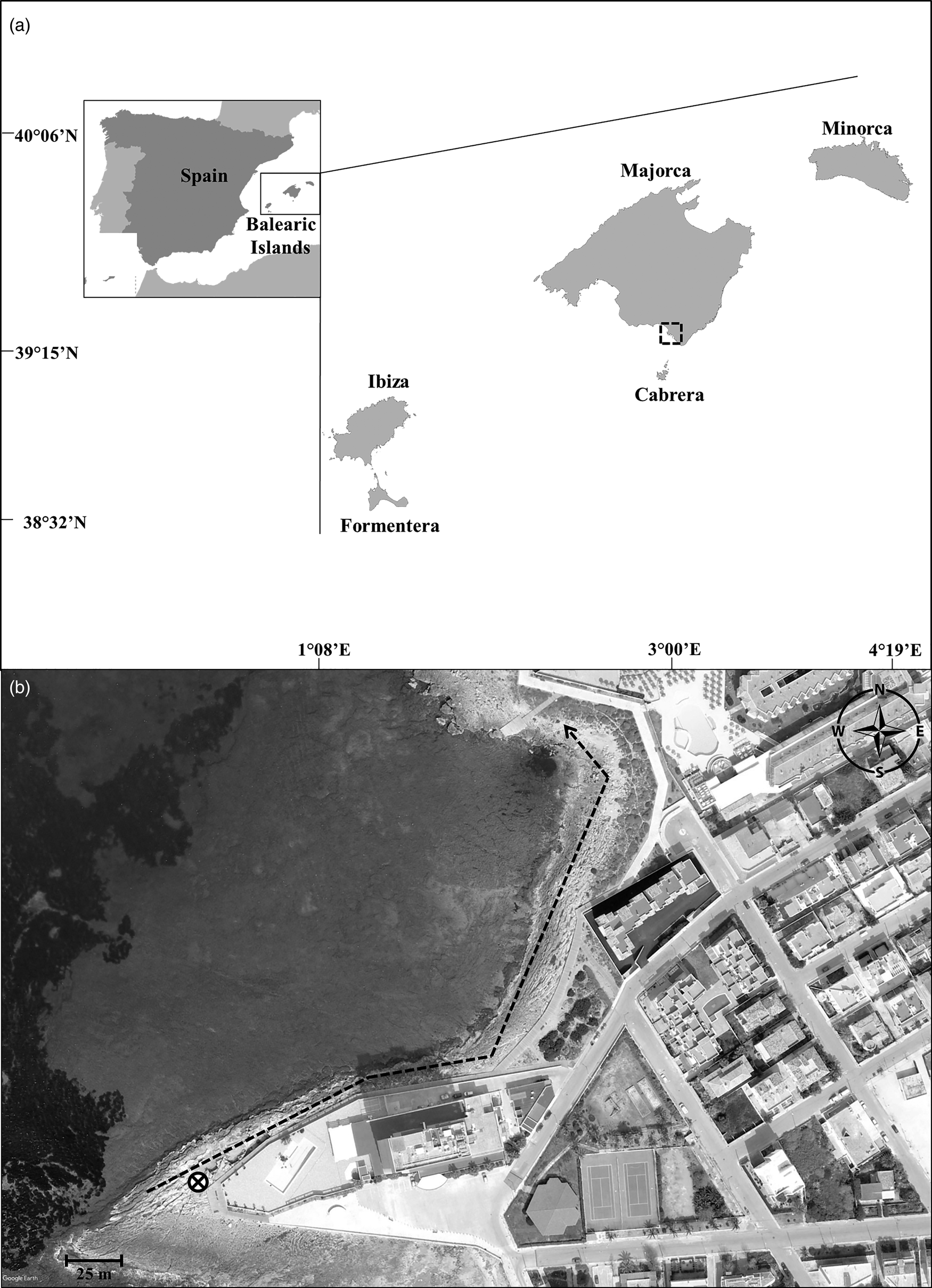
Figure 1. (a) Map of the Balearic Islands. Dotted square indicates the municipality of the study (Sa Colònia de Sant Jordi). (b) Location of the study. The dotted arrow indicates the transect along the supralittoral rook-pools. The cross icon indicates the HLC site.
Mosquito nuisance biting is a common problem during the high tourism season, reducing the quality of outdoor activities and neighbourhood lifestyle. This nuisance biting is commonly related to adults of four different species: the wetland mosquito Ae. caspius that produce high population densities when the soil phreatic surface increases; the common mosquito Culex pipiens s. l. (Linnaeus, 1758) from unattended swimming pools in single-family houses; the Asian tiger mosquito Ae. albopictus with a population increasing year by year (Sanz-Aguilar et al., Reference Sanz-Aguilar, Rosselló, Bengoa, Ruiz-Pérez, González-Calleja, Barceló, Borràs, Paredes-Esquivel, Miranda and Tavecchia2018), and the target species of the current study, Ae. mariae, in residential areas nearby supralittoral rock-pools.
Analysis of Ae. mariae breeding sites
A maximum of 15 rock-pools with the presence of Ae. mariae immature stages were randomly selected throughout a transect of 350 m across the supralittoral area (fig. 1b). These rock-pools were weekly checked from 27/07/2018 to 16/11/2018 (12 consecutive weeks) and from 22/03/2019 to 07/06/2019 (8 consecutive weeks).
Three main factors were determined in each rock-pool: the number of individuals of Ae. mariae immature stages (larvae and pupae) hereafter called ‘larvae’, abiotic parameters and biotic factors. The physical and chemical (abiotic) parameters analysed were: pH, temperature (°C), total dissolved solids (TDS) (ppt), conductivity (μS) and salinity (g l−1). These parameters were determined with a multiparametric PC-5 tester (XS Instruments, Italy). The biotic factors were determined by observation according to the authors’ criteria. These factors were: algae cover (0: no algae, 1: 25% covered by algae, 2: 50% covered by algae, 3: 75% covered by algae and 4: all the rock-pool covered by algae); presence of other fauna (1: presence, 2: absence) and presence of the seagrass Posidonia oceanica (L.) leaves (1: presence, 2: absence).
The abundance of larvae per rock-pool was estimated to describe the variations of immature stages of this species during the year and how this abundance may vary by month. This analysis was carried out using a Generalized Linear Mixed Model (GLMM) with a zero-inflated Poisson model to handle excess zero counts, considering rock-pools sampled on the same date as a random effect in order to avoid pseudo replication. Models were run using the R Development Core Team (2017) package ‘glmmTMB’ (Brooks et al., Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Maechler and Bolker2017).
In order to define a viability range of this species during their larval stage, we investigated how water physicochemical characteristics (abiotic parameters) affect the presence or absence of larvae. This analysis was carried out using GLMM with the R package ‘lme4’ (Bates et al., Reference Bates, Mächler, Bolker and Walker2015) with binomial distribution (logit link function), being the sample date of the rock-pool the random effect to consider the time variance. To avoid multicollinearity problems with the highly related abiotic variables such as conductivity, salinity and TDS, the variance inflation factor (VIF) was previously checked to detect multicollinearity in regression analysis (Dodge, Reference Dodge2008). VIF ranges from 1 upwards showing what percentage the variance (i.e. the standard error squared) is inflated for each coefficient. Following Ringle et al. (Reference Ringle, Wende and Becker2015), all variables with a VIF value >5 were immediately discarded. The best model indicated that the three abiotic factors tested (salinity, temperature and pH) had an important effect on the presence of larvae (table S1).
The number of larvae in the rock-pool may be associated with competition or predation and resource availability, hence the association between larval abundance of Ae. mariae and biotic factors were assessed. The number of larvae presented by each rock-pool was considered as an abundance measure. This analysis was carried out using a GLMM with a Poisson distribution (log link function) considering as well the sample date of the rock-pool as a random effect to consider the time variability. Abundance was influenced by the three biotic factors as indicated the best model (table S2).
Model selection in all analyses was performed using the Akaike's information criterion (AIC), considering models to be equivalent when the difference in AIC with the best model (ΔAIC) was <2 (Burnham and Anderson, Reference Burnham and Anderson2004; tables S1 and S2).
Adult mosquito sampling
Human landing captures (HLC) were performed weekly by two authors of the current study: MB and CB (same two authors during all collection events) from 28/06/2019 to 29/10/2019. People sampled mosquitoes from each other with a mouth aspirator (Silver, Reference Silver2008; WHO, 2013) from 30 min before sunset till 1 h after sunset, remaining 1:30 min exposed. The time of capture (minutes after the sunset) for each mosquito was recorded.
We examined the relationship between time (in minutes) when females of Ae. mariae landed after the sunset and the month of the year (June to October 2019) using a General Linear Model (GLM). Finally, we examined the variation in landing times after sunset and between months.
Ethical considerations
The human collectors consent to participate in the current study. During the study period, no transmission of mosquito-borne diseases was reported by local authorities for the location in which the study was conducted. Human landing collections were conducted in an area without registered cases of vector-borne diseases. The species sampled are not a potential vector of human pathogens.
Results
Breeding sites
Between July 2018 and June 2019, a total of 282 rock-pools were analysed recording a total of 4861 larvae of Ae. mariae. Some rock-pools were without water or without larvae, therefore the number of rock-pools checked varied between 12 and 15 per sampling period. June 2019 was peak larval abundance (67.84 ± 0.47 SD) showing a significant difference in comparison to the first sampling month (P < 0.0001). The lowest larval abundance was recorded in March, September and October (fig. 2). These three months also showed significant differences (P Setember = 0.004, P October = 0.03 and P March < 0.0001) to July 2018. November was discarded from the model due to the low number of observations (only three larvae in the study area).

Figure 2. Phenology of the estimated abundance of Ae. mariae during 2018 and 2019. Significance codes with respect to July 2018: ***P < 0.001; **P = 0.01; *P = 0.05.
The abiotic values varied between months (table 1). The physical and chemical characteristics of conductivity, salinity and TDS in the rock-pools water were highly correlated since they measure similar properties. Using VIF value, conductivity and TDS were discarded, which presented VIF values of 11.35 and 11.45, respectively. Algae cover of 75% in rock-pools showed the maximum number of larvae as the presence of other fauna (see below) and the presence of P. oceanica leaves (table 1).
Table 1. Variation in the abiotic factors of the sampled rook-pools and variation in the mean number of larvae depending on the biotic factors tested

Salinity, temperature and pH had a great effect on the presence of larvae, with salinity and pH the most important variables (fig. 3 and figs S1 and S3). Both variables had a positive effect on the presence of larvae in the rock-pools, however, salinity was a limiting factor since high concentration of salt in the rock-pools (>75 g l−1) drastically reduce the probability of larval presence.

Figure 3. Relationship between larvae presence in the rock-pools and abiotic factors. Salinity (on the top), temperature (middle), pH (on the bottom). In shaded grey the confidence intervals.
The three biotic factors considered had a strong effect on the abundance of larvae. A relatively high cover of algae (75%) was important to maintain a high abundance of larvae; however, this abundance decreased when algae covered the entire bottom of the rock-pool or when there was no algae coverage (fig. 4). Other fauna recorded in the rock-pools consisted of insects from orders Coleoptera and Diptera but no other mosquito species were recorded in rock-pools. This fauna observed had a significantly positive effect on the abundance of the larvae of Ae. mariae (P < 0.0001), while the presence of Posidonia leaves had the contrary effect, significantly decreasing the number of larvae (P < 0.0001; fig. 4).

Figure 4. Relationship between the number of larvae found in the rock-pools with the algae cover, and presence of fauna and Posidonia leaves. (*): when P values ≤0.05. Bars indicate standard deviation.
Adult activity
A total of 32 female Ae. mariae were collected by HLC. Only three individuals of Ae. caspius were recorded on October 29 but were not included in the analysis. Results showed that Ae. mariae activity was recorded up to 43 min after sunset (fig. 5). During the first HLC on June 28, females remained active for a period of 26 min after sunset (from minute 17 to minute 43), while on October 29, the activity was concentrated for 10 min after sunset (from minute 12 to minute 22). In fact, there were significant differences between the timing of Ae. mariae activity (GLM (Poisson); P < 0.0001), showing a reduction from 17 min after the sunset in June to 9 min in October. No HLC were recorded in September since the weather was not favourable for adult mosquito flight.
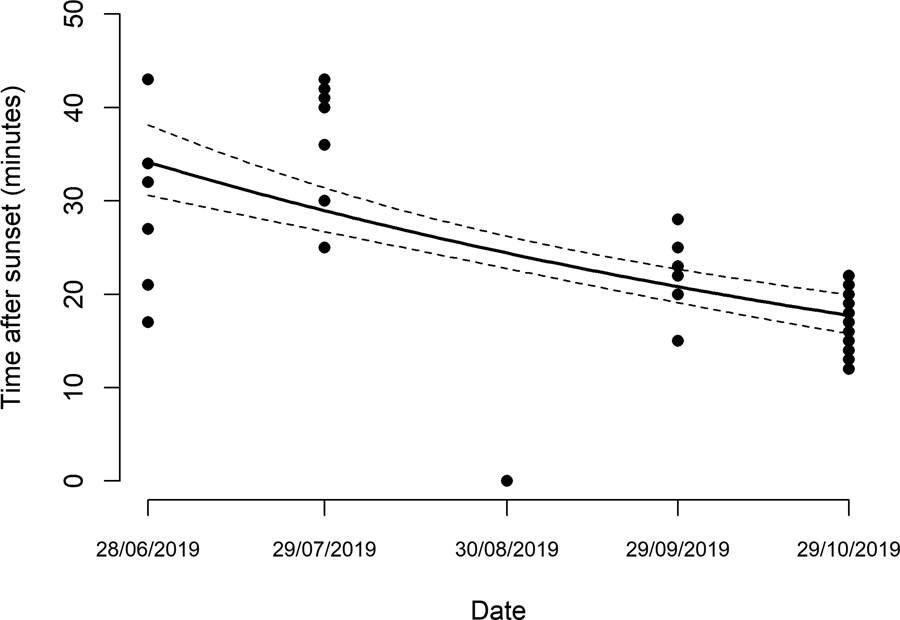
Figure 5. Number of adult Ae. mariae collected with human landing captures (HLC). Each dot is a collected mosquito. n, number of mosquitoes landed in each sampling session.
Discussion
Breeding sites of Ae. mariae immature stages were highly heterogeneous since variables vary from one month to another. These kinds of habitats are continuously altered due to rainfall, storms, waves, tides, insolation, etc., changing its environmental conditions (e.g. food source, microorganism communities, drying and physicochemical characteristics). The influence of these abiotic drivers is important since climate is becoming more suitable for mosquitos due to climate change (Coumou and Rahmstorf, Reference Coumou and Rahmstorf2012; Campbell-Lendrum et al., Reference Campbell-Lendrum, Manga, Bagayoko and Sommerfeld2015), including the increase of salinity of the Mediterranean Sea (Ozer et al., Reference Ozer, Gertman, Kress, Silverman and Herut2016).
The seasonal activity of this species occurred from March to October (Becker et al., Reference Becker, Petric, Zgomba, Boase, Madon, Dahl and Kaiser2010). An increase in the larval population in spring and the highest abundance were recorded in June during the current study. The best model for larvae presence included variables of temperature, salinity and pH.
Seawater has 37.5 g l−1 of salt and could increase in rook-pools due to evaporation and sea sprays, especially during months with high insolation. In addition, rock-pools with larvae present can be filled with rainwater, seawater or mixed, showing values ranging from 1 to 93.4 g l−1, highlighting the adaptive capability of this species. Rioux (Reference Rioux1958) found Ae. mariae larvae in rock-pools with salinity up to 200 g l−1, but we have not recorded these values in pools with larvae; however, we found a strong relationship between the number of observed larvae and the water temperature and salinity measured in the rock-pools (figs S1 and S2). High values of salinity reduced the probability of larval presence, similar to the results obtained with the sibling species Ae. phoeniciae (Rosenfeld et al., Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019). Interestingly, we observed a complete absence of larvae when salt was solidified on the surface of rock-pools. We hypothesize that this salt layer prevents larvae from breathing through the surface layer of the pool.
A mild positive effect of pH was recorded in our study, with Ae. mariae capable of tolerating a wide range of pH (fig. S3). Mosquito larvae can tolerate pH values ranging from 3 to 11 (Clements, Reference Clements1992). In fact, pH values could determine the species composition. Previous studies observed that Ae. phoeniciae and species from genus Culex have a preference for basic pH values (Grech et al., Reference Grech, Manzo, Epele, Laurito, Claverie, Ludueña-Almeida, Miserendino and Almirón2019; Rosenfeld et al., Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019) while others recorded negative or neutral effects (Burroni et al., Reference Burroni, Loetti, Freire, Jensen and Schweigmann2007; Gardner et al., Reference Gardner, Anderson, Hamer, Johnson, Varela, Walker and Ruiz2013).
Regarding the biotic factors, the presence of algae, Posidonia leaves and other fauna play an important role to maintain the presence of larvae in rock-pools. Margalef (Reference Margalef1949) studied the ecological limitations of this species and found that the absence of algae in the rock-pools was also a limiting factor, as did Rosenfeld et al. (Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019) for Ae. phoeniciae breeding sites. Algae in these sites could be a food source for mosquito larvae or even a shelter for other organisms that favour the presence of Ae. mariae. Conversely, a high coverage of algae decreased the number of larvae. This phenomenon could be related to an increase of other dipteran larvae that share a similar diet with Ae. mariae (e.g. Chironomids) (Naeem, Reference Naeem1988). This increase of dipteran larvae could also attract other fauna that can colonize the rook-pool and be potential predators or competitors of Ae. mariae larvae (Kolasa and Romanuk, Reference Kolasa, Romanuk, Holyoak, Leibold and Holt2005). The presence of Posidonia leaves in the rock-pools was a factor that slightly decreased the abundance of larvae, suggesting that floating Posidonia leaves could reduce the water surface for larvae breathing.
Margalef (Reference Margalef1949) also found a possible predation competence between larvae and other fauna. Fauna found in breeding sites of the current study were mainly specimens of Coleoptera of genus Ochthebius (Leach, 1815), Chironomidae and shore flies (family Ephydridae) which can compete or predate on Ae. mariae larvae (Linthicum et al., Reference Linthicum, Davies and Kamau1985; Rosenfeld et al., Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019). However, it was determined that the presence of other fauna had a positive effect on the abundance of Ae. mariae larvae suggesting a successful co-inhabit of these species in the rock-pool ecosystem (Rosenfeld et al., Reference Rosenfeld, Blaustein, Kneitel, Duchet, Horwitz, Rybak, Polevikov and Rahav2019). Further studies addressing this issue must be recommended to better understand the degree of these interactions.
The effort to perform HLC was justified due to the number of mosquitoes captured and the information obtained. It is important to highlight the reduction of time when this species is actively seeking hosts in accordance with the photoperiod, being aggressive during 26 min after sunset in June and a narrower interval of only 10 min in October. Previous studies demonstrated that winter diapause of Ae. mariae was determined by the photoperiod (Coluzzi et al., Reference Coluzzi, Di Deco and Gironi1975), however there is a lack of information about the relation of the photoperiod with the host-seeking behaviour of this species. Studies with Ae. albopictus and Aedes aegypti (L., 1762) recorded that this reduction could be determined by the short sunset and lower temperature during autumn months (Camara, Reference Camara2010; Costanzo et al., Reference Costanzo, Schelble, Jerz and Keenan2015).
This is the first study of the bioecology of Ae. mariae in the Mediterranean basin including a wide number of environmental drivers, thus, valuable data were obtained to improve our knowledge about the activity of this species. Control methods against Ae. mariae should focus on their biology and the heterogeneity of the larvae habitat. Integrated mosquito control is the best approach to reduce the nuisance to tourists and citizens, and a reduction of their larval habitat should be prioritized. The biotic and abiotic variations observed in this study should be considered in the planning of control methods, taking into account tides, waves and precipitation. Hence, the application of Bacillus-based formulations is the control method recommended by the authors. Control methods like physical barriers (polydimethylsiloxane, perlite, etc.) or filling these pools with expanding foam like in tree holes (CDC, 2016) were not recommended due to the aforementioned heterogeneity of this environment. Finally, the role of Ae. mariae as a vector of pathogens is poorly studied, therefore further studies addressing this subject should be considered in order to evaluate the importance of its host-seeking activity.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321001024
Acknowledgements
The authors thank the council of the municipality of Sa Colònia de Sant Jordi for letting us perform the present study.
Conflict of interest
None.









