INTRODUCTION
Mortar is a mixture of binder and aggregate in different proportions. The mortar-production process makes it possibile to establish the age of a structure by dating the binder. To obtain the accurate age of a mortar it is crucial to eliminate components that would cause an offset of the ages, e.g. carbonate aggregate, unburned limestone, dolomite fragments, or secondary crystallization, usually connected with rejuvenation (Sonninen and Jungner Reference Sonninen and Jungner2001; Nawrocka et al. Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005, Reference Nawrocka, Czernik and Goslar2009; Ringbom et al. Reference Ringbom, Heinemeier, Lindroos and Brock2011). Additional problems are caused by the admixtures of ceramic dust or terracotta (in the case of the cocciopesto type of mortars). Admixtures are responsible for the hydraulic nature of mortar and they cause greater reactivity of the mortar ingredients, not only during the setting but also in the period of its functioning in the wall (Binda and Baronio Reference Binda and Baronio1988; Ringbom et al. Reference Ringbom, Heinemeier, Lindroos and Brock2011; Michalska and Czernik Reference Michalska and Czernik2015). Knowledge of the different mortar components is essential during the preparation of samples and later during the interpretation of the results.
Methodological research into the possibility of dating carbonate binding materials began in the 1960s and 1970s (Labeyrie and Delibrias Reference Labeyrie and Delibrias1964; Baxter and Walton 1970; Folk and Valastro Reference Folk and Valastro1979) and is still continued by many groups, which have analyzed mortars with different compositions and states of preservation (Van Strydonck et al. Reference Van Strydonck, Dupas, Dauchot-Dehon, Pachiaudi and Marechal1986; Heinemeier et al. Reference Heinemeier, Jungner, Lindroos, Ringbom, von Konow and Rud1997, Reference Heinemeier, Ringbom, Lindroos and Sveinbjörnsdóttir2010; Nawrocka et al. Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005, Reference Nawrocka, Czernik and Goslar2009; Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Braskén and Sveinbjörnsdóttir2007, Reference Lindroos, Heinemeier, Ringbom, Brock, Sonck-Koota, Pehkonen and Suksi2011, Reference Lindroos, Orsel, Heinemeier, Lill and Gunnelius2014; Nawrocka-Michalska et al. Reference Nawrocka-Michalska, Michczyńska, Pazdur and Czernik2007; Goslar et al. Reference Goslar, Nawrocka and Czernik2009; Ringbom et al. Reference Ringbom, Heinemeier, Lindroos and Brock2011, Reference Ringbom, Lindroos, Heinemeier and Sonck-Koota2014; Hajdas et al. Reference Hajdas, Trumm, Bonani, Biechele, Maurer and Wacker2012; Nonni et al. Reference Nonni, Marzaioli, Secco, Passariello, Capano, Lubritto, Mignardi, Tonghini and Terrasi2013).
An important aspect of the research is to find a way to separate the binder carbonate, indicating the age of the structures, from other sources of carbon. The experimental research on samples of known age employs both mechanical (cryobreaking, grain fractions) and chemical (acid dissolution) attempts to separate the components when preparing the samples for 14C measurement.
Mechanical pretreatment involves alternating freezing and thawing steps to disintegrate the mortar structure. The first use of cryobreaking of mortars was applied by Nawrocka et al. (Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005) and further developed by Marzaioli et al. (Reference Marzaioli, Lubritto, Nonni, Passariello, Capano and Terrasi2011, Reference Marzaioli, Nonni, Passariello, Capano, Ricci, Lubritto, De Cesare, Eramo, Castillo and Terrasi2013) with additional ultrasonication to obtain the samples from suspension. The idea of dating the suspension (the CryoSonic protocol), proposed by Marzaioli et al. (Reference Marzaioli, Nonni, Passariello, Capano, Ricci, Lubritto, De Cesare, Eramo, Castillo and Terrasi2013) and Nonni et al. (Reference Nonni, Marzaioli, Secco, Passariello, Capano, Lubritto, Mignardi, Tonghini and Terrasi2013), was also applied in this study but with modifications and a different preparation procedure (Figure 1). The applied preparation is based on separation between different fractions also from suspension.
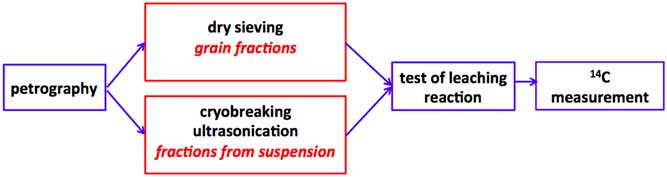
Figure 1 General scheme of applied DoM v.1 protocol for selection of samples and preparation for 14C measurements.
The chemical separation is an acid-leaching reaction of the carbonates (orthophosphoric or hydrochloric) in the function of time (Folk and Valastro Reference Folk and Valastro1979; Sonninen and Jungner Reference Sonninen and Jungner2001; Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Braskén and Sveinbjörnsdóttir2007; Nawrocka et al. Reference Nawrocka, Czernik and Goslar2009; Hodgins et al. Reference Hodgins, Lindroos, Ringbom, Heinemeier and Brock2011; Michalska and Czernik Reference Michalska and Czernik2015). Application of a test of leaching reaction of mortars before choosing a sample for dating allows for observing the differences in the rate and course of the decomposition for the mineralogically variegated materials.
In order to develop the application of the 14C method to a wider scope of variegated mortars, an intercomparison study has been undertaken by seven 14C laboratories (see in this issue Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017 and Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017). Each laboratory applied its own preparation and worked on the same set of samples: Finnish mortar (MDIC 1), Mallorca lime burial (MDIC 2), Swiss mortar mixer (MDIC 3), and Roman mortar (MDIC 4). According to the reference data (based on 14C dating of charcoal, wood, and bone fragments, as well as historical sources) the expected ages for all mortars were given (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017; Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017). Additionally the optically stimulated luminescence (OSL) has been applied to dating mortars (Urbanova et al. Reference Urbanova, Hourcade, Ney and Guibert2015). Mortar MDIC 1 of the Nagu church, based on the 14C measurement of a wood fragment and dendrochronological analysis, is dated to the first half of the 15th century AD (Sjöberg et al. Reference Sjöberg, Lindroos and Ringbom2011). The conglomerate from the burial site of Cova S’Estora MDIC 2, based on charcoal and bone dating, was established to be from 8th–4th century BC. Mortar MDIC 3 from the northern part of Cathedral Hill in Basel was dated (using charcoal) to the 10th–11th century AD, however, the later 14C measurements of other charcoal fragments indicated the 7th–8th century AD. The archaeological expectation for this mortar was 10th–11th century AD (Hüglin et al. Reference Hüglin2011). Mortar MDIC 4 from the city wall of the Roman city Aduatuca Tungrorum was dated (using charcoal) to the 4th century AD.
The preparation, methodology, and results of all involved scientific groups are presented in Hajdas et al. (Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017) and Hayen et al. (Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017).
This work presents the methodology applied by the Poznan team, combining the scientists from the Poznan Institute of Geology and Faculty of Physics, University of Adam Mickiewicz with the Poznań Radiocarbon Laboratory (Poland).
METHODS
A summary of the preparation methods applied by the seven groups (ABO, CIRCE, CIRCe, ETHZ, POZN, RICH, and MIL) for the four chosen samples is presented in Hajdas et al. (Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017).
The methodology applied by the Poznań group for MODIS samples involved petrographic observations, SEM-EDS analyses, different mechanical-chemical preparations, a test of leaching reaction for available fractions, and finally, on the basis on previous steps, 14C dating of the chosen samples (Figure 1).
Each sample preparation was preceded by thorough petrographic research (using an Olympus AX 70-Prons microscope) and observation by scanning electron microscope (SEM; Hitachi S-3700N). For many years, petrography has been used and described by different authors as an important step in the selection of mortars for dating (Nawrocka et al. Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005; Goslar et al. Reference Goslar, Nawrocka and Czernik2009; Ringbom et al. Reference Ringbom, Heinemeier, Lindroos and Brock2011; Nonni et al. Reference Nonni, Marzaioli, Secco, Passariello, Capano, Lubritto, Mignardi, Tonghini and Terrasi2013). SEM allows researchers to observe the morphology of the sample and to view the distribution of elements in a specimen by using an energy-dispersive X-ray spectrometer (EDS). The great advantage of this method is its non-destructive nature. The samples may be studied in their natural form, without necessity of preparing a thin section. By extension, it is possible to date exactly the same fragment of a sample that was previously observed under the microscope.
After detailed characterization, all of the mortars were carefully crushed into smaller fragments, and then depending on the mortars’ components and sample size, the appropriate preparation method was chosen. The general rule applied by the Poznan group in the case of mortar dating is to adapt the preparation to the specific composition of the sample.
The applied protocol is shown on Figure 1 and listed in Table 1, respectively, separately for all samples from each mortar.
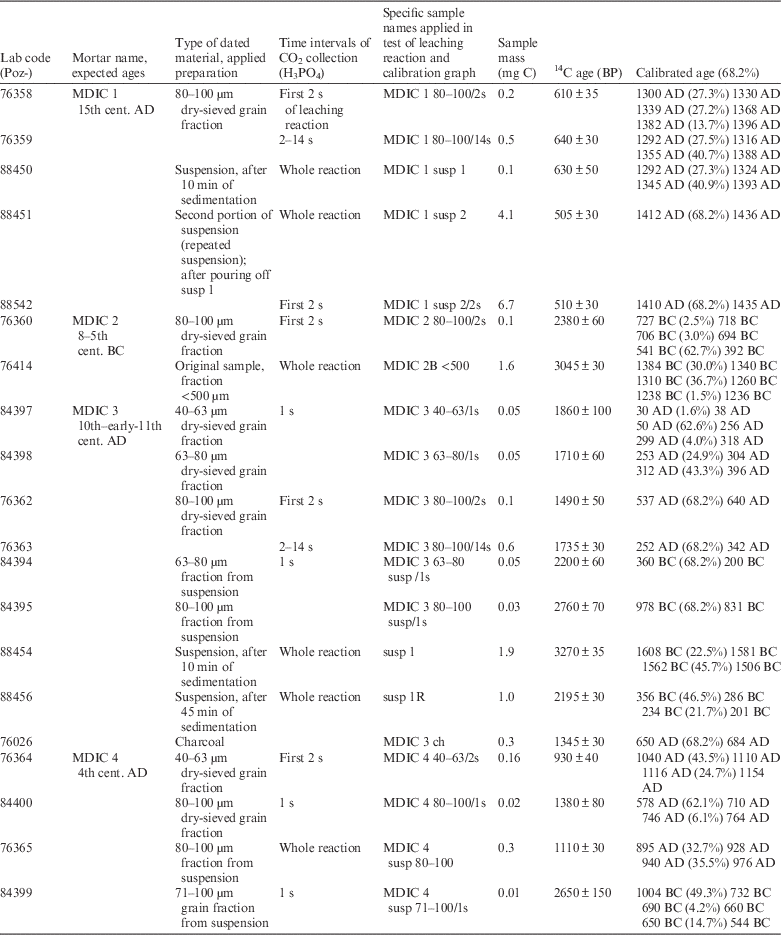
Table 1 Results of mortar dating and applied preparation protocol in Poznań, together with relative chronology.
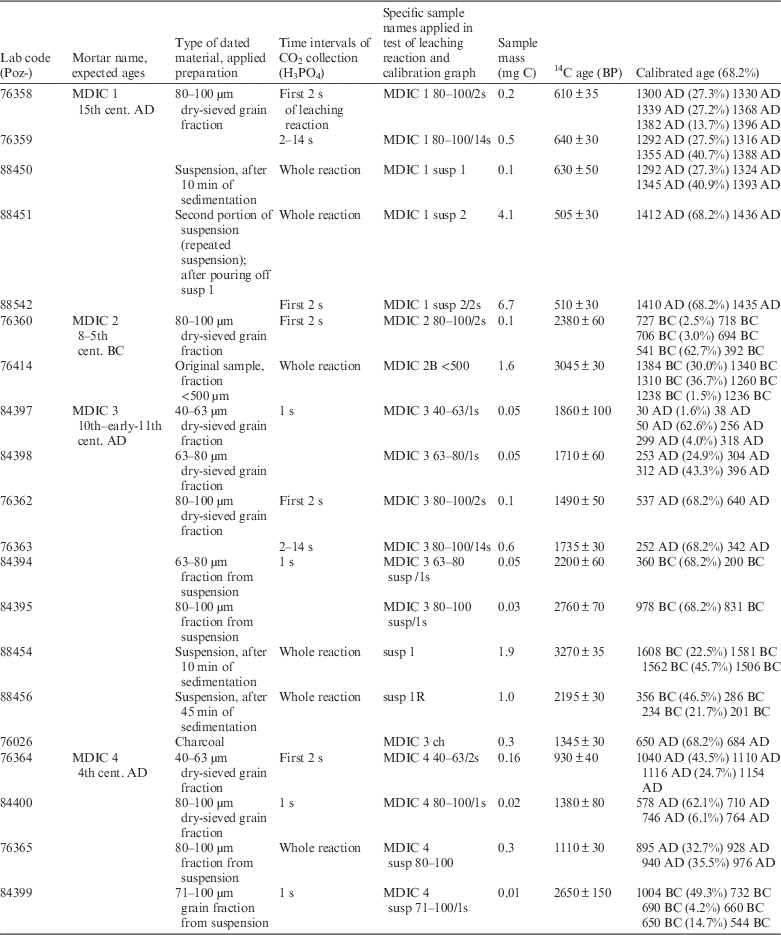
For samples with relatively simple composition in the context of dating (a small amount of components containing other sources of carbon than binder carbonates), the preparation was based on dry sieving to obtain grain fractions. The 40–63 μm, 63–80 μm, and 80–100 μm fractions were usually selected for hydrolysis (except, in the case of small samples, combined into wider grain ranges).
For other mortars with more complicated composition, additional steps of cryobreaking (Nawrocka et al. Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005) and an ultrasonic bath (Marzaioli et al. Reference Marzaioli, Nonni, Passariello, Capano, Ricci, Lubritto, De Cesare, Eramo, Castillo and Terrasi2013; Nonni et al. Reference Nonni, Marzaioli, Secco, Passariello, Capano, Lubritto, Mignardi, Tonghini and Terrasi2013) were applied to force the self-disintegration of mortar components and to obtain the suspension. Depending on the amount of material, multiple suspension samples from one mortar were collected. The relatively short time experimentally determined for the material to settle after remaining in the ultrasonic bath and mixing results in so-called “forced suspension” (and in this meaning is presented throughout manuscript). These kinds of samples contain slightly bigger grain size fractions than in the suspension obtained after a longer, natural sedimentation process. The prepared suspension samples included grain fractions from suspension as well as different portions of suspension collected at different times of sedimentation (10–45 min, see Table 1). The suspension collected after a short sedimentation was additionally divided into two portions from one mortar. The first portion was poured off after 10 min of sedimentation (susp 1), and then the residue was again flooded with deionized water, mixed, and after 5 min poured as a second portion of suspension (susp 2).
All the samples (bulk mortar, grain fractions, and suspension) were subjected to the test of chemical decomposition of carbonates. Hydrolysis was performed with an excess of orthophosphoric acid at 80°C. On the basis of the rate and course of these reactions and taking into account the petrographic composition, the most suitable fractions and time intervals of gas collecting for 14C measurement were chosen.
Typically, the CO2 was collected as rapidly as possible (after the first 1–2 s) to minimize the amount of CO2 from natural carbonates. Gas collected during the longer dissolution time interval was used as a control sample.
It must be noted that a sufficient amount of material required for such testing and dating was not always available. In such a case, the selection of the samples suitable for dating is only made based on petrography and experience from conducting studies of mortars with similar composition.
The gas collected during the hydrolysis and the charcoal fragment used to compare the obtained results were subjected to the standard procedures in the Radiocarbon Laboratory in Poznań (Goslar et al. Reference Goslar, Czernik and Goslar2004). The 14C results were calibrated to absolute ages using OxCal v4.3.2, the IntCal13 calibration curve (Ramsey and Lee Reference Ramsey and Lee2013; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013).
SAMPLE CHARACTERIZATION
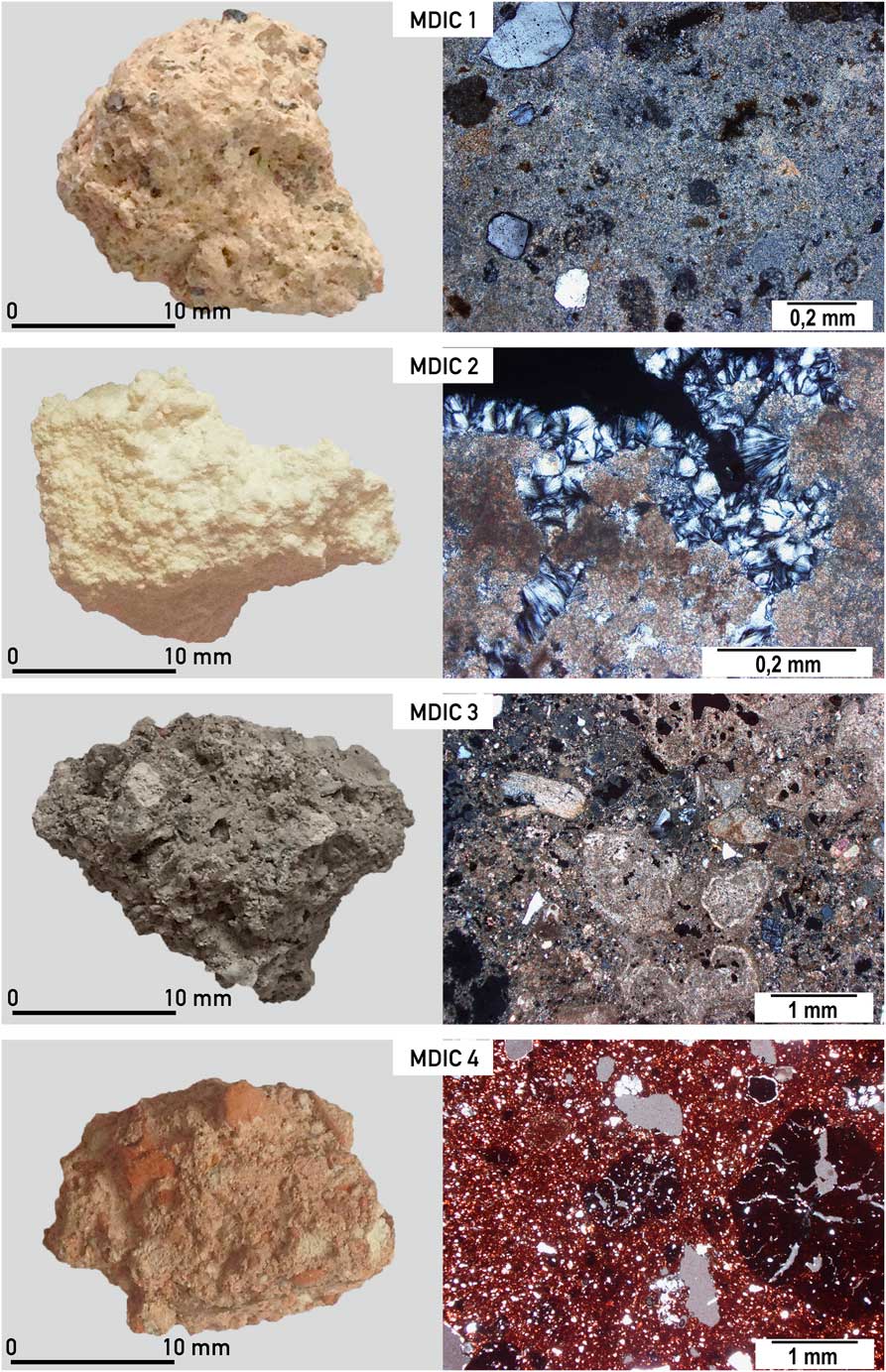
Four different samples (Figure 2) were subjected to thorough analyses:
-
∙ Sample MDIC 1: a wall’s bedding mortar from the church of Nagu in the Åboland archipelago, Finland;
-
∙ Sample MDIC 2: a lime conglomerate from a burial at Cova S’Estora (Son Pellisser) on the island of Mallorca, Spain;
-
∙ Sample MDIC 3: the remains of a medieval mortar mixer from Basel Cathedral Hill, Switzerland; and
-
∙ Sample MDIC 4: a rendering from a Roman wall excavated in the city of Tongeren, Belgium (Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017).
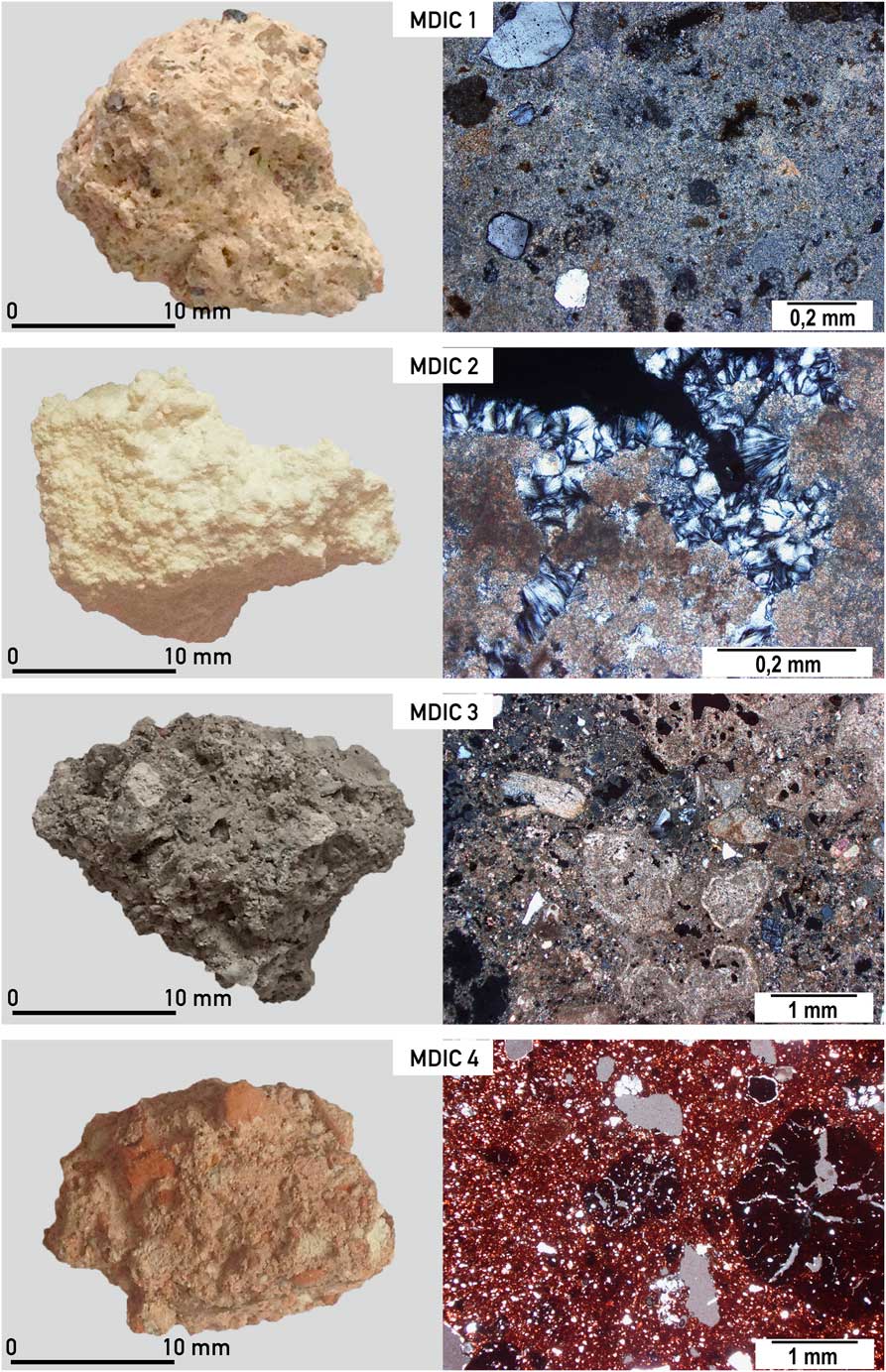
Figure 2 Macro and microscopic view of mortars from the intercomparison studies (MODIS) described in detail in the text; different structures of analyzed mortars are visible (from top to bottom): MDIC 1—small amount of aggregate, quartz, singular unburned fragment of limestone; MDIC 2—dolomitic mortar with hydromagnesite in the pores; MDIC 3—very heterogenic mortar, with different carbonaceous components, unburned fragments of limestone and shells; MDIC 4—cocciopesto mortar with ceramic dust and few cm in size fragments of crushed terracotta giving reddish color of the whole mortar. (Colors refer to online version.)
All these mortars have been characterized in detail (Figures 2 and 3; supplementary Figure S1). The most attention was focused on components that could affect the age of mortars and the differences among the mortars themselves.
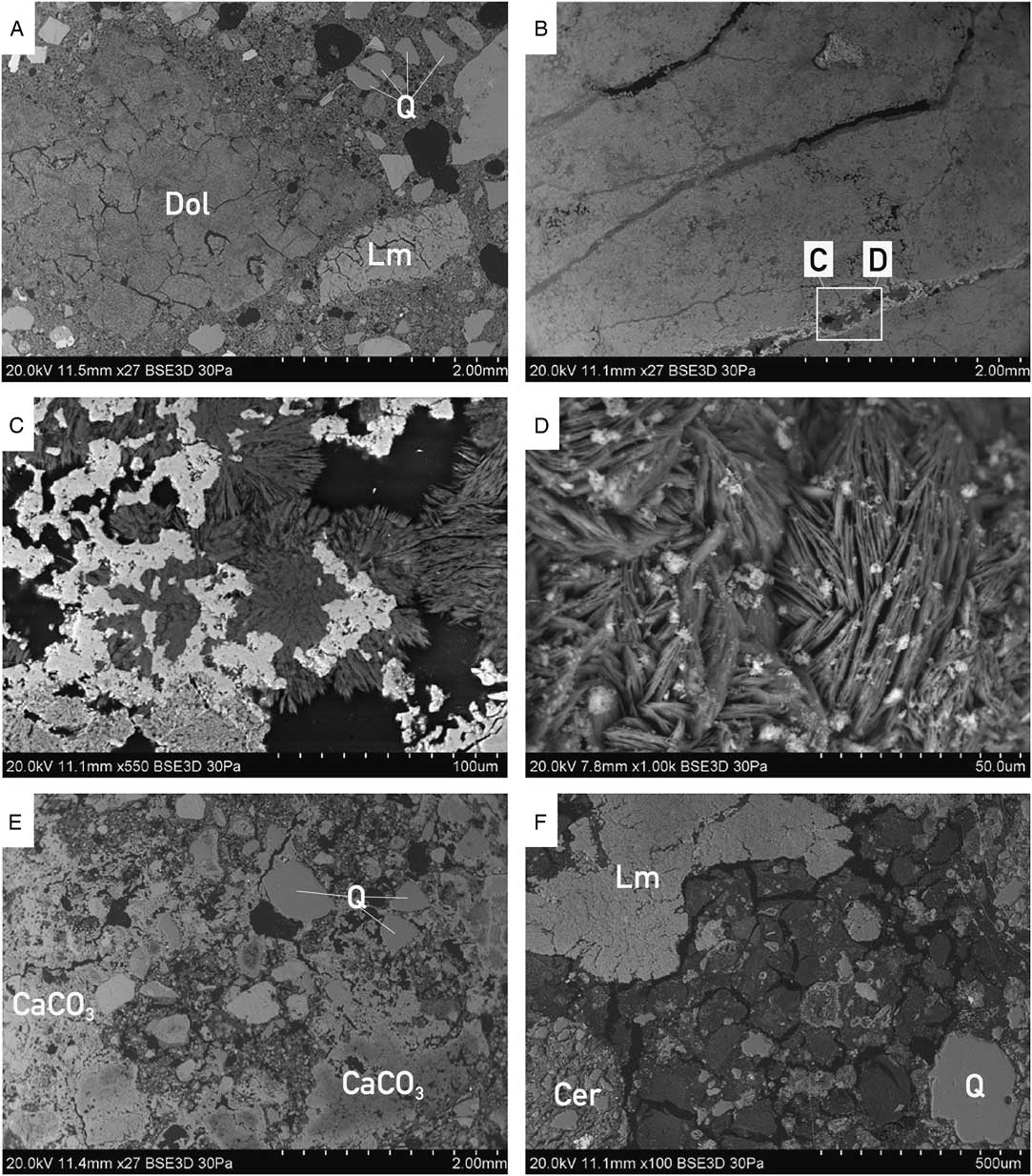
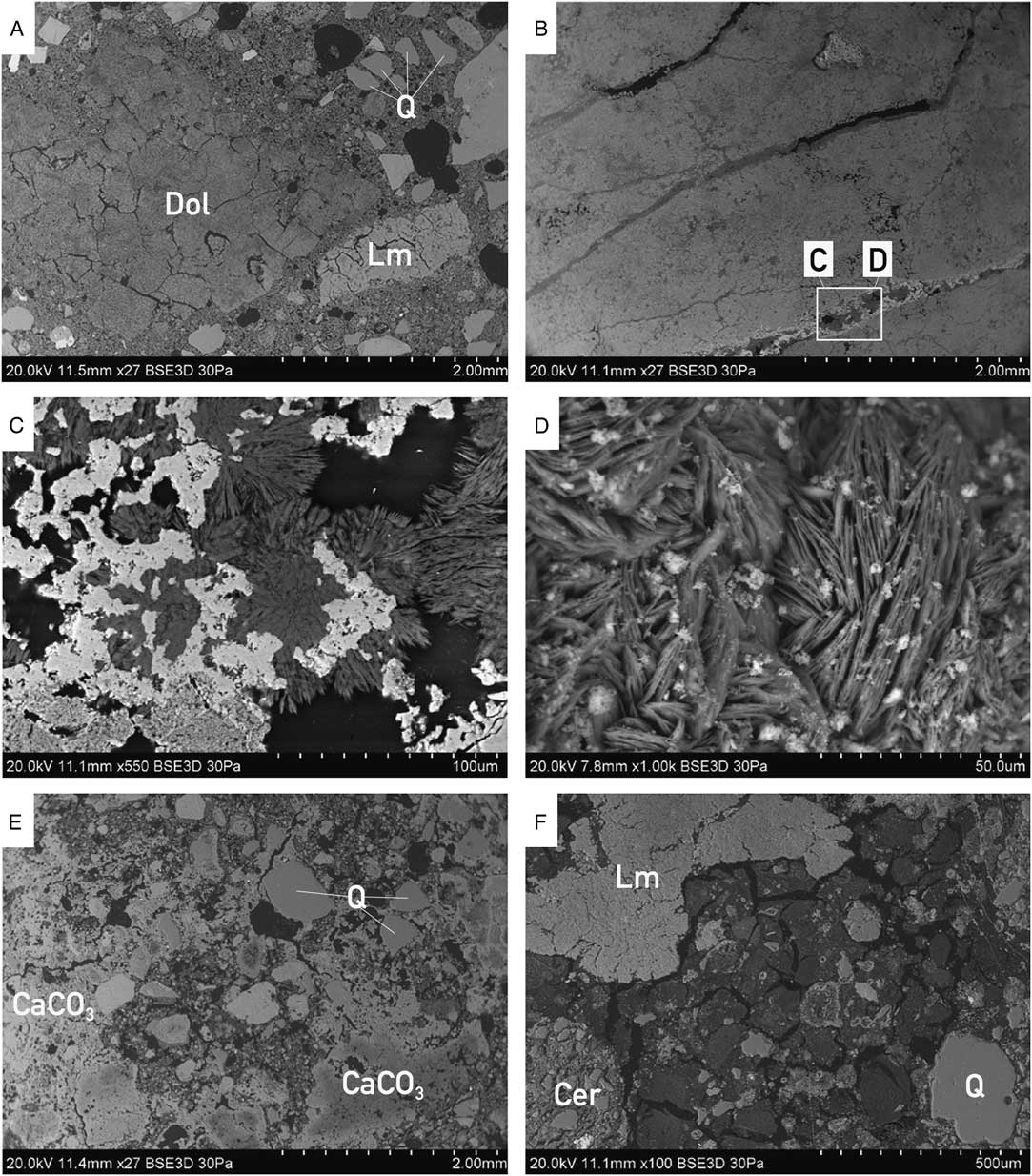
Figure 3 Morphology of bulk mortars with visible differences in contact binder-aggregate between all four mortars: from well-defined aggregate in sample MDIC1 (A), to sample MDIC2 (B) without any aggregate (see C and D for close-up views), to the very heterogenic mortar MDIC3 (E) with different generations of carbonates present (CaCO3), to the cocciopesto mortar MDIC4 (F) with typical, clearly visible shrinkage cracks within the binder. MDIC2 is a dolomitic sample with hydromagnesite present in pores and cracks: (D) image of globular hydromagnesite—the result of the free growth (without spatial limitation); Q = quartz, Lm = limestone, Dol = dolomite, Cer = ceramics.
Finnish Mortar (MDIC1)
This mortar is macroscopically light beige, with visible, poorly sorted aggregate and carbonate binder. Quartz, feldspars, amphibole, and some limestone (>100 μm) occur within the aggregate (Figure 2). According to local geology (Edelman Reference Edelman1985; Ehlers et al. Reference Ehlers, Lindroos and Jaanus-Järkkälä1986) limestone and dolomite could be present (Figure 3 and supplementary Figure S1); also skarns may occur (they consist of coarse-grained Ca-Mg-Fe silicates).
Based on EDS analyses and petrographic observation, mortar MDIC 1 could be characterized as slightly hydraulic, with the sand originating from the erosion of metamorphous rocks.
Sample of Mallorca (MDIC2)
As shown in Figures 2 and 3, this sample is macroscopically white. It does not contain an aggregate; only some not completely burned fragments (>200 μm) of the carbonate rocks in its structure are still visible. Petrographic observations and chemical analyses indicated a dolomitic character of the binder and the presence of hydromagnesite within the cracks (Figures 2 and 3). Dolomitic limes are described in the literature as more durable over the long term in comparison to the high-calcium limes (Dheilly et al. Reference Dheilly, Bouguerra, Beaudoin, Tudo and Quéneudec1999; Pavía et al. Reference Pavía, Fitzgerald and Howard2005; Diekamp et al. Reference Diekamp, Konzett and Mirwald2009). The burning of the dolomitic limestone itself is connected to the presence of calcium oxide and magnesium oxide, which are both relatively easily subjected to slaking. As far as calcium hydroxide reacts to CaCO3 collecting the CO2 from the air, the formation of MgCO3 is slower, delayed to the advantage of forming of the hydrous magnesium phases (e.g. hydromagnesite, artinite, brucite). This process depends to a large extent on the content of water in the mortar, air humidity, and CO2 concentration (Diekamp et al. Reference Diekamp, Konzett and Mirwald2009). Also, when a too-high temperature is applied during the burning process, a delay in hydration of an overburned MgO occurs, hence a delay in forming carbonates.
Swiss Mortar (MDIC3)
This mortar represents an extremely heterogeneous material and it is macroscopically grey. It contains a large amount of fine aggregate in the form of quartz sand. Within the structure of the mortar there are also relatively large fragments of charcoal, lime lumps, and numerous fragments of crushed limestones, some of which are partially burned (Figures 2 and 3). In the entire structure of mortar the secondary calcite is present (supplementary Figure S1). The sizes of carbonaceous components (different than the binder) are difficult to estimate in this mortar because they do not form a typical aggregate (from less than 10 μm up to 1 mm). As known from the literature, the use of metakaolin or silica fume (Babu and Prakash Reference Babu and Prakash1995; Wong and Abdul Razak Reference Wong and Abdul Razak2005), which is usually micro silica (sometimes also silica dust) has been recognized as a material that improves the durability, making the mortar matrix denser with refined pores. That kind of admixture is highly pozzolanic (Malhotra and Carette Reference Malhotra and Carette1982; Aly and Pavia Reference Aly and Pavia2015) and could react with calcium hydroxide to form calcium silicate hydrate (similar to Portland cement). Similarly, it is difficult to establish the presence of clay minerals or fine-grained loess material during the burning process of sample MDIC3 (e.g. from loess described from the relatively nearby area of Augusta Raucaria; Berner Reference Berner2010), which could significantly influence the mortar properties. The presence of different calcination phases in the binder and oolitic limestone, dolomite, and not completely burned carbonaceous fragment is responsible for the difficulty in obtaining the accurate 14C age of this mortar production. The calculated hydraulic index for this sample indicated the hydraulic character of the binder, which is the result of the raw material used for its production. The hydraulic index parameter itself, depending on the petrographic composition of the mortar, does not always reflect the actual character of the material (Ringbom et al. Reference Ringbom, Heinemeier, Lindroos and Brock2011), however in this instance it has been applied as an indicator of the binder properties, not as the absolute value for comparison with other parameters.
Roman Cocciopesto Mortar (MDIC4)
As shown in Figures 2 and 3, and in supplementary Figure S1, MDIC4 is a macroscopically reddish mortar, which is a result of the ground ceramics in the composition of the sample. There are fragments of broken ceramics, terracotta—from 1 to 5 cm in size—in the sample. The binder itself has a dolomitic character. Lime lumps and limestones also occur. The sample was subjected to the weathering re-crystallisation process, which was influenced, besides environmental conditions, by the presence of crushed bricks and ceramics (Binda and Baronio Reference Binda and Baronio1988). The smaller the components of the crushed ceramics, the bigger the reactivity is; for bigger ceramic fragments in the mortar the reactions may occur around the components themselves. In the entire mortar, the structures derived from dissolution are visible.
TEST OF LEACHING REACTION
The purpose of the leaching test is to investigate the rate and course of the reaction and, if possible, based on these tests and sample characteristics, choosing the most suitable granulation and time interval for CO2 collecting for measurement.
However, one must remember that the usage of these tests is limited and should be applied in combination with knowledge about sample compositions.
The rate and course of the reaction depends on the mortars’ components and admixtures. One of the assumptions of the chemical dissolution of mortars is based on the commonly faster decomposition of a delicate binder than the geological carbonate constituting the aggregate. However, in the case of very heterogenic mortars (like MDIC3) with a large amount of carbonaceous components the reaction course is not diagnostic because of the presence of different generations of carbonates in all fractions, from the beginning to the end of leaching. This type of chemical reaction does not allow us to distinguish individual components of different rates of the leaching.
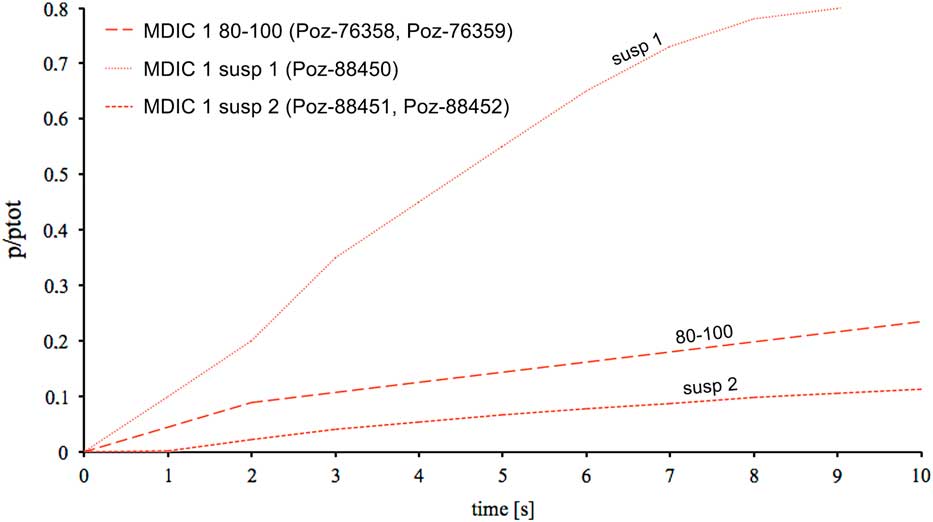
For mortar MDIC1, a test of the leaching reaction was performed for the grain fraction 80–100 μm and for two portions of suspension (Figure 4). The first portion of poured suspension material shows a high content of carbonaceous particles, probably connected with unburned carbonaceous aggregate presence, whereas the rate and speed of reaction in the case of the second portion from the same suspension fraction slow down (MDIC1 susp 2—sample obtained after previous removing of susp 1, see Table 1). The course of reaction for this mortar in fraction 80–100 μm (MDIC 1 80–100) is similar to the second portion of suspension material (MDIC1 susp 2), but runs more intensively and at a slightly higher rate, probably connected with the presence of singular, not totally burned, limestone fragments.
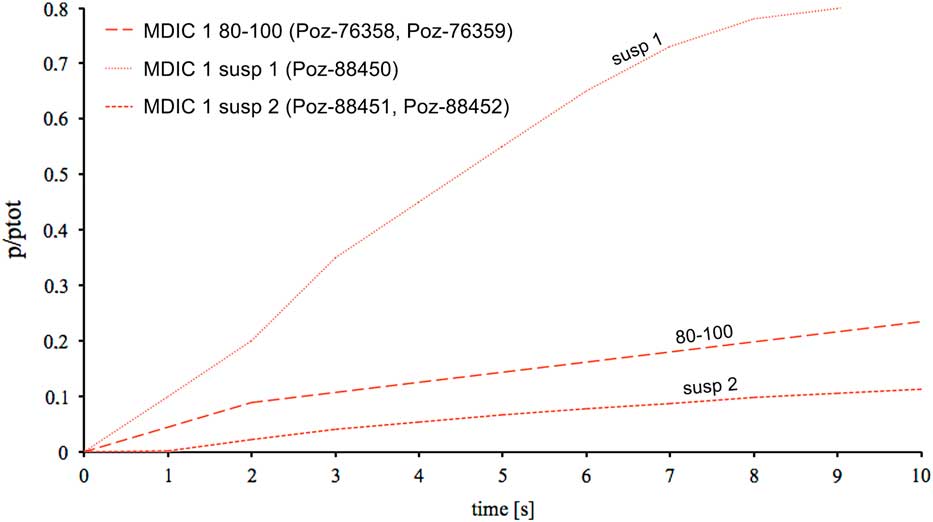
Figure 4 Leaching reaction of different fractions from Nagu mortar. Together with sample names, the laboratory numbers of dated fractions in different time ranges are given (see also Table 1). Cumulative CO2 pressure is represented by p/ptot and plotted versus time; ptot is the pressure of CO2 at the end of the reaction.
Fraction for dating from mortar MDIC2, almost pure dolomitic sample, was chosen on the basis on its petrographic characteristic, without the necessity of additional tests.
The reaction rate for all samples from mortar MDIC3 reflects the presence of significant amounts of carbonate from sources other than the burning process, dissolving during the entire reaction. There are no significant changes in the behavior of the different fractions or within a single sample that could help in an attempt to differentiate the carbonates from the binder and aggregate. Even samples from the suspension collected after different sedimentation times show the enormous influence of different carbonates during the entire reaction.
Taking into account very small fractions (e.g. <40 μm), the reaction may run intensively for all carbonate components (Baxter and Walton Reference Baxter and Walton1970; Folk and Valastro Reference Folk and Valastro1979; Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Braskén and Sveinbjörnsdóttir2007), but can also be affected by the presence of clay fraction (from natural admixtures) or ceramic dust (like MDIC4), which can significantly slow the leaching reaction and inhibit the release of CO2 (by forming a thin, impermeable layer).
The well-known problems with dating cocciopesto mortars (Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Brock, Sonck-Koota, Pehkonen and Suksi2011; Ringbom et al. Reference Ringbom, Lindroos, Heinemeier and Sonck-Koota2014) are also reflected in their course and rate of leaching. There is no simple connection between grain size, carbonate content, and reaction rate.
14C DATING RESULTS AND DISCUSSION
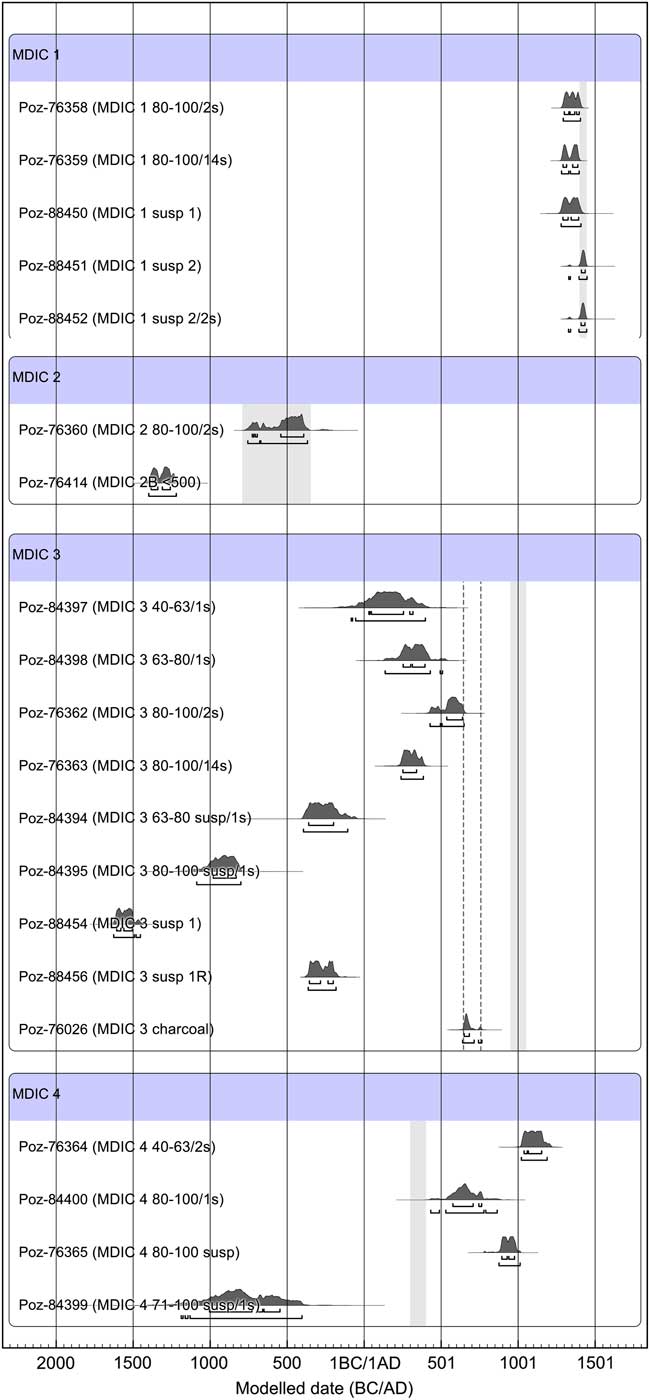
The obtained results of 14C dating are presented in Table 1 together with the applied preparation protocol, and in Figure 5 in graphical form.
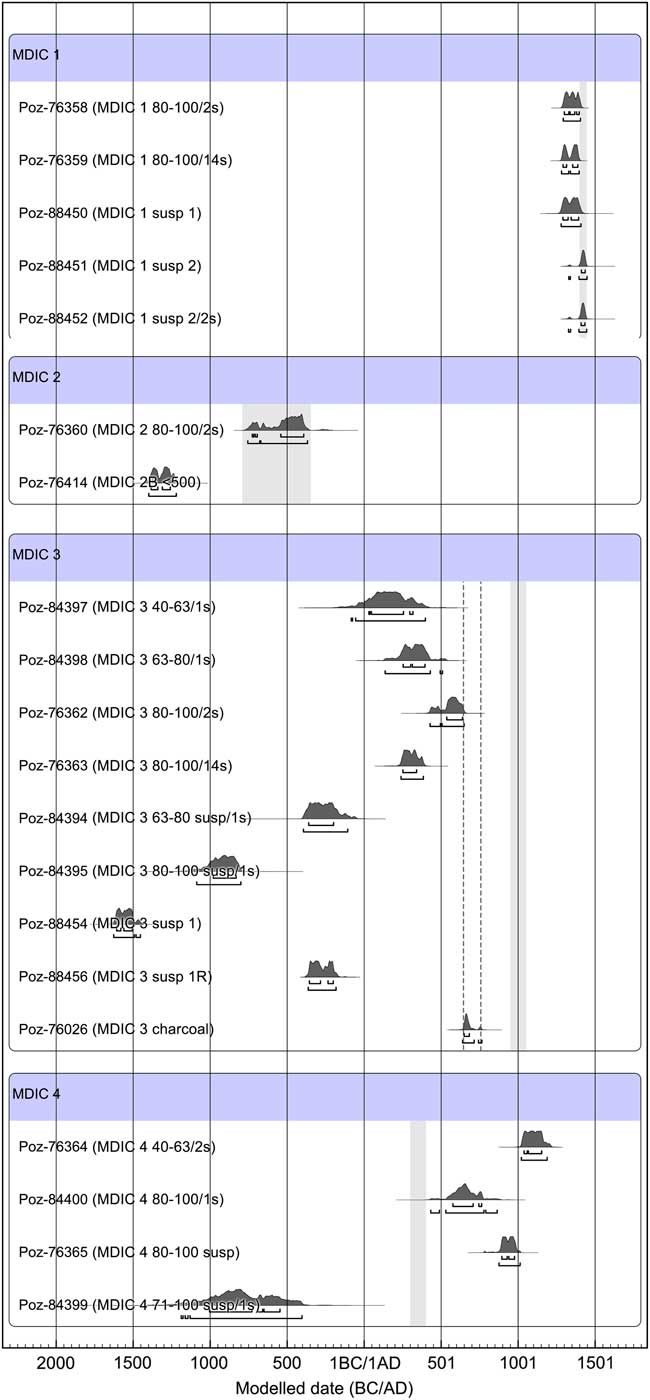
Figure 5 The result of calibration of 14C dates for samples of the intercomparison project MODIS, prepared by the Poznan team. The obtained results were confronted with reference data; in the case of mortar MDIC 3 to show the mixed sources of sample components and its highly heterogenic character, two ranges are marked: first from the left (dotted line) due to charcoal age obtained by dating in Poznan Radiocarbon Laboratory (similar to charcoal age obtained by ETHZ, Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017), and second, 10th–11th century AD, on the basis of archaeological indications.
The presented results indicate that for the sample MDIC1 the result obtained for the grain fraction 80–100 µm is slightly shifted toward the oldest ages (Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017). This fraction was dated twice, after CO2 collection during first 2 s of leaching reaction and after 14 s of the same reaction. Both samples gave similar 14C ages, showing the influence of singular unburned carbonates fragments already in the first CO2 portion collected during first 2 s of leaching. The interesting results gave experimentally chosen suspension portion poured after 10 min of sedimentation process (MDIC 1 susp 1), and the second portion from the same samples—after removing first susp 1 (MDIC 1 susp 2, Poz-88451, Poz-88452). The reaction rate for the sample MDIC 1 susp 1 was very fast and its course was very intense in comparison to MDIC 1 susp 2 (Figure 4). These courses of reaction together with petrographic observations and presence of partially unburned limestone fragments imply that the first portion contained contamination of geologically old carbonates. This contamination was removed already in the second one (MDIC 1 susp 2), which resulted a 14C age corresponding to the reference age. In this case good sampling, with well-defined relative chronology, depth within the wall, and homogeneity of structure, are reflected by the obtained 14C ages. This explains why mortar MDIC1 was successfully 14C dated by all participating laboratories (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017; Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017).
For the Mallorca sample (MDIC 2), the actual age is the age received from the measurement of the fraction 80–100 μm, taken from the first 2 s of the dissolution reaction (MDIC 2 80-100/2s, Poz-76360). The sample MDIC 2B <500 obtained from intercomparison program already pre-sieved, was used as a control sample. CO2 for measurement from this sample was collected at the end of leaching reaction and resulted in overestimated 14C ages (MDIC 2B <500, Poz-76414). In comparison with other tested samples, the course and rate of leaching reaction of sample MDIC 2 susp 1 indicated that its 14C age could still be overestimated. If the available sample were bigger, the dating of MDIC 2 susp 2 (after removing susp 1) could be more promising.
The samples from Switzerland (MDIC3) and Belgium (MDIC4) have far more complicated compositions than the first ones (MDIC1 and MDIC2). For the extremely heterogenic sample from Basel (MDIC 3), the best, but still too-old result, compared to that expected, was obtained from the dating of the dry-sieved fraction 80–100 μm, collected in the first seconds of the dissolution (MDIC3/80-100/2s, Poz-76362). It should be noted that all obtained ages are overestimated, however, this effect is bigger in the suspension material (MDIC 3/susp/63–80/1s; MDIC 3/susp/80–100/1s; MDIC 3 susp 1; MDIC 3 susp 1R). Taking into account the course of reaction for sample MDIC 3 1R, one could assume that if collection of bigger samples from suspension after 45 min of sedimentation were possible, the dating would be of CO2 collected from the first 2 s of the reaction instead of CO2 collected at the end of the reaction. Regardless of the preparation used, dating of mortar MDIC3 gives ages older than expected, which can be traced back to its origin from mortar mixer, built on older strata. This mortar contains a lot of carbonates from different origin. The charcoal results are also ambiguous. The 14C age obtained from dating charcoal from mortar in Poznan Radiocarbon Laboratory is 1345±30 BP, calibrated to 650 AD to (68.2% conf. level) 684 AD, whereas the age expected by archaeologists is 10th–11th century AD. The similar result for charcoal dating was obtained by ETHZ 1313±22 BP (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017), whereas charcoal dated prior to the intercomparison project gives different ages: 1055±45 BP and 1105±40 BP (Hayen et al. Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017).
For the cocciopesto sample MDIC 4, where the expected date is 4th century AD, the obtained results are in general far too young. Only one suspension fraction sample MDIC 4 71-100 susp/1s (Poz-84399) indicates too-old ages.
The analysis of the dry-sieved grain fraction 80–100 μm (MDIC 4 80-100/1s; Poz-84400) showed the closest result to the expected age, but still too young, calibrated 578 AD to (62.1% conf. level) 710 AD, 746 AD to (6.1% conf. level) 764 AD.
Most of the samples gave rejuvenated results, connected to high reactivity of mortars containing ceramic dust in their composition. The interpretation of rate and course of leaching reaction is difficult. The smallest fraction <40 μm react with acid slower than other grain fractions. According to petrography and the visibly large amount of ceramic dust and terracotta fragments in this mortar, the delayed hardening effect of cocciopesto to be expected (Lindroos et al. Reference Lindroos, Heinemeier, Ringbom, Brock, Sonck-Koota, Pehkonen and Suksi2011; Ringbom Reference Ringbom, Lindroos, Heinemeier and Sonck-Koota2014). All laboratories, regardless of the preparation method, showed the delayed hardening effect. The nature of mortar MDIC 4, recrystalization of binder, and general high reactivity of that kind of mortar (Binda and Baronio Reference Binda and Baronio1988) together with the presence of lime lumps, and not totally burned limestone and dolomite fragments, make the 14C dating difficult.
For both mortars MDIC 3 and MDIC 4, the dry-sieved, fine-grained fractions (40–63 μm, 63–80 μm) gave dating results farther from the expected age than the coarser faction (80–100 μm). In fact, the closest results to the expected age for both mortars MDIC 3 and MDIC 4 are from 14C dating of fractions 80–100 μm, after CO2 collection during the first 1–2 s of leaching. The suspension fractions are much more exaggerated than dry-sieved fractions for these two mortars.
SUMMARY AND CONCLUSION
The procedure used by the Poznan group (DoM v.1) was based on petrographic observations and SEM-EDS analyses, followed by different mechanical-chemical preparation, and for the chosen mortars, tests of leaching reaction for available fractions.
The course and rate of reaction of the mortars could be helpful in sample selection for dating only in the case of well-defined sample components. In the case of very small samples, the test of leaching reaction was limited to accessible material, and then the sample selection was done using only petrography and the reaction rate for other fractions from a particular mortar.
Finally, the dated material was dry-sieved grain fractions, grain fraction from suspension, and total suspension collected at different times of sedimentation and in different portions (repeated suspension).
In the case of mortar MDIC 1, the only contamination is singular pieces of unburned or not totally burned limestone and dolomite.
The obtained results indicate presence of geological carbonates in the first second of reaction for dry-sieved grain fraction and for the first portion of suspension (collected after 10–15 min of sedimentation). The second portion of suspension, after pouring off the first one, independently of time of CO2 collection at the beginning of leaching or at the end (MDIC 1 susp 2, Poz-88451, Poz-88452), gives the accurate age of mortar production. It can be assumed that dating of the second portion of suspension will allow the unburned limestone contamination to be eliminated.
The mortar MDIC 2 as a pure dolomitic one with only traces of not totally burned fragments, lime lumps and hydromagnesite within the pores, deprived of aggregate. Taking the composition of mortar into account the fraction 80–100 μm was chosen for dating and gives a successful result.
Based on mineralogy of mortars MDIC 3 and MDIC 4 they are considered to be problematic with respect to 14C dating. Sample MDIC 3 is very heterogenic with different carbonates involved, whereas MDIC 4 is a cocciopesto mortar with (as already known from the literature) problems of delayed hardening and rejuvenation of the results. For both mortars, the dry-sieved, fine-grained fractions (40–63, 63–80) gave dating results farther from the expected age than the coarser fraction (80–100). Ages of the suspension fractions are much more offset from expected than are the ages of the dry-sieved fractions.
Summarizing, in the case of mortars with one source of age distortion (e.g. dead carbon from carbonaceous aggregate), the suspensions are promising. However, the type (portion or fractions) of suspension also depends on the mortars’ components. In the case of sample MDIC1, removing of the first suspension portion and dating second one gave successful results. This observation could not be confirmed on sample MDIC 2 due to the lack of available material.
In the case of difficult mortars, as MDIC3 and MDIC4, where already dead carbon effect and recrystallization may have occurred, the results closest (but still far from expected) were obtained by dating dry-sieved grain fractions. The offsets of 14C ages of suspension are highly exaggerated. This can be explained by a higher proportion of ceramic dust, clay minerals, and different phases of recrystallization in the suspension than in coarser fractions. The clay minerals form the smallest fraction and can easily get through to the suspension, resulting in their lower amount of carbonates in comparison to the dry-sieved grain fractions. The natural clays as well as ceramic dust present in mortars are connected with forming a thin, impermeable layer, during the reaction with acid, reducing the amount of CO2 released from binder carbonates in the first few seconds of reaction.
Taking into account the presented results and comparing them with all 14C measurement performed by all other laboratories involved in the MODIS project (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017) the necessity of good sampling is very clear.
The most homogeneous sample in this first intercomparison project of mortars dating, MDIC1, was successfully dated by all laboratories involved. Similarly, close agreement was obtained for sample MDIC2 but the complicated materials represented by mortars MDIC3 and MDIC4 gave results reflecting sample components and their nature, different from the relative chronology and expected ages (Hajdas et al. Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017).
The good quality of the mortars samples, which meets high standards, is one of the most important conditions required to provide good dating results.
Future research could focus on the wider use of repeated suspension and isotopic fractionation of oxygen and carbon in mortar components (Van Strydonck and Dupas Reference Van Strydonck and Dupas1989), combined with the knowledge of local geological structres as a source of raw material for mortar production (Van Strydonck et al. Reference Van Strydonck, Dupas, Dauchot-Dehon, Pachiaudi and Marechal1986; Nawrocka et al. Reference Nawrocka, Michniewicz, Pawlyta and Pazdur2005; Michalska et al. 2013).
ACKNOWLEDGMENTS
The authors gratefully thank all colleagues involved in the MODIS program. Many thanks to both reviewers for their comments and suggestions of needed supplementation, e.g. additional dating of suspension samples and code names given for the applied procedure. The reference data were cited according to two articles in this issue: Hajdas et al. (Reference Hajdas, Lindroos, Heinemeier, Ringbom, Marzaioli, Terrasi, Passariello, Capano, Artioli, Addis, Secco, Michalska, Czernik, Goslar, Hayen, Van Strydonck, Fontaine, Boudin, Maspero, Panzeri, Galli, Urbanova and Guibert2017) and Hayen et al. (Reference Hayen, Van Strydonck, Boaretto, Lindroos, Heinemeier, Ringbom, Hueglin, Michalska, Hajdas, Marzaioli, Maspero, Galli, Gilberto, Ch, Guilbert and Caroselli2017).
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2017.128