INTRODUCTION
The apicomplexan Sarcocystis neurona is an important pathogen causing severe neurological disease in horses and marine mammals. The normal life cycle of S. neurona alternates between opossums, the definitive host, and a variety of small mammals such as raccoons, skunks, cats and armadillos that can serve as intermediate hosts (Dubey et al. Reference Dubey, Saville, Lindsay, Stich, Stanek, Speer, Rosenthal, Njoku, Kwok, Shen and Reed2000, Reference Dubey, Lindsay, Saville, Reed, Granstrom and Speer2001a ,Reference Dubey, Saville, Stanek, Lindsay, Rosenthal, Oglesbee, Rosypal, Njoku, Stich, Kwok, Shen, Hamir and Reed b ; Cheadle et al. Reference Cheadle, Yowell, Sellon, Hines, Ginn, Marsh, Dame and Greiner2001, Reference Cheadle, Ginn, Lindsay and Greiner2002). Similar to these natural intermediate hosts, horses become infected when they ingest food and water contaminated with S. neurona sporocysts shed in the opossum faeces. In horses, S. neurona may reach the central nervous system, resulting in a clinical disease called equine protozoal myeloencephalitis (EPM) that is endemic in the Americas (Beech, Reference Beech1974; Dubey et al. Reference Dubey, Lindsay, Saville, Reed, Granstrom and Speer2001a ). In S. neurona-infected horses, clinical signs and severity of EPM are dependent on the area of the CNS affected. Most common clinical symptoms include head tilt, depression, muscle atrophy, paralysis, lameness and seizures. Despite treatment of affected horses, complete recovery does not always occur and potential lifelong debility and risk of EPM relapse exists (Fenger, Reference Fenger1998; MacKay, Reference MacKay2006).
Apicomplexans are purine auxotrophs that fulfil their purine requirements by salvaging from their host cells. Purine salvage is accomplished by complementary pathways involving either hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT) or adenosine kinase (AK), neither of which is essential for parasite survival (Krug et al. Reference Krug, Marr and Berens1989). Toxoplasma gondii deficient in AK activity can grow in the presence of the adenine analogue adenine arabinoside (Ara-A) (Pfefferkorn and Pfefferkorn, Reference Pfefferkorn and Pfefferkorn1978). Similarly, T. gondii lacking functional HXGPRT are capable of growing in the presence of 6-thioxanthine (6-TX), a toxic analogue of xanthine (Pfefferkorn and Borotz, Reference Pfefferkorn and Borotz1994). Importantly, parasites that lack functional HXGPRT are sensitive to mycophenolic acid (MPA), which blocks inosine-5′-monophosphate (IMP) dehydrogenase and prevents purine salvage by the AK pathway. Consequently, the HXGPRT gene can be used for positive-negative selection of transgenic parasites (Donald et al. Reference Donald, Carter, Ullman and Roos1996; Donald and Roos, Reference Donald and Roos1998).
Basic methods for DNA transfection and transient expression of reporter molecules such as β-galactosidase, luciferase and yellow fluorescent protein (YFP) have been described previously for S. neurona (Gaji et al. Reference Gaji, Zhang, Breathnach, Vaishnava, Striepen and Howe2006). Further, selection of stable transformants of S. neurona has been accomplished by expressing mutant dihydrofolate reductase-thymidylate synthase (DHFR-TS) to confer resistance to pyrimethamine (Gaji et al. Reference Gaji, Zhang, Breathnach, Vaishnava, Striepen and Howe2006). To enhance molecular genetic capabilities for investigating the biology of S. neurona, a bioinformatics approach was used to identify sequences in the S. neurona genome that encode enzymes of the purine salvage pathway. The SnHXGPRT gene sequence was determined, and Sn∆HXG clones of S. neurona were developed to enable efficient positive-negative selection of stable transgenic parasites.
MATERIALS AND METHODS
Sarcocystis neurona strain SN3
Sarcocystis neurona strain SN3 was propagated in bovine turbinate (BT) cell monolayers. Upon lysis of the infected BT monolayer, the extracellular merozoites were harvested by passing through 23 and 25 G needles and filter-purified to remove the host cell debris (Hoane et al. Reference Hoane, Carruthers, Striepen, Morrison, Entzeroth and Howe2003). Freshly isolated merozoites were used for transfection. When not required immediately, merozoites were pelleted and stored at −20 °C until further use.
In silico identification of purine salvage enzymes
The S. neurona genome has been sequenced and is available through EuPathDB (www.eupathdb.org) and GenBank (Accession # JAQE01000000). A custom searchable database of the draft genome of S. neurona was generated using CLC Genomics Workbench version 6.0 (CLC Bio, Cambridge, MA). Apicomplexan orthologues of the enzymes involved in purine salvage were obtained from the eukaryotic pathogen database (EuPathDB.org; Table 1). Tblastn searches of the draft genome sequence of S. neurona were conducted using T. gondii purine salvage enzyme sequences as the primary queries. Reciprocal searches against the apicomplexan protein sequences in EuPathDB were performed using the newly identified S. neurona purine salvage enzyme sequences as queries.
Table 1. In silico identification of purine salvage enzymes of Sarcocystis neurona

a partial sequence.
Characterization of the HXGPRT gene and gene locus in S. neurona
The structural organization of the SnHXGPRT gene was determined in silico and primers were designed to amplify regions of the gene locus (Supplemental Table S1). Total RNA was isolated from SN3 strain merozoites using Trizol reagent (Life Technologies, Grand Island, NY). SnHXGPRT-specific primers were used to generate cDNA, which served as template for PCR. A single SnHXGPRT PCR product was obtained and sequenced. The accuracy of the SnHXGPRT nucleotide sequence was confirmed by comparison to the S. neurona genome sequence.
Knockout (KO) plasmid construct for disruption of the SnHXGPRT gene
To generate Sn∆HXG parasites, a gene-targeting plasmid consisting of the YFP gene flanked by the UTR regions of SnHXGPRT was constructed in pBluescript. The YFP gene amplified from pSnSAG1/YFP-YFP (Gaji et al. Reference Gaji, Zhang, Breathnach, Vaishnava, Striepen and Howe2006) was ligated to 3·2 kb of 5′-UTR and 3·4 kb of 3′-UTR of the SnHXGPRT locus amplified from S. neurona genomic DNA, yielding the plasmid pSnHXG-UTRs-YFP. Primers and restriction enzymes used to construct pSnHXG-UTRs-YFP are listed in Supplemental Table 1. The proper orientation of the YFP gene in pSnHXG-UTRs-YFP was confirmed by restriction enzyme digestion and PCR.
Transfection of S. neurona merozoites and negative selection using 6-TX
Sarcocystis neurona merozoites were harvested and resuspended in complete cytomix for use in transfections, as described previously (Howe and Sibley, Reference Howe and Sibley1997; Gaji et al. Reference Gaji, Zhang, Breathnach, Vaishnava, Striepen and Howe2006). Approximately 40 μg of KpnI-linearized pSnHXG-UTRs-YFP was combined with 2×107 merozoites and transfected by electroporation. The transfected merozoites were allowed to recover at room temperature for 10 min and then inoculated into 125-mm culture dishes containing BT cells. At 8 days post-transfection, the SnHXGPRT-deficient population was selected with 80 or 160 μg mL−1 of 6-TX in culture medium supplemented with dialysed bovine serum (Donald et al. Reference Donald, Carter, Ullman and Roos1996). After 52 days of 6-TX selection, single-cell clones of the surviving parasites were isolated in a 96-well plate.
Confirmation of HXGPRT disruption in S. neurona
The Sn∆HXG single-cell clones from the 96-well plate were expanded in a 24-well plate, and genomic DNA was extracted. To confirm disruption of the SnHXGPRT gene, a series of PCRs was performed with primer pairs targeting the SnHXGPRT locus and the YFP transgene (Supplemental Table S1). Single-cell clones showing disruption of the HXGPRT locus were tested for sensitivity to IMP dehydrogenase inhibition by culturing them in the presence of MPA (25 μg mL−1) and xanthine (50 μg mL−1) (Donald et al. Reference Donald, Carter, Ullman and Roos1996).
Heterologous complementation of Sn∆HXG
The ptubXFLAG::HX plasmid consisting of the TgHXGPRT minigene driven by the T. gondii dhfr promoter (Bhatti and Sullivan, Reference Bhatti and Sullivan2005) was used to complement the Sn∆HXG parasites. Briefly, Sn∆HXG merozoites were transfected with 40 μg of ptubXFLAG::HX linearized with EcoRV. Post-transfection, the cultures were subjected to MPA selection (25 μg mL−1 MPA and 50 μg mL−1 xanthine) from day 3 onwards to isolate a TgHXGPRT-complemented S. neurona (Sn-TgHXGc) population. After 30 days of selection with MPA, single-cell clones were isolated in a 96-well plate. Amplification primers targeting the expression cassette of ptubXFLAG::HX were used to confirm that MPA-resistant Sn-TgHXGc clones harboured the heterologous HXGPRT sequence from T. gondii.
Plaque assay
Plaque assays were performed to assess growth of Sn∆HXG and Sn-TgHXGc parasites. Approximately 1000 merozoites of wild type SN3 and the transgenic clones were inoculated in triplicate into wells of a 24-well plate containing BT cell monolayers. Parasites were grown either in the absence or presence of MPA, and the plaques were counted 10 days post-inoculation.
RESULTS
Genes for purine salvage enzymes in S. neurona
Sequences encoding enzymes involved in purine salvage by S. neurona were identified by Tblastn searches of the custom S. neurona genome database (Table 1). The two key enzymes AK and HXGPRT that aid in complementary purine salvage pathways were identified in the S. neurona genome. The S. neurona AK and HXGPRT sequences showed 39 and 65% amino acid identity to their respective T. gondii orthologues. Adenosine monophosphate (AMP) and IMP, which are the initial products of the purine salvage pathways, are inter-convertible in T. gondii and facilitated by the enzymes AMP deaminase, adenylosuccinate synthetase and adenosuccinate lysase; all three enzymes were identified in the S. neurona genome sequence. Similarly, orthologues to IMP dehydrogenase and GMP synthase were identified. Surprisingly, no orthologue for purine nucleoside phosphorylase was identified in the draft sequence of the S. neurona genome.
Characterization of the SnHXGPRT gene
Based on the draft genome sequence of S. neurona, the genomic locus of SnHXGPRT spans 4765 bp. Sequence of a PCR product obtained from S. neurona merozoite cDNA suggested that SnHXGPRT is a 232 amino acid protein encoded by a 699 bp open reading frame derived from 8 exons of the genomic locus (GenBank Accession No. KF406342). Sarcocystis neurona SN3 strain merozoites grew readily in the presence of MPA but were inhibited by 6-TX (data not shown), thus suggesting that S. neurona possess a functional HXGPRT enzyme.
SnHXGPRT can be manipulated for positive-negative selection
Transfection of S. neurona wild-type merozoites with the pSnHXG-UTRs-YFP gene-targeting plasmid resulted in isolation of S. neurona that were resistant to 6-TX. Analysis of single-cell clones with PCR primers that target SnHXGPRT or YFP (Supplemental Table S1) confirmed disruption of the native locus and replacement with the YFP transgene in several 6-TX-resistant clones (Fig. 1). The Sn∆HXG parasite clones were susceptible to MPA treatment, suggesting that these parasite lines indeed lack functional HXGPRT. Although the YFP gene was amplified from these Sn∆HXG parasite clones, YFP expression was not detected by fluorescence microscopy or western blot analysis of either merozoite or schizont stages (Supplemental Fig. S1). Clone Sn∆HXG.B7 was maintained in culture for further analyses.
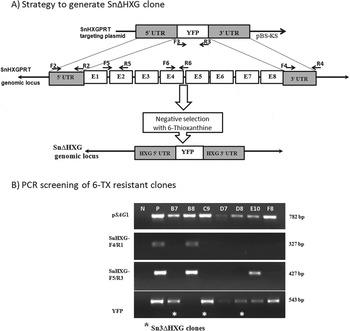
Fig. 1. Generation of S. neurona Sn∆HXG clones. (A) A linearized gene-targeting plasmid, pSnHXG-UTRs-YFP, consisting of the YFP gene flanked by ∼4 kb UTRs of SnHXGPRT gene was used to target the native HXGPRT locus in the S. neurona genome. E1 to E8 represent exons of the SnHXGPRT gene. SnHXGPRT-deficient parasites were selected by growing the transfected parasites in culture medium containing 160 μg mL−1 6-TX. The diagram also shows the location of primers used to generate the knockout construct and to screen the 6-TX-resistant clones. Primer information is provided in supplemental table S1. (B) Positive amplification of the YFP gene and no amplification of the native HXGPRT locus helped identify the HXGPRT knockout clones. * indicates S. neurona single-cell clones with disrupted HXGPRT locus. A primer pair targeting the promoter region of the S. neurona surface antigen SnSAG1 was used as a positive control.
TgHXGPRT restores HXGPRT-mediated purine salvage in S. neurona
To assess whether the T. gondii TgHXGPRT minigene would complement SnHXGPRT-deficient S. neurona, the ptubXFLAG::HX plasmid was transfected into the Sn∆HXG.B7 clone. The transfected population yielded parasites that were resistant to MPA, thus indicating that purine salvage was restored via the heterologous HXGPRT from T. gondii. PCR amplification performed on single-cell clones of the Sn-TgHXGc parasites confirmed the presence of the TgHXGPRT minigene (Fig. 2). Plaque assays performed to compare the growth efficiencies of SnHXGPRT-deficient and TgHXGPRT-complemented S. neurona revealed that MPA completely inhibited the growth of Sn∆HXG parasites while there was no significant reduction in the plaque counts of Sn-TgHXGc parasites (Fig. 3). Although there was no difference in the number of plaques when grown in the presence of 6-TX, the plaques for the Sn-TgHXGc and wild-type parasites were noticeably smaller in size and schizonts exhibited delayed development (data not shown).
Fig. 2. Heterologous complementation of Sn∆HXG parasites. The T. gondii HXGPRT minigene driven by the Tgdhfr promoter was transfected into Sn∆HXG merozoites, and the transformants were selected in culture medium containing 25 μg mL−1 MPA and 50 μg mL−1 xanthine. Primer pairs targeting the native SnHXGPRT locus and the Tgdhfr_HXGPRT of the transfected plasmid were used in PCR to confirm that the MPA-resistant clones lacked the native SnHXGPRT locus but carried Tgdhfr_HXGPRT minigene. Primers targeting the promoter region of SnSAG1 were used as a positive control. Primer information is provided in supplemental table S1.

Fig. 3. Plaque assay to determine growth efficiencies of HXGPRT-deficient (SN∆HXG) and TgHXGPRT-complemented (Sn-TgHXGc) S. neurona. Approximately 1000 merozoites of wild-type SN3 and the transgenic clones were inoculated in triplicate into wells of a 24-well plate with confluent BT cell monolayers and were grown either in the absence or presence of MPA. Plaque counts at 10 days post-inoculation revealed that MPA completely inhibited the growth of SN∆HXG parasites (no plaques observed) while TgHXGPRT was able to complement the HXGPRT-deficient parasites.
DISCUSSION
Purine salvage is crucial for survival of apicomplexans, and enzymes involved in this process have been successfully exploited as therapeutic targets as well as selection markers for molecular genetics studies (Donald and Roos, Reference Donald and Roos1998; Gherardi and Sarciron, Reference Gherardi and Sarciron2007). Apicomplexans utilize complementary purine salvage pathways involving HXGPRT and/or AK as key enzymes. Interestingly, however, not all members of the Apicomplexa encode both HXGPRT and AK (Chaudhary et al. Reference Chaudhary, Darling, Fohl, Sullivan, Donald, Pfefferkorn, Ullman and Roos2004). Bioinformatic searches of the various apicomplexan genomes indicated that Cryptosporidium and Theileria species encode AK alone. Similarly, Plasmodium species rely solely on HXGPRT for purine salvage. Toxoplasma gondii and Eimeria species use both HXGPRT and AK for purine salvage. In this paper, we report in silico identification of purine salvage enzymes of S. neurona, which suggested that purine salvage in this parasite can occur by either pathway involving HXGPRT or AK. These in silico findings were supported by the ability of wild-type S. neurona to grow in the presence of MPA, which requires functional HXGPRT. Likewise, HXGPRT-deficient S. neurona were viable and could be propagated in cell culture without any apparent reduction in growth efficiency, suggesting that the complementary purine salvage pathway involving AK is functional.
The failure to identify a PNP homologue in the S. neurona genome is significant. PNP is an important enzyme that is involved in converting inosine to hypoxanthine and guanosine to guanine (Berens et al. Reference Berens, Krug, Marr, Marr and Müller1995), both of which serve as substrates for HXGPRT. In T. gondii, hypoxanthine is reported to be the major purine source, next only to adenosine (Chaudhary et al. Reference Chaudhary, Darling, Fohl, Sullivan, Donald, Pfefferkorn, Ullman and Roos2004). The potential absence of PNP in S. neurona questions the capability of this parasite to generate inosine and guanosine-derived substrates for HXGPRT, and further suggests that this parasite must heavily rely on AK for purine salvage and/or is limited to xanthine as the primary substrate for HXGPRT. This analysis of extensive transcriptome data from merozoite and schizont stages of S. neurona also supports the absence of PNP in S. neurona (S. Dangoudoubiyam, unpublished data). Nevertheless, it is possible that the S. neurona PNP sequence has diverged significantly from its sister genera, making it difficult to identify by standard bioinformatic approaches.
In this study, we have used a gene-targeting plasmid for disruption of the native HXGPRT locus, thus demonstrating the feasibility of double-homologous recombination in S. neurona. It was intended that YFP expression would be driven by the native HXGPRT promoter following integration at this locus. Although proper integration of the YFP gene was confirmed in the Sn∆HXG clones, expression of YFP could not be detected either by fluorescence microscopy or western blots. Moreover, sequencing of the PCR products from one of the Sn∆HXG clones revealed that the YFP gene was in frame and contained no mutations. Point mutations were observed in the SnHXGPRT 5′-UTR within 1 kb upstream of the YFP gene, but it is not known if this was responsible for silencing YFP expression. Despite the lack of YFP expression in the Sn∆HXG clones, this will not hinder their use as a molecular genetics tool for positive-negative selection in S. neurona.
An earlier study that used pTgSAG1 to drive expression of luciferase in S. neurona suggested that heterologous promoters were not efficient in this parasite (Gaji and Howe, Reference Gaji and Howe2009). In contrast to this, HXGPRT function in Sn∆HXG parasites was successfully restored using HXGPRT of T. gondii driven by the TgDHFR promoter, thereby demonstrating the feasibility of complementation using heterologous promoters and genes. Recently, other promoters such as pTgTUB and pTgIMC have also been found to be functional in S. neurona (S. Dangouboubiyam, unpublished data), which further indicates that certain T. gondii promoters are capable of driving expression in S. neurona. This could prove advantageous for studies in comparative apicomplexan biology by diminishing the need for generating transgene constructs driven by S. neurona promoters.
HXGPRT and AK exist as single copy genes in apicomplexans, and prior work has shown that either enzyme alone is sufficient for parasite survival with no obvious fitness defects (Chaudhary et al. Reference Chaudhary, Darling, Fohl, Sullivan, Donald, Pfefferkorn, Ullman and Roos2004). Plaque assays of Sn∆HXG and Sn-TgHXGc parasites in MPA were as anticipated, but 6-TX in the growth medium only delayed development of Sn-TgHXGc parasites, as was evident by the smaller plaque sizes. This finding is consistent with 6-TX being a parasitistatic drug and not parasiticidal (Pfefferkorn et al. Reference Pfefferkorn, Bzik and Honsinger2001).
Previously developed methods for DNA transfection, expression of reporter molecules, and selection of stable transformants (Gaji et al. Reference Gaji, Zhang, Breathnach, Vaishnava, Striepen and Howe2006) have established S. neurona as a practical model for comparative studies of the Apicomplexa (Vaishnava et al. Reference Vaishnava, Morrison, Gaji, Murray, Entzeroth, Howe and Striepen2005). The Sn∆HXG clones described herein will facilitate positive-negative selection of transgenes and are a valuable addition to the molecular genetic tools available for manipulating S. neurona. Moreover, the recently sequenced genome of S. neurona further enhances investigation of this parasite. Collectively, these new resources are anticipated to accelerate gene discovery and functional studies in S. neurona, while also enabling additional comparative analyses that will contribute to the understanding of apicomplexan biology.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/S0031182014000687.
ACKNOWLEDGEMENTS
We thank Dr Anthony Sinai for providing ptubXFLAG::HX plasmid for use in heterologous complementation of HXGPRT in S. neurona. The S. neurona Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession JAQE00000000. The version described in this paper is version JAQE01000000. Published as Kentucky Agricultural Experiment Station Article No. 14-14-026 with approval of the Director.
FINANCIAL SUPPORT
This work was supported by a grant from the United States Department of Agriculture National Institute of Food and Agriculture (award 2009-65109-05918 to D. K. H.) and funds from the Amerman Family Equine Research Endowment.







