Introduction
The New Jersey cranberry industry is the third largest in the United States, covering 1,250 ha and producing approximately 29.6 million kg at a total value of US$27.8 million in 2016 (USDA-NASS 2018). Cranberry production in New Jersey is localized in the Pine Barrens, where sandy acidic soil (pH 4 to 5), good drainage, and abundant rainfall averaging 100 cm yr−1 are optimal for this native species (USDA-NRCS 2018b). Weed control in cranberry remains a challenge, as herbicide options are very limited compared with row crops or other specialty crops. Additionally, the absence of soil cultivation in the cranberry cropping system creates a favorable environment for the development of perennial weed species.
Carolina redroot is a monocotyledonous herb found in coastal plain acidic sandy soil from the Gulf Coast to Massachusetts (USDA-NRCS 2018a). In New Jersey, it is particularly abundant in cranberry beds, where it can rapidly form monoculture patches because of its rhizome capacity to generate new shoots. Previous work has demonstrated that Carolina redroot seed germination rate was no higher than 0.5% and that seed longevity was no longer than 130 d, suggesting that rhizome sprouting is the main propagation mechanism for this species (Boughton et al. Reference Boughton, Boughton, Griffith and Bernath-Plaisted2016). Carolina redroot spread has been linked to the development of fairy ring (Thanatophytum sp.) disease in cranberry beds (Oudemans et al. Reference Oudemans, Polashock and Vaiciunas2010) as well as other “stand opening” conditions under which the cranberry canopy is damaged. Openings in the cranberry ground coverage are colonized by Carolina redroot, which can rapidly spread through rhizomatic propagation. This weed has been shown to dominate areas previously occupied by native grass species following disturbance by feral swine that appear to target patches occupied by Carolina redroot for feeding. (Bankovich et al. Reference Bankovich, Boughton, Boughton, Avery and Wisely2016; Boughton and Boughton Reference Boughton and Boughton2014). In a similar way, cranberry vines are damaged and uprooted when overwintering waterfowl feed on Carolina redroot rhizomes, creating additional open areas that can be rapidly overrun by weeds and cause important production and economic losses (D Schiffhauer, personal communication).
Few publications have documented control of Carolina redroot with POST herbicides. In a greenhouse study, Welker (Reference Welker1979) noted that glyphosate at 2,200 g ae ha−1 provided good control the year following the application, while paraquat at 1,100 or 2,200 g ai ha−1 had good initial control but only fair residual control. Similar work by Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013) has shown Carolina redroot shoot and rhizome control at 63 DAT to be 72% and 91% with paraquat at 560 g ai ha−1, 69% and 88% with glyphosate at 1,260 g ae ha−1, and 59% and 73% with glufosinate at 660 g ai ha−1, respectively. In pastures, Carolina redroot control was 70% to 85% with triclopyr at 1.1 kg ha−1 and a mix of dicamba at 560 g ai ha−1 plus 2,4-D at 1,600 g ha−1 (Ferrell et al. Reference Ferrell, Sellers and Walter2009). In cranberry, Meggitt and Aldrich (Reference Meggitt and Aldrich1959) reported that Carolina redroot density was not affected by spring application of amitrol at 1,100 g ai ha−1 but decreased by 84% with fall application at the same rate. Studies conducted on New Jersey cranberry beds infested with Carolina redroot have shown that napropamide applied PRE at 6,720 g ai ha−1 provided 78% control at 83 DAT without causing injury to cranberry vines (Besançon et al. Reference Besançon, Carr and Schiffhauer2017). However, regrowth of Carolina redroot occurred in early summer following dissipation of napropamide activity and justifies the need for an efficient POST herbicide.
Data evaluating the Carolina redroot control effectiveness of POST herbicides currently registered for cranberry are limited. Because this weed spreads primarily through rhizomatic propagation, the use of glasshouse studies for evaluating herbicide efficacy on underground structures is warranted. Therefore, a greenhouse trial was set up for screening efficacy of various POST herbicides at controlling emerged Carolina redroot and preventing the development of new rhizomes.
Materials and Methods
Studies were conducted at the Philip E. Marucci Center for Blueberry and Cranberry Research at Rutgers University in Chatsworth, NJ (39.42°N, 74.30°W) in 2017.
Plant Material
Carolina redroot divisions averaging 4.2 cm in height were collected from a commercial cranberry bog in Chatsworth, NJ (39.43°N, 74.32°W) on May 5 and May 19, 2017. The rhizome of each plant was cut at a 3-cm length and buried 1-cm deep when plants were transplanted into 11-cm2, 10-cm-deep, black plastic pots containing 1 L of a 1:1 (v/v) mix of SunGro Canadian sphagnum peat moss (Sun Gro Horticulture, Agawam, MA) and Woodmansie sand (coarse-loamy, siliceous, semiactive, mesic Typic Hapludults) obtained from a local gravel pit and used for sanding cranberry beds. Organic matter content (3.9%) and pH (4.4) of the potting mix are representative of New Jersey cranberry bog soils, based on soil analysis conducted by the Ocean Spray cooperative over multiple years in various cranberry beds (D Schiffhauer, personal communication). Foliar growth and emergence of new leaves were monitored following transplanting. Plants were kept for 10 d in the greenhouse to allow sufficient time for establishment of a root system. This was assessed at the end of the period by unpotting 6 plants not used in this study and examining the root development. At this time, plants averaged 11 cm in height with three or four fully extended leaves.
Treatments
Treatments were applied 11 d after planting of Carolina redroot and consisted of 12 herbicide and rate combinations applied POST (Table 1) and a nontreated check (UNT). All herbicides are labeled for use on cranberry, except for fomesafen, flumioxazin, and pronamide. However, these three herbicides were included because studies are currently being conducted to determine crop tolerance and support efforts to receive a registration on cranberry (H Sandler, personal communication). Graminicides were not included in this study, because previous work has demonstrated the lack of Carolina redroot control with sethoxydim (Meyers et al. Reference Meyers, Jennings, Monks, Ballington and Jordan2013). Mesotrione, quinclorac, and 2,4-D were applied with 1% crop oil concentrate, whereas carfentrazone-ethyl, clopyralid, and diquat were sprayed with 0.25% nonionic surfactant. Glyphosate was mixed with dry ammonium sulfate at 870 g ai ha−1. Two runs were conducted 15 d apart with herbicides applied on May 15, 2017, for the first run and May 30, 2017, for the second run. Treatments were applied with a CO2-pressurized backpack sprayer fitted with a single 11002 VS nozzle tip (TeeJet® Technologies, Springfield, IL) delivering 187 L ha−1 at 210 kPa. Following herbicide application, plants were left to dry for 2 h outside the greenhouse and were not watered for 24 h to avoid herbicide wash off from the leaf surface. Pots were then watered twice daily to provide adequate soil moisture to the plants. During the time of the experiment, no other weed than Carolina redroot emerged from the potting mix, eliminating the need for hand weeding.
Table 1 POST herbicides and adjuvants applied on Carolina redroot.
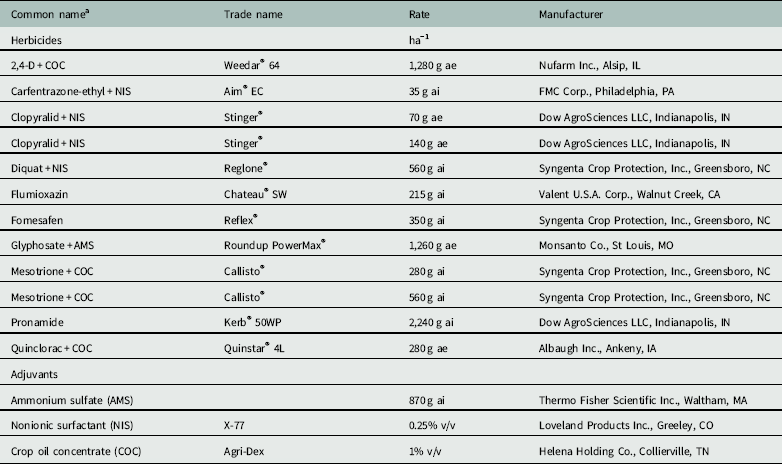
a AMS, dry ammonium sulfate; COC, crop oil concentrate; NIS, nonionic surfactant.
Statistical Analysis
The study was conducted as a randomized complete block design with 10 replications in two experimental runs. Each pot was considered as a single replication unit. Aboveground vegetation control was visually rated at 2, 7, 14, 21, 42, and 63 DAT on a 0% (no control) to 100% (death of all plants) scale with 5% increments, based on a composite estimation of growth inhibition and foliar injury (Frans et al. Reference Frans, Talbert, Marx and Crowley1986). The same timing was also used to measure plant height. All plants were harvested at 63 DAT by carefully removing them from the pots, washing soil residues under a stream of clear tap water, and separating the aboveground shoots from the rhizomes and roots at the soil level. A visual estimation of Carolina redroot root/rhizome control was conducted based on a scale similar to the one previously described for shoot control. The cumulative length of newly formed rhizome was also measured for each plant. The number of secondary shoots that emerged from newly formed rhizomes was also counted for each plant. Fresh weight of the aboveground and belowground parts was recorded before placing them in paper bags and drying them at 65 C for 96 h. Following heat drying, shoot and root/rhizome dry weight was measured.
Data were subjected to ANOVA using the generalized linear mixed model (GLIMMIX) procedure in SAS v. 9.4 (SAS Institute, Cary, NC) to determine whether experimental runs could be combined for regression analysis. Herbicide treatments were considered fixed variables, whereas experimental run, replication within a run, and their interaction were considered random effects (Carmer et al. Reference Carmer, Nyquist and Walker1989). Because of unequal variance, visual estimates of weed control were arcsine square-root transformed prior to ANOVA (Grafen and Hails Reference Grafen and Hails2002). The transformed means were back transformed for presentation purposes. Mean comparisons were performed using Fisher’s protected LSD test when F-values were statistically significant (P≤0.05).
Results and Discussion
In the absence of significant interaction between runs and treatments, data were combined across runs for Carolina redroot shoot control and plant height at the different evaluation dates and for root/rhizome control, number of secondary shoots, and dry biomass of main shoot and root/rhizome at 63 DAT.
Shoot Control
Compared with the UNT, diquat consistently provided the greatest level of Carolina redroot control at any evaluation date, ranging from 94% at 7 DAT to 99% at 63 DAT (Table 2). Mesotrione activity on Carolina redroot in the form of newly emerging chlorotic leaves was initially noted at 14 DAT with 17% and 13% control for the 280 g ai ha−1 and 560 g ai ha−1 rates, respectively. Greater than 90% control was noted by 63 DAT with both rates of mesotrione. Flumioxazin and 2,4-D were also very effective with an increase from 17% and 28% control at 7 DAT, respectively, to 87% control at 63 DAT. Carolina redroot control did not exceed 35% by 63 DAT with fomesafen, whereas other herbicides (quinclorac, clopyralid, and pronamide) never provided significant control of Carolina redroot shoots at any evaluation date. Similarly, Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013) reported no more than 8% Carolina redroot control with fomesafen and 9% with clopyralid. The current data and Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013) both show that glyphosate provided some control of Carolina redroot, but its activity was noted well after herbicide was applied. Control with glyphosate was only 24% at 42 DAT before increasing to 40% at 63 DAT.
Table 2 Carolina redroot aboveground vegetation control relative to the untreated check at 7, 14, 28, 42, and 63 d after POST application (DAT).

a Nonionic surfactant was included with clopyralid, carfentrazone-ethyl, and diquat treatments. Crop oil concentrate was included with mesotrione, quinclorac, and 2,4-D treatments. Ammonium sulfate was included with glyphosate treatment.
b Data were pooled across runs (N=20), and means within a column followed by the same letter are not different according to Fisher’s protected LSD (P≤0.05).
c Control was rated on a 0% (no control) to 100% (death of all plants) scale with 5% increments.
Plant Height
Diquat was the only treatment that reduced plant height at all evaluation dates, with shoots completely degraded by 42 DAT (Table 3). Significant reduction of plant height was noted with 2,4-D and flumioxazin with 45% and 38%, respectively, at 14 DAT and 83% at 63 DAT. Carolina redroot shoot control never exceeded 34% with fomesafen, but height measurements indicate that plant growth was affected more than suggested by visual observations, with significant reduction starting at 14 DAT and reaching 43% at 63 DAT. Influence of mesotrione on plant height was noticeable only at 42 DAT with 70% and 84% reduction for 280 and 560 g ai ha−1 rates, respectively, compared with the UNT. Late herbicide effect was also noted for glyphosate, but to a far lesser extent than mesotrione, with a significant reduction of 34% at 63 DAT in comparison with the UNT. No significant effect of quinclorac, clopyralid, carfentrazone-ethyl, and pronamide was observed on Carolina redroot height at any rating dates.
Table 3 Carolina redroot aboveground shoot height at 7, 14, 28, 42, and 63 d after POST herbicide treatment (DAT).

a Nonionic surfactant was included with clopyralid, carfentrazone-ethyl, and diquat treatments. Crop oil concentrate was included with mesotrione, quinclorac, and 2,4-D treatments. Ammonium sulfate was included with glyphosate treatment. UNT, nontreated check.
b Data were pooled across runs (N=20), and means within a column followed by the same letter are not different according to Fisher’s protected LSD (P≤0.05).
Root/Rhizome Control
Significant control of belowground parts was noted for all treatments that provided control of Carolina redroot aboveground shoots (Table 4). Mesotrione provided 88% to 98% root/rhizome control with rates of 280 g ai ha−1 and 560 g ai ha−1, respectively. Diquat and 2,4-D also provided significant root/rhizome control with 86% and 90%, respectively. Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013) reported only 24% root/rhizome control with flumioxazin applied at 430 g ai ha−1. Although a lower rate was used in the present study (215 g ai ha−1), results indicate higher control (85%) with flumioxazin. Plants collected for this study were 45% smaller than those dug out by Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013). This may have helped to improve herbicide spray coverage, resulting in increased aboveground shoot and root/rhizome control compared with results from the literature. Although aboveground vegetation control with glyphosate was significantly less than for five other herbicide treatments at 63 DAT, glyphosate significantly reduced the development of Carolina redroot root and rhizome system, with 87% control. Previous studies looking at absorption and translocation of [14C]glyphosate in various perennial weed species reported that glyphosate readily moves through the symplast and accumulates in the roots of Canada thistle [Cirsium arvense (L.) Scop.], field bindweed (Convolvulus arvensis L.), hedge bindweed [Calystegia sepium (L.) R. Br.], and hemp dogbane (Apocynum cannabinum L.) (McAllister and Haderlie Reference McAllister and Haderlie1985; Sandberg et al. Reference Sandberg, Meggitt and Penner1980; Schultz and Burnside Reference Schultz and Burnside1980). Additionally, Claus and Behrens (Reference Claus and Behrens1976) observed that increasing rates of glyphosate from 280 to 1,120 g ae ha−1 resulted in higher mortality of quackgrass [Elymus repens (L.) Gould] rhizome buds, with all buds killed at the highest glyphosate rate. In accordance with reports from the literature, this study suggests that glyphosate applied at 1,260 g ae ha−1 can prevent the development of Carolina redroot rhizome, thus depriving the plant of its primary propagation mechanism. Carolina redroot root/rhizome control for other herbicides ranged from 0% to 2% with clopyralid to 43% with fomesafen. These results are comparable to those obtained by Meyers et al. (Reference Meyers, Jennings, Monks, Ballington and Jordan2013), who reported 3% and 30% root/rhizome control with clopyralid and fomesafen, respectively.
Table 4 Carolina redroot biomass, cumulative rhizome length,and number of secondary aboveground shoots at 63 d after POST herbicide treatment.Footnote a

a Data were pooled across runs (N=20), and means within a column followed by the same letter are not different according to Fisher’s protected LSD (P≤0.05).
b Nonionic surfactant was included with clopyralid, carfentrazone-ethyl, and diquat treatments. Crop oil concentrate was included with mesotrione, quinclorac, and 2,4-D treatments. Ammonium sulfate was included with glyphosate treatment. UNT, nontreated check.
c Control was rated on a 0% (no control) to 100% (death of all plants) scale with 5% increments.
Shoot and Root/Rhizome Dry Biomass
Carolina redroot shoot and root/rhizome dry biomass averaged 0.58 g and 1.30 g, respectively, for the UNT (Table 4). All herbicide treatments, with the exception of quinclorac, significantly reduced shoot dry biomass compared with the UNT. Mesotrione at 280 g ai ha−1 and diquat or mesotrione at 560 g ai ha−1 produced the greatest reduction of shoot dry biomass with 74% and 79% reduction, respectively, compared with the UNT. Glyphosate, flumioxazin, and 2,4-D also significantly reduced shoot dry biomass by 55%, 60%, and 72%, respectively. Other herbicides only resulted in minor shoot dry biomass reduction, ranging from 17% with clopyralid at 70 g ai ha−1 to 38% with fomesafen. The visual root/rhizome rating at 63 DAT was supported by root/rhizome dry biomass data that showed reductions by 78% with glyphosate or flumioxazin, 83% with diquat or mesotrione at 280 g ai ha−1, and 88% with mesotrione at 560 g ai ha−1 compared with the UNT. With a 42% reduction of the root/rhizome dry biomass compared with the UNT, pronamide had a higher quantitative effect on the development of the Carolina redroot root system than reflected by the qualitative evaluation of root/rhizome control rating taken at 63 DAT. Other herbicides provided nonsignificant control of belowground parts, with no more than 24% root/rhizome dry biomass reduction for quinclorac, clopyralid, and carfentrazone-ethyl.
Cumulative Rhizome Length
Compared with the UNT, 2,4-D, diquat, flumioxazin, glyphosate, and mesotrione at 560 g ai ha−1 decreased the cumulative length of rhizomes by 87% to 92% (Table 4). Significant reduction of rhizome development was also noted with fomesafen and mesotrione at 280 g ai ha−1, but to a far lesser extent, with 78% and 26% cumulative length decreases, respectively. Finally, quinclorac, carfentrazone-ethyl, pronamide, and clopyralid, regardless of applied rate, did not significantly affect growth of the rhizomatic parts. Because rhizome serves as an important sink for carbohydrates produced by the plant, reduction of its development can have substantial effect on a plant’s ability to propagate through the production of secondary shoots from the rhizome.
Emergence of Aboveground Secondary Shoots
Carolina redroot propagation occurs mainly through the development of rhizomes from which clonal plants will develop (Boughton et al. Reference Boughton, Boughton, Griffith and Bernath-Plaisted2016). Therefore, it is important to evaluate the impact of herbicide treatments on a plant’s ability to generate aboveground secondary shoots from viable rhizome structures. With the exception of clopyralid and pronamide, treatments that significantly reduced the root/rhizome dry weight had a reduced number of secondary shoots compared with the UNT (Table 4). Development of secondary shoots was not affected by application of quinclorac, clopyralid, and pronamide, with an average of 2.1 secondary shoots per plant, a similar number to what was noted for the UNT. Carfentrazone-ethyl significantly reduced shoot and root/rhizome dry weight by 26% and 19%, respectively, and had a more pronounced effect on the number of secondary shoots, which decreased by 50%, compared with the UNT. Previous work on alligatorweed [Alternanthera philoxeroides (Mart.) Griseb.], a perennial aquatic weed, with carfentrazone-ethyl applied at 56 g ai ha−1 was shown to reduce overall plant dry biomass to 43% of the nontreated check and plant growth by 40% at 4 wk after treatment (Richardson et al. Reference Richardson, Roten, West, True and Gardner2008). The authors concluded that although alligatorweed regrew rapidly following carfentrazone-ethyl application, higher rates could potentially provide adequate control, which could also be the case for Carolina redroot. The carbohydrate depletion resulting from the root/rhizome dry weight reduction with 2,4-D, flumioxazin, fomesafen, and diquat helped reduce the number of secondary shoots between 70% and 90%. Only glyphosate and mesotrione, regardless of rate applied, completely inhibited the emergence of new Carolina redroot shoots from the rhizome. This observation parallels the high level (above 85%) of rhizome control noted with these two herbicides in the present study.
Boughton et al. (Reference Boughton, Boughton, Griffith and Bernath-Plaisted2016) reported that the germination of Carolina redroot seeds was very low (average of 0.35) despite seed production exceeding 2,400 seeds per inflorescence. These findings suggest that rhizome sprouting is the dominant mechanism for Carolina redroot to spread. Therefore, applications of herbicides that greatly inhibit the development of the rhizome and its ability to produce clonal plantlets can provide control and reduce the extent of Carolina redroot patches in cranberry beds. Mesotrione has been labeled for PRE and POST weed control in cranberry since 2008 with excellent crop tolerance (Majek and Ayeni Reference Majek and Ayeni2004). Sandler (Reference Sandler2017) demonstrated that a combination PRE–POST herbicide program with a low rate of napropamide PRE followed by mesotrione POST may be the most cost-beneficial program in new cranberry plantings. Ongoing field research with various PRE herbicides shows that napropamide might provide control of Carolina redroot until early summer (Besançon et al. Reference Besançon, Carr and Schiffhauer2017).
Results of the present study indicate that mesotrione, flumioxazin, and glyphosate are promising options for POST control of Carolina redroot. Wiper applicators could be considered for applying glyphosate, especially because glyphosate formulation and low viscosity make it suitable for this type of application (Harrington and Ghanizadeh Reference Harrington and Ghanizadeh2017). To minimize the risk of crop injury, wiper applications should be made when Carolina redroot’s flower stalk reaches its complete development and stands well above the cranberry canopy. However, further research is warranted to determine the potential for cranberry injury that can be caused by glyphosate dripping from the wiper or by contact between treated plants and cranberry vines. It is also unknown whether the Carolina redroot growth stage at the time of herbicide application would allow a sufficient amount of glyphosate to be translocated to the aboveground parts for effective Carolina redroot control. Work is underway in New Jersey, Wisconsin, and Massachusetts to evaluate flumioxazin for crop safety and weed control efficacy in fall- and spring-applied field trials. Studies have also been initiated in New Jersey in 2018 with mesotrione alone or combined with PRE herbicides to validate results from this study under field conditions and allow the development of Carolina redroot use pattern recommendations. Additionally, mesotrione was granted a Federal Insecticide, Fungicide, and Rodenticide Act §24(c) Special Local Need Label in 2018 for spot treatment applications in cranberry that locally increase herbicide concentration and provide effective control of dodder (Cuscuta spp.) or perennial weeds, such as eastern poison-ivy [Toxicodendron radicans (L.) Kuntze] (Sandler Reference Sandler2010). This might prove to be an effective management tool for eradicating early infestation patches of Carolina redroot that develop within cranberry necrotic zones caused by the fairy ring disease or along ditches surrounding cranberry beds.
Acknowledgments
The author acknowledges the technical support of Pine Island Cranberry Company Inc. and Peter Oudemans for the use of Carolina redroot plants and greenhouse space required for this research. Additional technical support by Baylee Carr is also greatly appreciated. This research was funded by the New Jersey Blueberry and Cranberry Research Council. No conflicts of interest have been declared.






