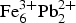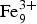Introduction
The Moctezuma mine, Sonora, Mexico (29°48′N, 109°40′W), is the type locality for 21 Te oxysalt minerals, constituting approximately one quarter of all known secondary Te minerals (Christy et al., Reference Christy, Mills and Kampf2016; Pasero et al., Reference Pasero2017). This extraordinary mineralogical diversity was one of the driving forces behind increased study of Te that has occurred from the 1960s onwards. Despite this mineralogical diversity, a number of Te oxysalts first described at Moctezuma still have unknown crystal structures. The determination of these crystal structures will provide further information about the formation of these minerals and their relationship to other Te oxysalts. The present investigation uses synchrotron X-ray diffraction, first used successfully on a secondary Te mineral in determining the structure of quetzalcoatlite (Burns et al., Reference Burns, Pluth, Smith, Eng, Steele and Housley2000).
Eztlite was first reported as having the formula ![]() ${\rm Fe}_6^{3 +} {\rm Pb}_{\rm 2}^{2 +} $(Te4+O3)3(Te6+O6)(OH)10·nH2O, where n ≈ 8 (Williams, Reference Williams1982). It is one of six secondary Te minerals reported to contain both the +4 and +6 oxidation states of Te (Pasero et al., Reference Pasero2017). However, of these species, only carlfriesite has a confirmed crystal structure that shows both oxidation states are indeed present (Effenberger et al., Reference Effenberger, Zemann and Mayer1978). The structures of 26 synthetic compounds with mixed Te oxidation states are known (Christy et al., Reference Christy, Mills and Kampf2016), confirming that a greater variety in mixed-valence species than currently known in nature is possible.
${\rm Fe}_6^{3 +} {\rm Pb}_{\rm 2}^{2 +} $(Te4+O3)3(Te6+O6)(OH)10·nH2O, where n ≈ 8 (Williams, Reference Williams1982). It is one of six secondary Te minerals reported to contain both the +4 and +6 oxidation states of Te (Pasero et al., Reference Pasero2017). However, of these species, only carlfriesite has a confirmed crystal structure that shows both oxidation states are indeed present (Effenberger et al., Reference Effenberger, Zemann and Mayer1978). The structures of 26 synthetic compounds with mixed Te oxidation states are known (Christy et al., Reference Christy, Mills and Kampf2016), confirming that a greater variety in mixed-valence species than currently known in nature is possible.
This redefinition of eztlite follows on from other recent studies of existing secondary Te minerals (e.g. Christy et al., Reference Christy, Mills and Kampf2016; Kampf et al., Reference Kampf, Mills and Rumsey2017).
Specimen descriptions
Two eztlite specimens were analysed in this investigation. One was a type specimen (probably part of an original, single holotype) from the Moctezuma mine, registered in the collections of the Natural History Museum, London, specimen number BM 1984,468, which showed evidence of striations where a previous investigator had scraped away material for analysis. No obvious single crystals were observed, although eztlite was clearly present as blood-red crusts (Fig. 1a), surrounded by mixed Fe and Te oxides. A large number of small (<100 µm) aggregates of poughite are also found on this specimen.

Fig. 1. (a) A view of the eztlite type specimen, BM 1984,468, showing red eztlite (a thin coating of poorly crystalline material next to the arrow), with orange, red and black iron–tellurium oxides on quartz. Several small aggregates of yellow poughite are also visible in the bottom half of the image. Field of view 3.5 mm. (b) A view of the M54056 specimen, showing dark red eztlite aggregates which are surrounded by Fe oxide films and other Fe oxides on quartz.
A second eztlite specimen, which also originated from the Moctezuma mine, was recently purchased and is registered in the collections of Museums Victoria, registration number M54056. This specimen was used to obtain crystals of higher quality than were present on the type specimen, allowing for single-crystal analysis of eztlite to be performed. This specimen contains a cluster of sparkling, deep-red aggregates of prismatic eztlite crystals, on the surface of a rock otherwise rich in quartz, calcite and Fe oxides. Several spherical aggregates of emmonsite are also present. There are >100 eztlite aggregates covering an area of ~1 mm2, with no eztlite obviously present on the rest of the specimen (see Fig. 1b). Iridescent Fe-oxide films, powdery orange Fe oxides and botryoidal hematite surround the eztlite aggregates.
Chemistry
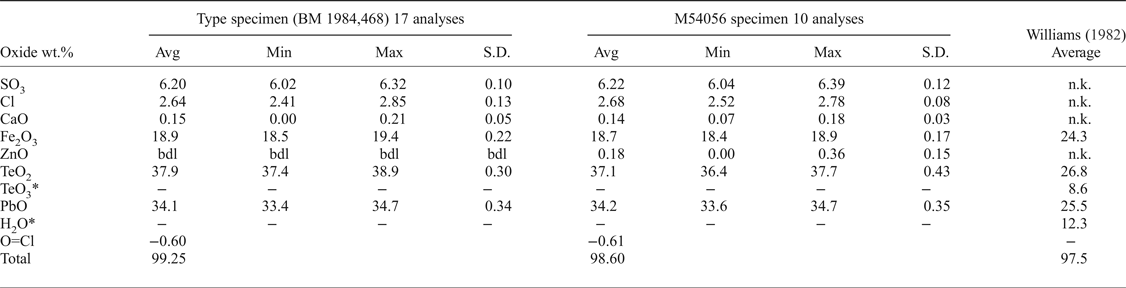
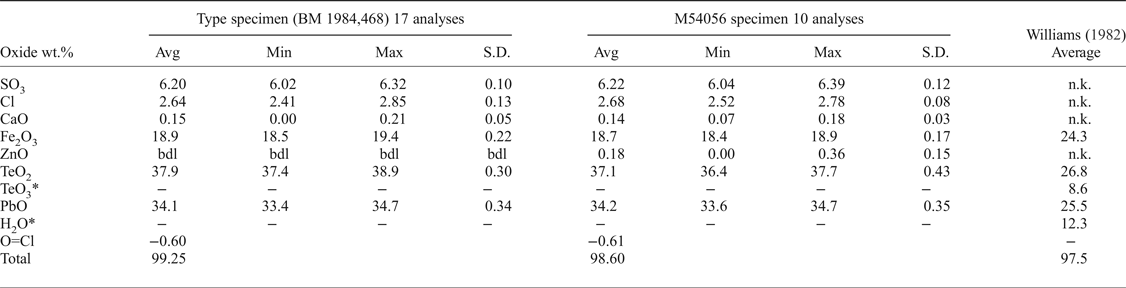
Quantitative chemical analyses of both eztlite specimens were performed on a Cameca SX100 Electron Microprobe in wavelength-dispersive mode, with an accelerating voltage of 12 kV, a specimen current of 10 nA, a beam diameter of 5 µm and PAP matrix correction (Pouchou and Pichoir, Reference Pouchou, Pichoir, Heinrich and Newbury1991) at the Imaging and Analysis Centre, Core Research Laboratories, Natural History Museum, London. The standards used for each element were: baryte (S), halite (Cl), wollastonite (Ca), hematite (Fe), sphalerite (Zn), TeO2 (Te) and vanadinite (Pb). The sample was assumed to contain significant OH (Williams, Reference Williams1982) so low values of voltage and current were used to reduce expected dehydration during analysis. Consequently, results are recorded to only one decimal place for Fe, Te and Pb. These beam conditions input less energy into the sample but give lower counting statistics for determined elements. Analytical results (Table 1) for the two samples are near identical, with both containing essential Pb2+, Fe3+, Te4+, S and Cl. M54056 contains minor Zn, and both specimens contain minor Ca. No other elements were detected using energy-dispersive spectroscopy or the electron microprobe. There was insufficient eztlite for CHN or infrared analyses. Raman spectroscopy was attempted, but excessive fluorescence prevented meaningful results from being obtained. Thus the crystal-structure analysis (see below) was used to show that eztlite is anhydrous.
Table 1. Electron microprobe data for the two eztlite specimens, including the analysis by Williams (Reference Williams1982) for comparison.
* TeO3 and H2O were only detected by Williams and are not actually present in eztlite.
S.D. – standard deviation; bdl – below detection limit; n.k. – not known
The chemical analysis of eztlite yields an unambiguous elemental composition that is significantly different to that reported by Williams (Reference Williams1982). The empirical formula (based on 15O anions per formula unit) for the type specimen is Pb1.94Ca0.03Fe3.01Te3.02S0.99Cl0.95O15.00 and for M54056 is Pb1.97Ca0.03Zn0.03Fe3.01Te2.98S1.00Cl0.97O15.00. Both formulae may be approximated as ![]() ${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} {\rm Te}_3^{4 +} $ SClO15. As all Te4+ centres are in trigonal pyramidal geometry (see below), the end-member formula of eztlite is
${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} {\rm Te}_3^{4 +} $ SClO15. As all Te4+ centres are in trigonal pyramidal geometry (see below), the end-member formula of eztlite is ![]() ${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} $(Te4+O3)3(SO4)O2Cl, which requires PbO 34.75%, Fe2O3 18.99%, TeO2 37.27%, SO3 6.23%, Cl 2.76%, total 100 wt.%.
${\rm Pb}_{\rm 2}^{2 +} {\rm Fe}_3^{3 +} $(Te4+O3)3(SO4)O2Cl, which requires PbO 34.75%, Fe2O3 18.99%, TeO2 37.27%, SO3 6.23%, Cl 2.76%, total 100 wt.%.
Compared to Williams (Reference Williams1982), the analyses of type eztlite (BM 1984,468) contain significantly lower Fe2O3 (18.89% compared to 24.3%), significantly higher PbO (34.10% compared to 25.5%) and similar overall Te (37.87% TeO2 compared to 26.8% TeO2 and 8.6% TeO3). Williams (Reference Williams1982) and his analyst, Marjorie Duggan, analysed for Al, Mn, Fe, Te (both valences) and Pb by wet chemical methods when determining the chemical composition of eztlite. No mention is made of analysing for S or Cl. The lack of these two elements yields an ideal mass deficit (SO3 + Cl) of 8.99 wt.%, which corresponds roughly to the 12.3 wt% H2O determined by the Penfield method on a 945 µg sample of ‘eztlite’ (Williams, Reference Williams1982). Presumably the Penfield analysis incorporated some hydrous material along with eztlite, or analysed a hydrous phase instead of eztlite, as eztlite is now known to be anhydrous. The presence of a second phase is also a possible source of the erroneous presence of Te6+ that Williams (Reference Williams1982) determined in eztlite.
Crystallography
Powder diffraction
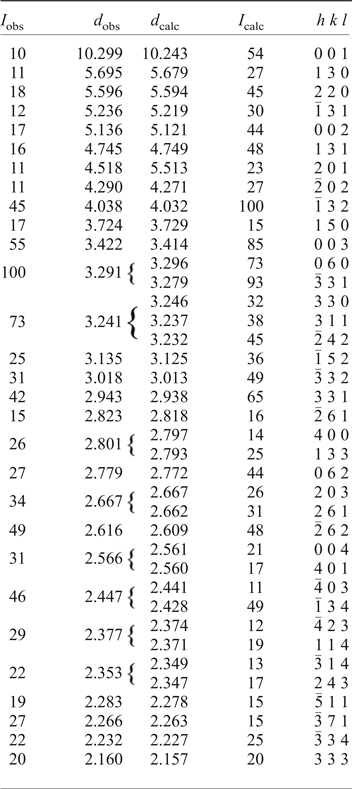
An eztlite aggregate comprising eleven sub-spherical eztlite grains obtained from the type specimen (BM 1984,468) was attached to a non-diffracting amorphous-carbon fibre (10 µm diameter) that was glued to a glass support rod. This sample was mounted on a Rigaku R-Axis curved image-plate diffractometer (®Rigaku Oxford Diffraction) at the Natural History Museum (London), and a dataset collected using CuKα radiation. A Gandolfi-type randomized sample movement was achieved by rotations on the φ and ω axes. Observed d hkl and reflection intensities collected to 2θ = 90° were derived by profile-fitting using Highscore Plus software (Degen et al., Reference Degen, Sadki, Bron, König and Nénert2014), although the dataset used was truncated at 2θ = 60° due to poorly defined, low-intensity peaks at higher angles. High background resulted in lower than expected relative intensities for peaks at low values of 2θ. The intensities of calculated and observed patterns commonly showed discrepancies, although this is not unexpected as the maximum intensity peak was different for the observed and calculated patterns. Nonetheless, the d values were mostly in good agreement.
The unit-cell parameters of eztlite were refined using Chekcell (Laugier and Bochu, Reference Laugier and Bochu2004) from the powder data and are a = 11.468(2) Å, b = 19.769(3) Å, c = 10.510(2) Å, β = 102.74(1)° and V = 2324.0(3) Å3. These parameters are in good agreement with the synchrotron single-crystal unit cell and with the pattern calculated from the structure using the PowderCell program (Kraus and Nolze, Reference Kraus and Nolze1996). This result confirms that the material analysed on the type specimen is identical to that on M54056. A comparison of observed and calculated peaks for the powder diffraction data is given in Table 2. Although the powder lines collected by Williams (Reference Williams1982) match peaks observed in this study, showing that the powder pattern he collected was from pure eztlite, he indexed a different monoclinic cell with the unit-cell parameters a = 6.58, b = 9.68, c = 20.52 Å, β = 90.25° and V = 1307 Å3.
Table 2. Powder X-ray diffraction data for eztlite to 45°2θ.
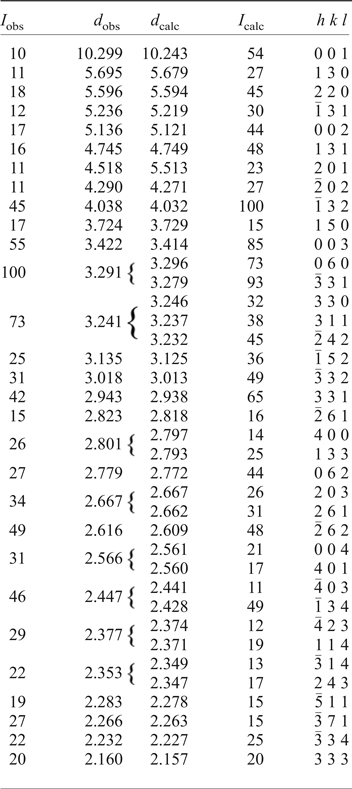
Only I calc >15% are shown.
Single-crystal diffraction
The single-crystal X-ray diffraction experiment was carried out on the micro-focus macromolecular MX2 beamline at the Australian Synchrotron, part of ANSTO. A 4 µm × 4 µm × 5 µm pale-red single crystal of eztlite was selected from specimen M54056. Data were collected at 100 K by a Dectris EigerX 16M detector using monochromatic radiation with a wavelength of 0.71073 Å. Further details of data collection and structure refinement are provided in Table 3.
Table 3. Crystallographic information relating to data collection and refinement of eztlite.
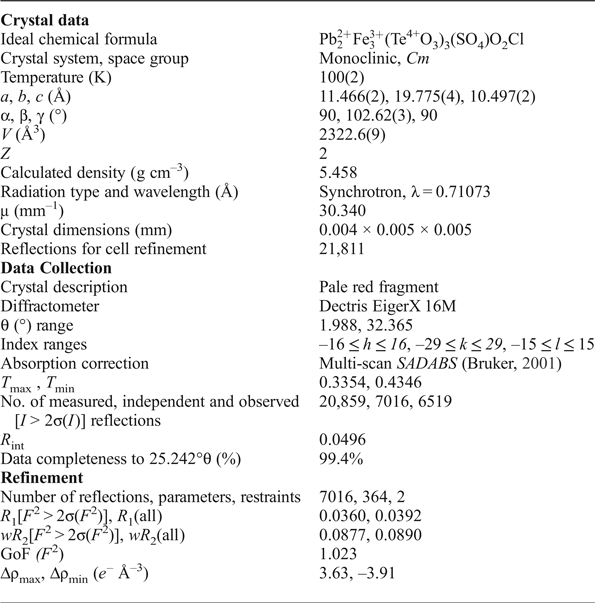
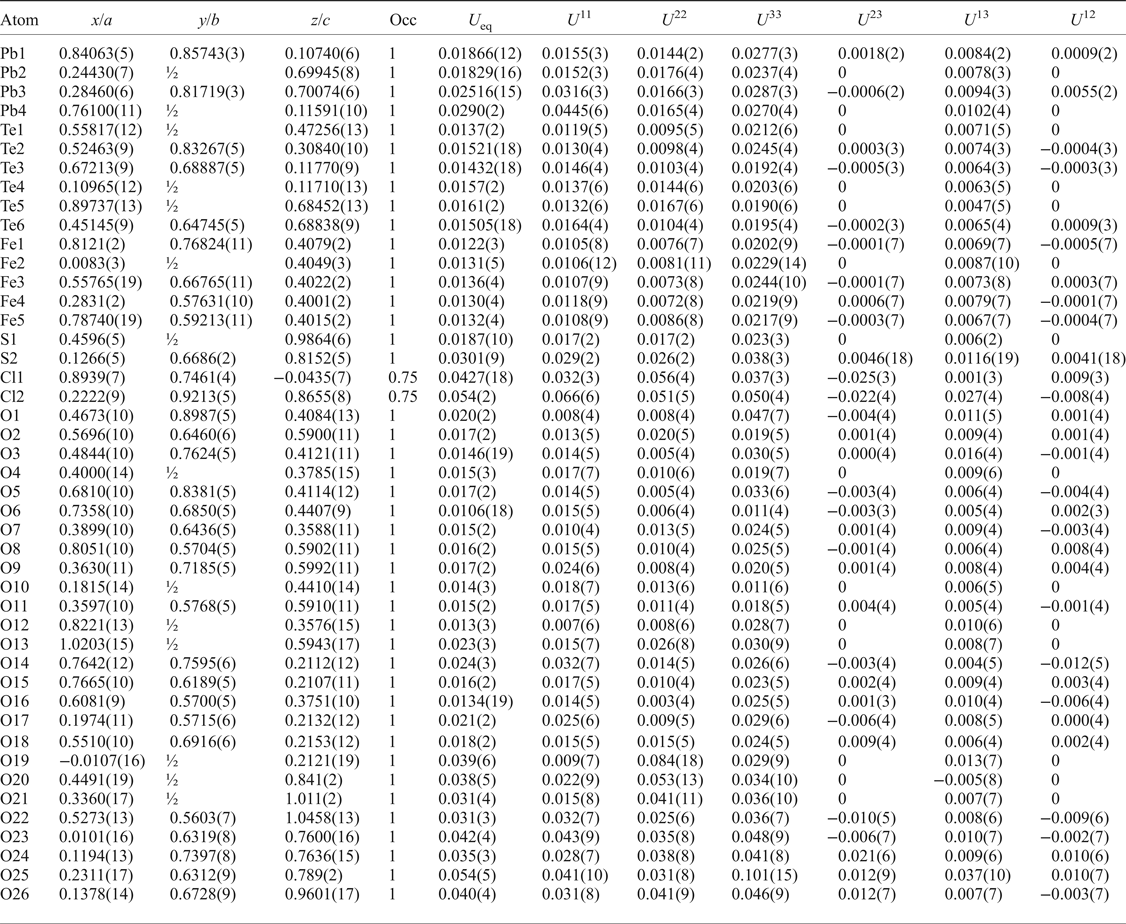
After processing the data using XDS (Kabsch, Reference Kabsch2010), XPREP (Bruker, Reference Bruker2001) and SADABS (Bruker, Reference Bruker2001), 20,859 reflections were found with an R int of 0.0496. Structure solution in space group Cm was carried out by direct methods using SHELXT (Sheldrick, Reference Sheldrick2015a) and four Pb, six Te, five Fe, two S, one Cl and 27 O atoms were located. Structure refinement by full-matrix least-squares was implemented by SHELXL (Sheldrick, Reference Sheldrick2015b), using neutral atomic-scattering factors. The final structure contains two Cl and 26 O atoms as one of the ‘oxygen’ atoms was determined to be a Cl site. All atom positions and anisotropic displacement parameters (U ij) were refined to final R 1 and wR 2 values of 0.0360 and 0.0877, respectively. After refining until the weighting scheme converged, and omitting all reflections with calculated F 0/F C errors greater than 5.00, one atom (O16) remained as non-positive definite. This is attributed to the position of O16 at an apex site in the mitridatite-like layers (see next section), which is postulated to cause the observed distortion. Despite this, all bond lengths to O16 are chemically sensible (Table 4). A summary of bond valences is provided in Table 5, using the parameters of Brese and O'Keefe (Reference Brese and O'Keeffe1991) for Pb–Cl bonds, Krivovichev and Brown (Reference Krivovichev and Brown2001) for Pb–O bonds, Mills and Christy (Reference Mills and Christy2013) for Te4+–O and Te4+–Cl bonds, and the parameters of Gagné and Hawthorne (Reference Gagné and Hawthorne2015) for Fe3+–O and S–O bonds. Fractional atom coordinates, site occupancies and atom displacement parameters (U ij) for all atoms are shown in Table 6.
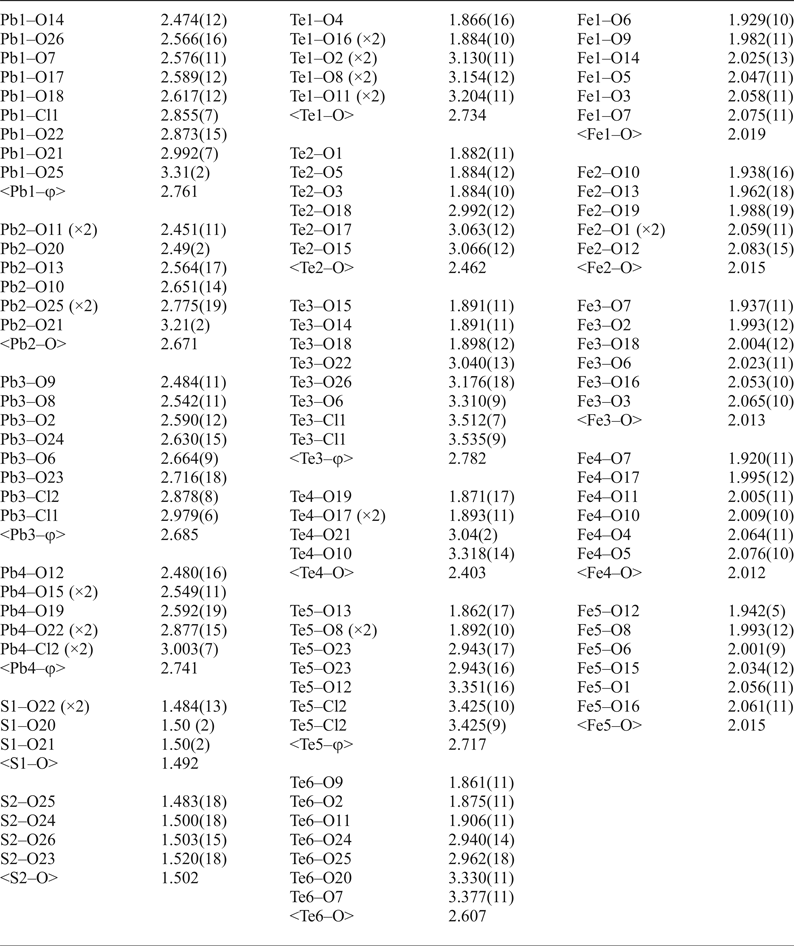
Table 4. Bond lengths (Å) for eztlite*.
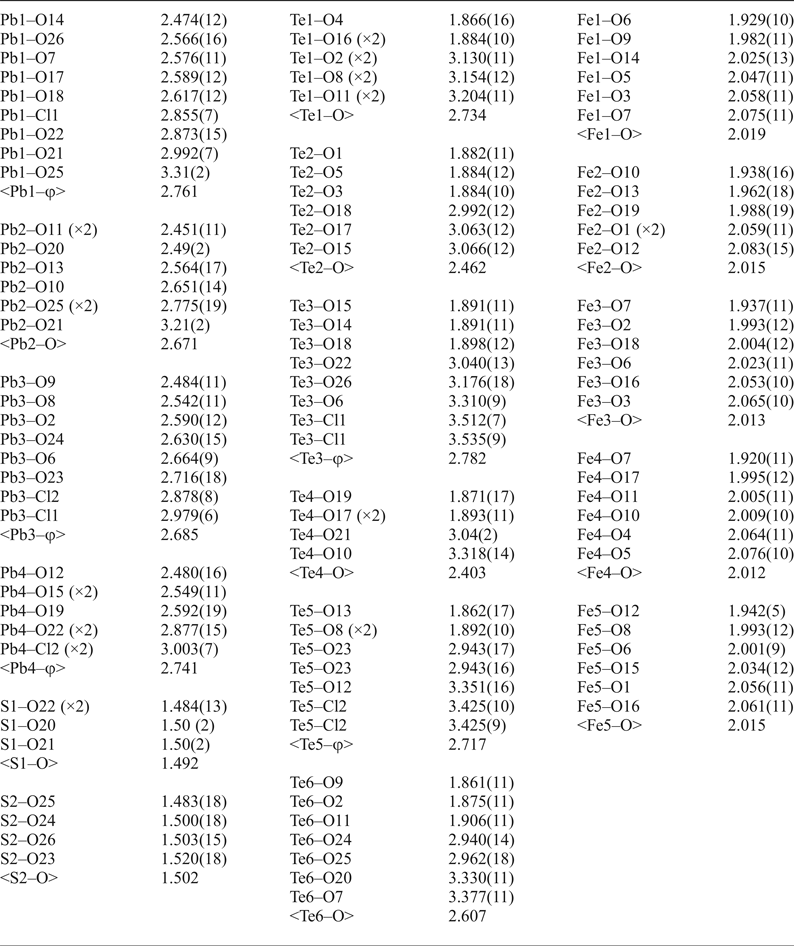
*For Te and Pb, φ denotes average bond length incorporating both O and Cl.
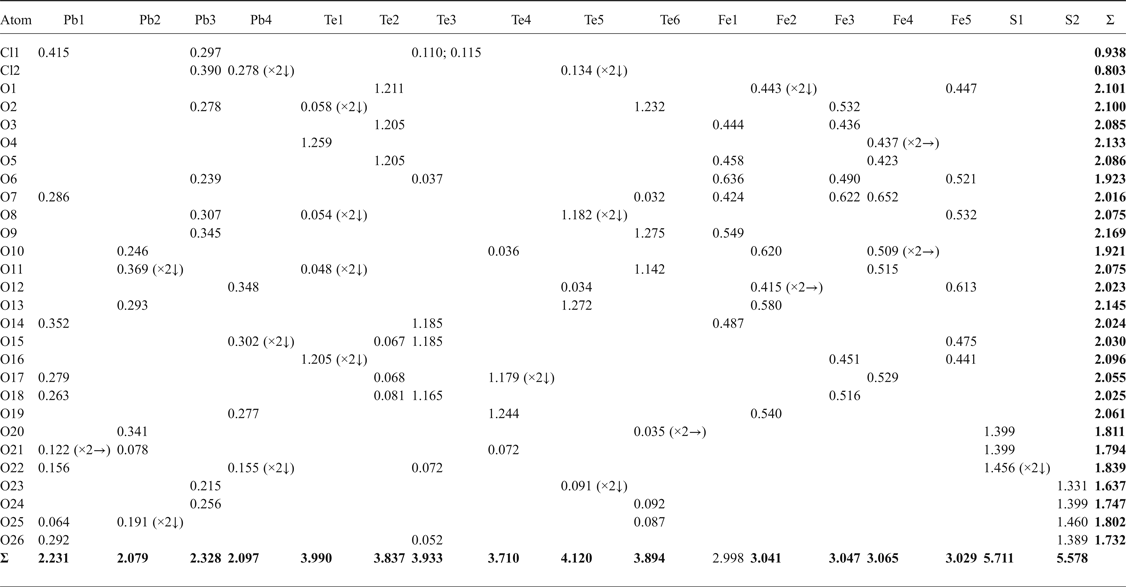
Table 5. Bond-valence sums (in valence units, vu) for eztlite.
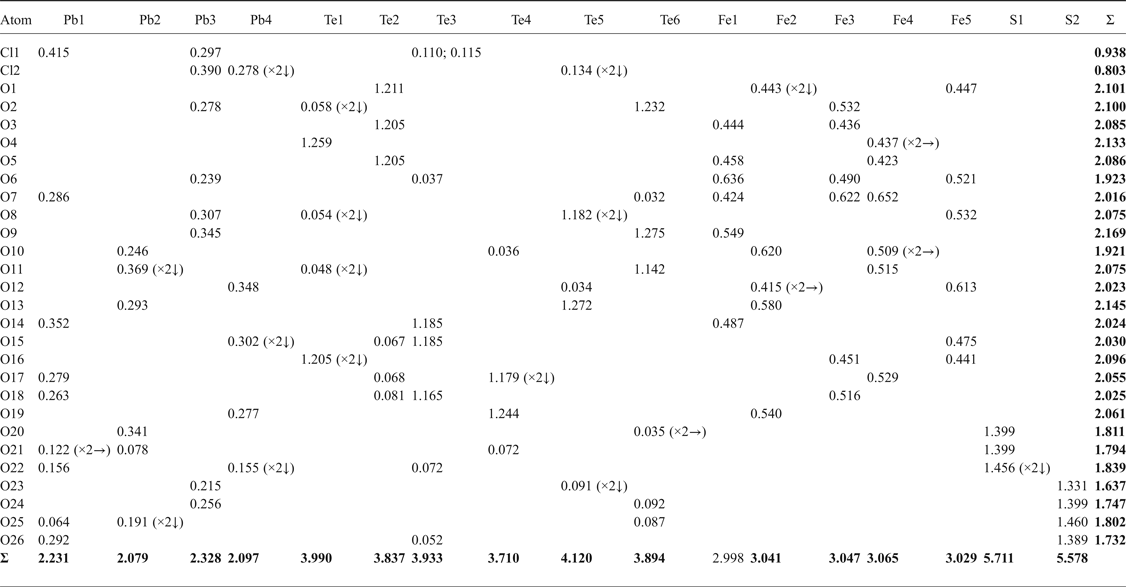
Table 6. Fractional atom coordinates, occupancies and displacement parameters for the atomic sites of eztlite.
The crystallographic files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Crystal-structure description
The crystal structure of eztlite is best described as a complex layered arrangement, with layers formed from Fe3+O6 octahedra and Te4+O3 trigonal pyramids. This arrangement is pseudo-trigonal, however other, less-ordered components in the structure result in a monoclinic habit overall. The interlayer regions are occupied by 8- and 9-coordinate Pb cations, sulfate tetrahedra, and chloride anions coordinated chiefly with Pb.
The layers in eztlite (see Fig. 2a) are analogous to the layers formed in mitridatite-type structures (see Fig. 2b). Mitridatite is a secondary Ca–Fe–phosphate mineral with the formula Ca2![]() ${\rm Fe}_3^{3 +} $(PO4)3 O2·3H2O (Moore and Araki, Reference Moore and Araki1977; Huminicki and Hawthorne, Reference Huminicki, Hawthorne, Kohn, Rakovan and Hughes2002). The other three group members are arseniosiderite, the As analogue (Moore and Ito, Reference Moore and Ito1974); kolfanite, the dihydrate (Voloshin et al., Reference Voloshin, Men'shikov, Polezhaeva and Lentsi1982); and robertsite, the Mn3+ analogue (Andrade et al., Reference Andrade, Morrison, Di Domizio, Feinglos and Downs2012). None of these minerals contain Pb, sulfate or chloride, instead containing interlayer water and Ca cations. In eztlite, the arrangement of nonameric triangles formed from edge-sharing Fe3+O6 octahedra, each of which in isolation has the formula [
${\rm Fe}_3^{3 +} $(PO4)3 O2·3H2O (Moore and Araki, Reference Moore and Araki1977; Huminicki and Hawthorne, Reference Huminicki, Hawthorne, Kohn, Rakovan and Hughes2002). The other three group members are arseniosiderite, the As analogue (Moore and Ito, Reference Moore and Ito1974); kolfanite, the dihydrate (Voloshin et al., Reference Voloshin, Men'shikov, Polezhaeva and Lentsi1982); and robertsite, the Mn3+ analogue (Andrade et al., Reference Andrade, Morrison, Di Domizio, Feinglos and Downs2012). None of these minerals contain Pb, sulfate or chloride, instead containing interlayer water and Ca cations. In eztlite, the arrangement of nonameric triangles formed from edge-sharing Fe3+O6 octahedra, each of which in isolation has the formula [![]() ${\rm Fe}_9^{3 +} $O36]45– and which are linked to three adjacent triangles through corner-sharing at the central vertices of each side, is identical to that found in mitridatite. Te4+O3 trigonal pyramids decorate the triangular arrangement of Fe3+O6 octahedra, with four Te4+O3 groups associated directly with each nonamer (see Fig. 2a). The incorporation of tellurite polyhedra into the two-dimensional layers results in the empirical layer formula of [Fe3+(Te4+O3)O]1–. Mitridatite-type structures contain either PO4 or AsO4 tetrahedra, hence the three-coordinate Te deviates somewhat from mitridatite, though tellurite groups are found in similar crystallographic locations to the phosphate and arsenate in mitridatite (see Fig. 2b). Another difference between the structure types is that in mitridatite-type structures, the central tetrahedron points in the opposite direction to the three others, whereas in eztlite, all four of the Te4+O3 groups are orientated in the same direction with respect to the Te atom position. Eztlite crystallizes in Cm, which is a maximal subgroup of Cc, the space group in which mitridatite crystallizes.
${\rm Fe}_9^{3 +} $O36]45– and which are linked to three adjacent triangles through corner-sharing at the central vertices of each side, is identical to that found in mitridatite. Te4+O3 trigonal pyramids decorate the triangular arrangement of Fe3+O6 octahedra, with four Te4+O3 groups associated directly with each nonamer (see Fig. 2a). The incorporation of tellurite polyhedra into the two-dimensional layers results in the empirical layer formula of [Fe3+(Te4+O3)O]1–. Mitridatite-type structures contain either PO4 or AsO4 tetrahedra, hence the three-coordinate Te deviates somewhat from mitridatite, though tellurite groups are found in similar crystallographic locations to the phosphate and arsenate in mitridatite (see Fig. 2b). Another difference between the structure types is that in mitridatite-type structures, the central tetrahedron points in the opposite direction to the three others, whereas in eztlite, all four of the Te4+O3 groups are orientated in the same direction with respect to the Te atom position. Eztlite crystallizes in Cm, which is a maximal subgroup of Cc, the space group in which mitridatite crystallizes.
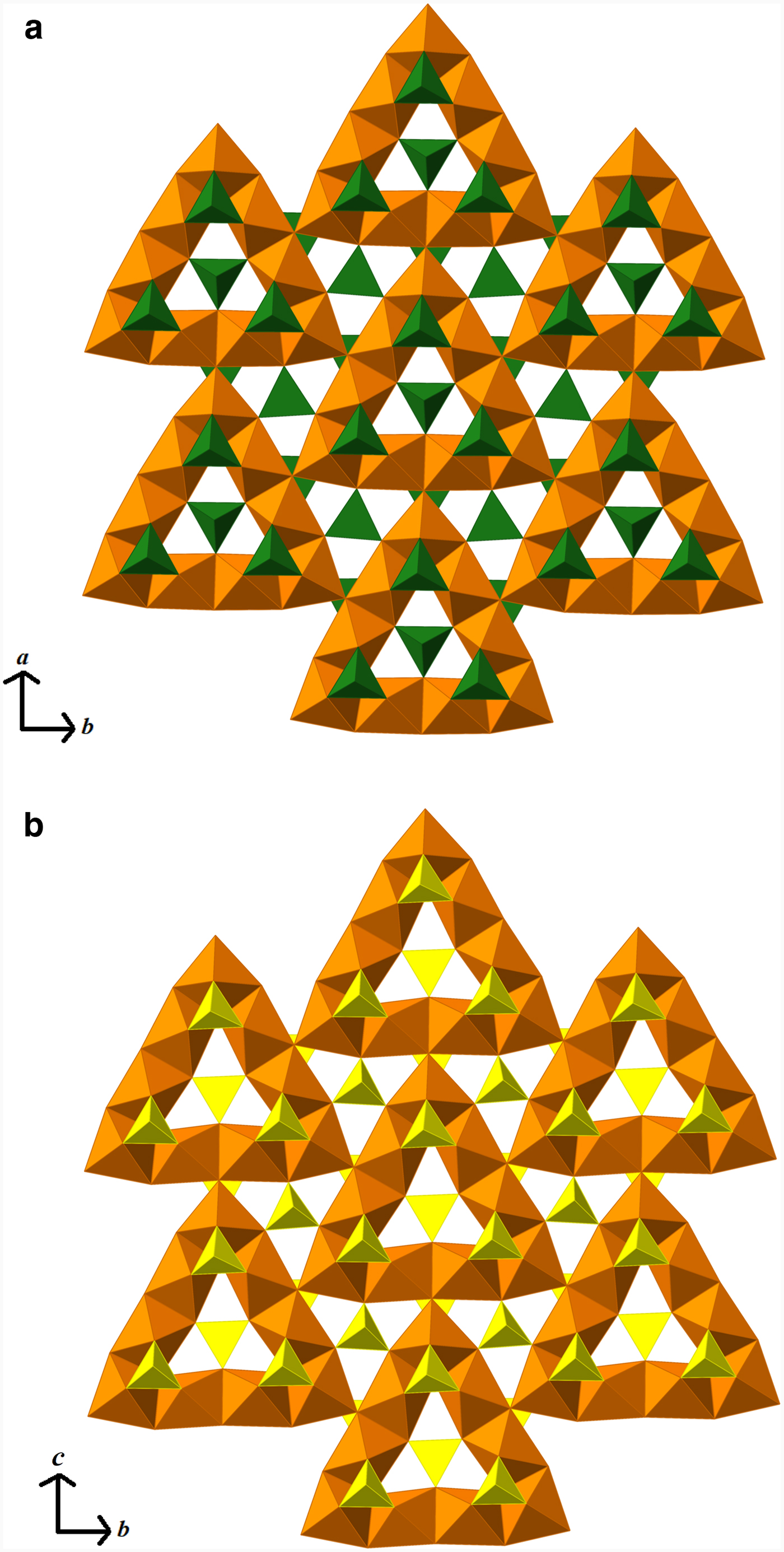
Fig. 2. (a) Eztlite layer, comprising Fe3+O6 octahedra in orange and Te4+O3 trigonal pyramids in dark green. Note that the tellurium atoms are orientated closest to the viewer in the centre of each nonameric unit. (b) Mitridatite layer, comprising Fe3+O6 octahedra in orange and PO4 tetrahedra in yellow. Note that the phosphorus atoms in the centre of the nonameric units are orientated into the page. (Note that mitridatite was defined along different axes to eztlite; a swapped with c)
Pb2+ cations are found above and below each mitridatite-like layer, bonding to oxygen atoms both in the layers and at the vertices of the sulfate tetrahedra, and also to chloride ions (see Fig. 3a). The sulfate tetrahedra and chloride ions are found between the layers of lead cations. One third of the sulfate tetrahedra are oriented in the opposite direction to the others and the chloride ions are close to equidistant from both layers. This is in contrast to mitridatite-type structures, in which calcium and water fill the interstitial space (see Fig. 3b). Each of the four lead atoms in eztlite is found in a different coordination environment, in over-bonded environments with an average Pb bond-valence sum of 2.184 valence units (vu). Pb2 and Pb4 are in asymmetric configuration due to the stereoactive 6s 2 lone pair, but Pb1 and Pb3 do not display obvious asymmetry. Pb1 is in 9-fold coordination (PbO8Cl), while the other three are in 8-fold coordination: Pb2 as PbO8 and Pb3 and Pb4 as PbO6Cl2.
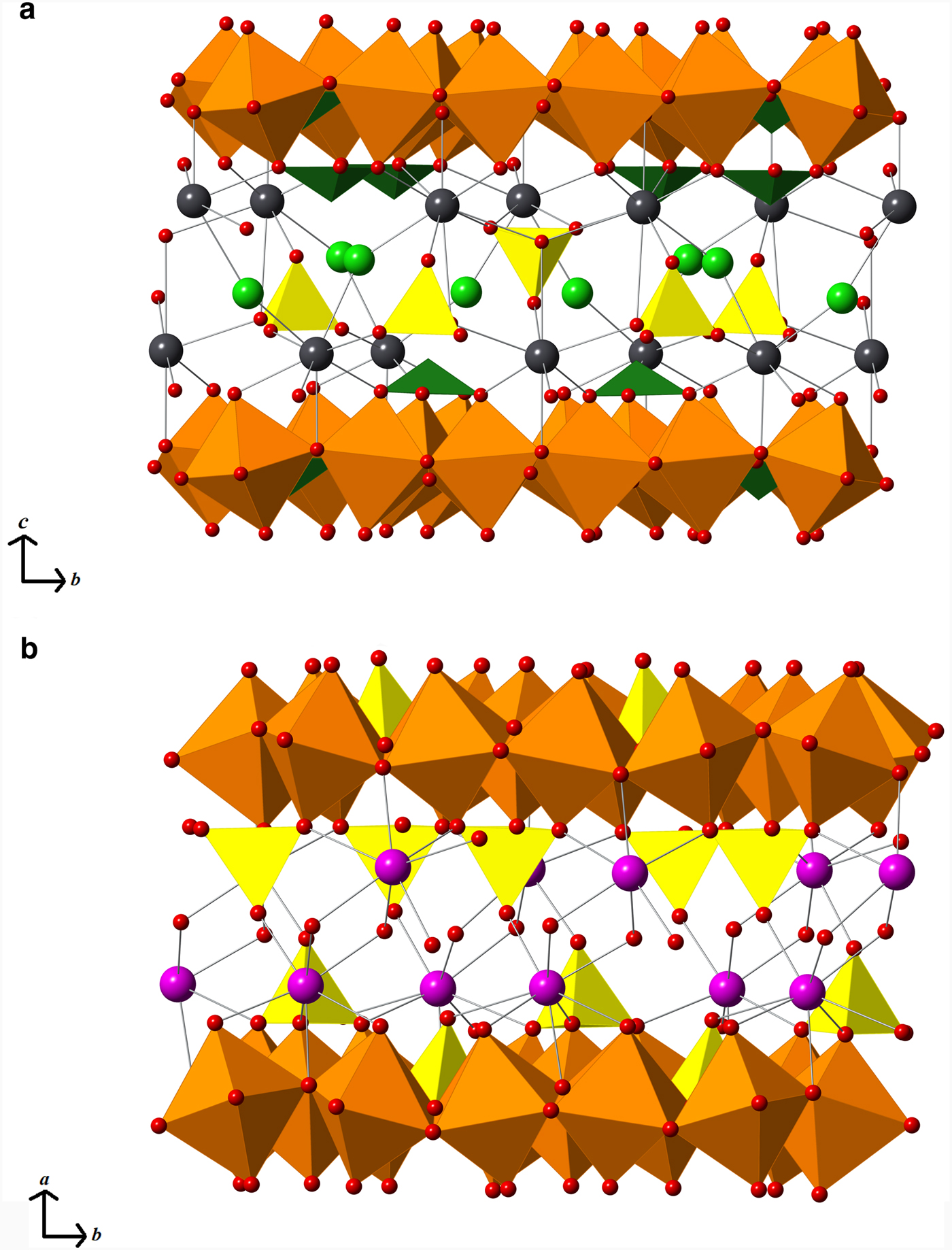
Fig. 3. (a) Two eztlite layers showing interlayer species (Fe3+O6 in orange and Te4+O3 in dark green). Pb2+ cations are shown in dark grey and Cl atoms in lime-green. Sulfate tetrahedra are shown in yellow (not to be confused with yellow phosphate tetrahedra in mitridatite). Oxygen atoms are shown as small red spheres. (b) Two mitridatite layers showing interlayer species (Fe3+O6 in orange). Yellow phosphate tetrahedra extend further into the layers than do the tellurite trigonal pyramids in eztlite. Ca2+ cations are in magenta. Non-bonded interstitial water molecules omitted for clarity. (Note that mitridatite was defined along different axes to eztlite.)
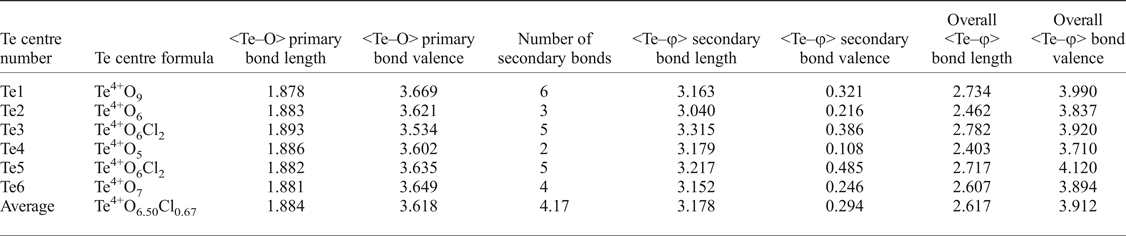
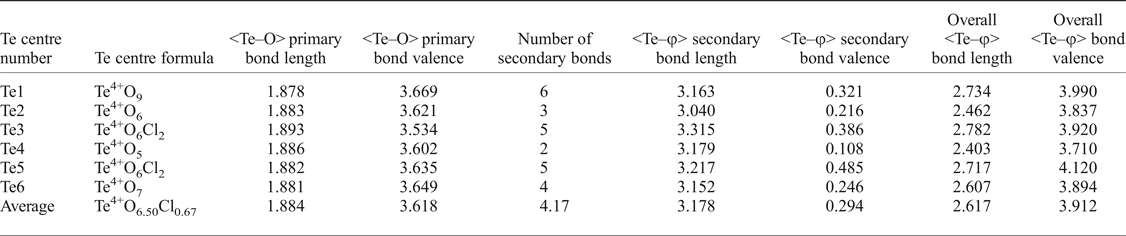
It is also worth further examining the bonding of the Te atoms (see Table 7 for a Te bonding summary). Although each Te possesses three short, primary bonds to allow for the formation of the Te4+O3 trigonal pyramids, the average bond-valence sum for Te incorporating only primary bonds is 3.618 vu, indicating that the remaining valence of the Te cations is filled with longer secondary bonds to both oxygen and chlorine atoms. Tellurium secondary bonds thus play an important role in increasing the stability of the eztlite structure by providing crosslinks between the mitridatite-like layer and interstitial species. The majority, but not all, of these secondary bonds are on the same side of the Te atom as the lone pair. The number of secondary bonds varies between 2 and 6, with an average of 4.17 secondary bonds per Te atom, which fits well with the range observed by Christy and Mills (Reference Christy and Mills2013). Bond-valence sums for Te vary between 3.710 vu for Te4 (which is under-bonded due to only having two secondary linkages) to 4.120 vu for Te5, with an average bond valence of 3.912 vu, which is an average increase of 0.294 vu per Te atom once secondary bonds are incorporated. The Te3 and Te5 cations have the greatest proportion of their bond valence made up by secondary bonding as both of these Te centres have two Te–Cl secondary bonds which contribute more than 0.1 vu each.
Table 7. Summary of tellurium bonding in eztlite with bond lengths (Å) and bond valences (vu).
Relationship to other Te oxysalt structures
Eztlite contains isolated neso tellurite (Te4+O3)2– groups that are part of a larger structural group (a layer based on edge and corner linking of Fe3+O6 octahedra, with tellurite decoration, in this case). The minerals juabite (Roberts et al., Reference Roberts, Gault, Jensen, Criddle and Moffatt1997; Kampf and Mills, Reference Kampf and Mills2011) and rodalquilarite (Feger et al., Reference Feger, Kolis, Gorny and Pennington1999; Kampf and Mills, Reference Kampf and Mills2011) are also in this category, but display no structural similarities to eztlite (Christy et al., Reference Christy, Mills and Kampf2016). Although also containing Te4+, sulfate and chloride in a layered structure, nabokoite is markedly different to eztlite. All octahedral Cu sites in nabokoite are isolated from each other, and instead of Te4+O3 trigonal pyramids, nabokoite contains perfect tetragonal Te4+O4 pyramids (Pertlik and Zemann, Reference Pertlik and Zemann1988).
Eztlite may be related to cuzticite (reported formula ![]() ${\rm Fe}_2^{3 +} $Te6+O6·3H2O), which was first described in the same paper as eztlite, and is noted to sometimes occur in close proximity to eztlite (Williams, Reference Williams1982). Cuzticite is also being studied currently by the authors to determine its elemental composition and structure.
${\rm Fe}_2^{3 +} $Te6+O6·3H2O), which was first described in the same paper as eztlite, and is noted to sometimes occur in close proximity to eztlite (Williams, Reference Williams1982). Cuzticite is also being studied currently by the authors to determine its elemental composition and structure.
As discussed above, eztlite displays far more similarity to minerals with a mitridatite-like structure (Huminicki and Hawthorne, Reference Huminicki, Hawthorne, Kohn, Rakovan and Hughes2002, Moore and Araki, Reference Moore and Araki1977) than to any known Te oxysalt structures.
Acknowledgements
This study has been partly funded by The Ian Potter Foundation grant ‘tracking tellurium’ to SJM and a Museums Victoria 1854 Student Scholarship awarded to OPM, which we gratefully acknowledge. The single-crystal work was undertaken using the MX2 beamline at the Australian Synchrotron, part of ANSTO, and made use of the Australian Cancer Research Foundation (ACRF) detector. Microprobe work was funded through Natural Environment Research Council grant NE/M010848/1 ‘Tellurium and Selenium Cycling and Supply’ to Chris J. Stanley (Natural History Museum, London). The picture of eztlite on specimen M54056 (Fig. 1) was taken with the assistance of David Paul (Museums Victoria, Australia).
Associate Editor Ferdinando Bosi, and three reviewers (Peter Leverett, Anthony Kampf and one anonymous reviewer) are thanked for their comments and suggestions which improved the manuscript and reported crystal structure.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.108