Introduction
Increasing industrialization and urbanization, swift and often unplanned, has been accompanied by the extraction and distribution of mineral substances from natural deposits (Brenner & Schmid, Reference Brenner and Schmid2014), leading to a rapid increase in anthropogenic toxic metal emissions and, consequently, in increased air and water pollution levels (Moore et al., Reference Moore, Gould and Keary2003; Stankovic & Stankovic, Reference Stankovic, Stankovic, Lichtfouse, Schwarzbauer and Robert2013). Lead is one of the most abundant heavy metals on Earth and may affect every organ in the body, mainly nervous, excretory and circulatory systems (ATSDR, 2007). Lead is found in several compounds and alloys, and it is used in several industrial processes, including the production of batteries, paint, cables, petrol, ceramics, electronics and plastics (Meyer et al., Reference Meyer, Brown and Falk2008). The distribution of lead in the environment varies among and within countries, depending on historical and current uses of this metal. Elevated contaminant levels in the environment are not necessarily indicative of adverse effects. Only a fraction of the metal in the environment is bioavailable for potential intake by the biota and exertion of adverse effects within the receptor organism (Baker et al., Reference Baker, Herrchen, Hund-Rinke, Klein, Kördel, Peijnenburg and Rensing2003).
To determine the risk of exposure to heavy metals is often a complex task, but the use of animal species as bioindicators offers a potentially simple solution for the difficulty of measuring bioavailability and summarizing complex patterns of contamination in the environment (Sures, Reference Sures2004; Vidal-Martínez & Wunderlich, Reference Vidal-Martínez and Wunderlich2017). Bioindicators of accumulation are biological monitors that accumulate a pollutant in their tissues without significant adverse effects, and are therefore used to measure the amount of a pollutant that is biologically available (Beeby, Reference Beeby2001; Vidal-Martínez & Wunderlich, Reference Vidal-Martínez and Wunderlich2017). In recent years, intestinal parasites of vertebrates have received increasing attention as indicators of heavy metals, mainly acanthocephalans of the orders Moniliformida (Scheef et al., Reference Scheef, Sures and Taraschewski2000; Sures et al., Reference Sures, Jürges and Taraschewski2000; Torres et al., Reference Torres, Eira, Miquel, Foronda and Feliu2011; Teimoori et al., Reference Teimoori, Yaraghi, Makki, Shahbazi, Nazmara, Rokni, Mesdaghinia, Moghaddam, Hosseini and Rakhshanpour2014) and Echinorhynchida (Siddall & Sures, Reference Siddall and Sures1998; Schludermann et al., Reference Schludermann, Konecny, Laimgruber, Lewis, Schiemer, Chovanec and Sures2003; Sures & Reimann, Reference Sures and Reimann2003; Thielen et al., Reference Thielen, Zimmermann, Baska, Taraschewski and Sures2004), and cestodes of the order Cyclophyllidea (Sures et al., Reference Sures, Scheible, Bashtar and Taraschewski2003; Eira et al., Reference Eira, Torres, Vingada and Miquel2005; Jankovská et al., Reference Jankovská, Miholová, Bejček, Vadlejch, Šulc, Száková and Langrová2010a; Torres et al., Reference Torres, Foronda, Eira, Miquel and Feliu2010, Reference Torres, Eira, Miquel, Foronda and Feliu2011). These helminths have the ability to bioconcentrate different metals at concentrations surpassing that of their hosts (Sures et al., Reference Sures, Grube and Taraschewski2002, Reference Sures, Scheible, Bashtar and Taraschewski2003; Torres et al., Reference Torres, de Lapuente, Eira and Nadal2004; Al-Quraishy et al., Reference Al-Quraishy, Gewik and Abdel-Baki2014; Teimoori et al., Reference Teimoori, Yaraghi, Makki, Shahbazi, Nazmara, Rokni, Mesdaghinia, Moghaddam, Hosseini and Rakhshanpour2014). However, few studies involving helminths as environmental quality indicators have been conducted in terrestrial habitats (Vidal-Martínez & Wunderlich, Reference Vidal-Martínez and Wunderlich2017).
In urban environments, the Rattus spp./Hymenolepis diminuta system was the first model to be used for detection of lead pollution involving a cestode (Sures et al., Reference Sures, Grube and Taraschewski2002, Reference Sures, Scheible, Bashtar and Taraschewski2003; Al-Quraishy et al., Reference Al-Quraishy, Gewik and Abdel-Baki2014; Čadková et al., Reference Čadková, Miholova, Száková, Valek, Jankovska and Langrova2014). This system is widely distributed, easy to collect and identify, especially in urban ecosystems (Feng & Himsworth, Reference Feng and Himsworth2014; Hancke & Suárez, Reference Hancke and Suárez2016), meeting some of the criteria set for a good bioindicator (Beeby, Reference Beeby2001). Most of the studies using this model have been based on comparing sites with similar environmental characteristics but different lead concentrations.
In the urban matrix of the city of Buenos Aires (Argentina), two landscape units can be distinguished as being associated with housing quality and urban planning: residential neighbourhoods with urban services such as garbage removal, sanitation networks, electricity and plumbing; and shanty towns with precarious houses and an inadequate supply of basic urban services (Fernández et al., Reference Fernández, Cavia, Cueto and Suárez2007; Cavia et al., Reference Cavia, Cueto and Suárez2009). These characteristics affect the establishment and proliferation of animal populations. Previous studies conducted in Buenos Aires city have found that Rattus rattus is the dominant species in residential neighbourhoods, while Rattus norvegicus is the most abundant in shanty towns (Cavia et al., Reference Cavia, Cueto and Suárez2009). At the same time, differences in environmental conditions that characterize each of these landscape units also alter the parasite burden in rats. According to Hancke & Suárez (Reference Hancke and Suárez2016), the abundance of H. diminuta within the urban matrix is highest in shanty towns, possibly due to a greater abundance of intermediate hosts.
The determination of lead concentration in the Rattus spp./H. diminuta system in the city of Buenos Aires would help to infer the bioavailability of this metal in different landscape units of this city. Although studies of heavy-metal pollution have been performed in the city of Buenos Aires, they took place in specific areas and were not followed up over time (Lavado et al., Reference Lavado, Rodríguez, Scheiner, Taboada, Rubio, Alvarez, Alconada and Zubillaga1998; Ratto et al., Reference Ratto, Marceca, Moscatelli, Abbruzese, Bardi, Bossi, Bres, Cordón, Di Nano, Murruni, Potarsky and Williams2004, Reference Ratto, Marbán, González and Giuffré de López Camelo2006; Smichowski et al., Reference Smichowski, Gómez, Dawidowski, Giné, Bellato and Reich2004; López et al., Reference López, Perelman, Rivara, Castro and Faggi2006; Perelman et al., Reference Perelman, Castro, Navarro, Rechi, Arriaga, López, Carretero and Faggi2006). The overall goal of the present study was to use the Rattus spp./H. diminuta model as a tool to assess environmental lead pollution in shanty towns and residential neighbourhoods of the city of Buenos Aires.
Materials and methods
Study area
Fieldwork was conducted in the city of Buenos Aires, Argentina (34°37′S, 58°24′W). This city covers an area of 200 km2 with 2,890,151 inhabitants (INDEC, 2010). The climate is temperate with a mean annual temperature of 17.4°C and mean annual precipitation of 1146 mm. The matrix of the city is composed of buildings, houses and paved streets, with internal patches formed by parks, green spaces and shanty towns (Cavia et al., Reference Cavia, Cueto and Suárez2009).
Rodent trapping
Rodents were collected from surveys carried out as part of a rodent control programme in the city of Buenos Aires from 2003 to 2006. Sampling was conducted in six sites of two different landscape units: three shanty towns and three residential neighbourhoods. Rodents were captured using live cage traps as described by Cavia et al. (Reference Cavia, Cueto and Suárez2009). Traps were placed inside houses and in their yards, in stores or factories, and were monitored every morning for four consecutive days. Captured animals were sacrificed with anaesthesia, identified, sexed, measured and weighed. All animals were fixed in formaldehyde and a week later preserved in 70% ethanol and stored in the collection of the Laboratory of Urban Rodent Ecology of the Buenos Aires University. The intestines of all adult Rattus spp. (17.5–26.0 cm) were reviewed for the isolation and identification of H. diminuta. Parasites and a sample of the liver of each parasitized rat were isolated for lead determination, and were stored in 10% formaldehyde that had been tested for heavy-metal content before its use.
Analytical procedure
Lead extraction from rat livers and H. diminuta specimens was performed according to a modification of the methodology proposed by Torres et al. (Reference Torres, Eira, Miquel, Foronda and Feliu2011). About 200 mg of liver and the whole parasite biomass found in a host were each placed in a 10-ml flask with 2 ml of nitric acid (Suprapur, Merck, Buenos Aires, Argentina). The samples were gradually heated using an infrared lamp for 5 h. After the digestion, samples were diluted in distilled water to a volume of 10 ml. Lead determinations were carried out using inductively coupled plasma mass spectrometry (ICP-MS). Analytical blanks were prepared under the same conditions. The detection limits of the measurements (mean ± 3 SD of blanks) were 3.05 μg/l. To prevent inaccuracies in the determination of lead based on wet weight in parasites and tissues, the concentrations of heavy metals were applied to dry weights, according to Jankovská et al. (Reference Jankovská, Miholová, Bejček, Vadlejch, Šulc, Száková and Langrová2010a). However, to compare our data with those obtained by other authors, wet weight conversion factors were calculated for parasites and livers (μg/g wet weightparasite = μg/g dry weight × 0.23 and μg/g wet weightliver = μg/g dry weight × 0.29, respectively).
Statistical analysis
To study the factors affecting lead concentration in rats and H. diminuta in the city of Buenos Aires, a general linear mixed model (GLMM) was performed. Lead concentration in rat liver and parasites was considered as a response variable, whereas tissue (liver or parasite), landscape units, sex, weight of rats and dry weight of parasites, and their interactions, were explanatory variables. Sampling sites were included as a random factor. Since lead concentrations were not normally distributed, and had variance heterogeneity, data were Ln-transformed. Results were considered to be statistically significant when P < 0.05. Data were analysed using the gls and lme functions of the nlme R-packages interfaced by InfoStat Statistical Software version 2015 (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2015).
The relation between lead concentration in parasites and in the host's liver was calculated using the bioconcentration factor proposed by Sures et al. (Reference Sures, Siddall and Taraschewski1999), as the ratio of the mean metal concentrations in the parasite and the host liver (C[parasite]/C[liver]). This was calculated for each landscape unit separately.
To study the effect of H. diminuta on lead concentration in the host, a Student's t-test was performed to compare the lead concentrations in livers between infected and uninfected rats. To avoid external or environmental effects, only rats trapped in one of the shanty towns were used. All the statistical analyses were performed using InfoStat Statistical Software version 2015 (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2015).
Results
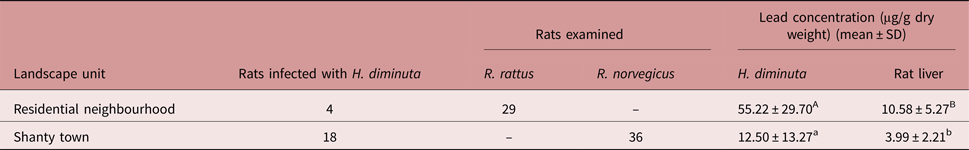
Sixty-five rats were captured and 22 of them were infected with H. diminuta. Fifty per cent of the captured animals in shanty towns (n = 36) were parasitized, while only 14% (n = 29) were infected in residential neighbourhoods (table 1).
Table 1. Number of rats infected with Hymenolepis diminuta and mean values of lead concentration in rat livers and parasites, relative to landscape unit (different letters indicate significant differences, P < 0.05). All concentrations are presented as μg/g dry weight.
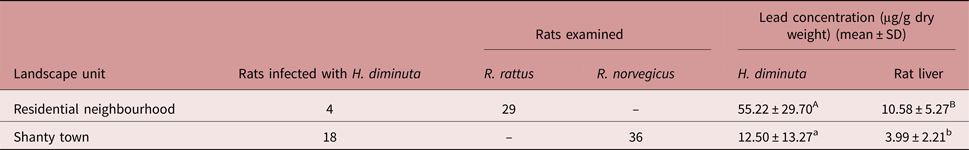
The mean lead concentration of H. diminuta was highest in residential neighbourhoods (table 1), where the specimen with highest lead level was also found (79.6 μg/g dry weight). The lowest mean lead concentration was detected in livers of rats from shanty towns (table 1), where the specimen with lowest lead level was also found (1.5 μg/g dry weight).
The GLMM analysis showed that landscape unit and tissue type have a significant effect on lead concentration, being higher in residential neighbourhoods, and higher in H. diminuta tissue (df = 4, F = 9.55, P = 0.037 and df = 37, F = 22.84, P < 0.0001, respectively).
When comparing lead levels in H. diminuta and rats, the bioconcentration factor revealed a fivefold higher lead level in H. diminuta compared to host livers from residential neighbourhoods, while in shanty towns this relationship was 3:1.
Finally, no significant differences were found between the mean lead concentration in livers of uninfected rats (n = 11) and infected rats (n = 6; t = −0.72, df = 15, P = 0.480) in animals trapped in one of the shanty towns.
Discussion
The results of this study show the feasibility of using bioindicators to characterize heavy-metal pollution in urban ecosystems when environmental data are scarce. Lead concentration in H. diminuta and in its hosts, the rats, was higher in residential neighbourhoods compared to shanty towns. Although data of environmental lead contamination in this study area were not available, the results obtained so far allowed us to hypothesize the possible causes for the observed patterns. In Argentina, the principal sources of lead in cities are service pipes, peeling paint and industry (Mattalloni et al., Reference Mattalloni, De Giovanni and Virgolini2014). Since shanty towns lack urban basic services and the industries in Buenos Aires city are more likely to be located within residential neighbourhoods, a higher exposure to lead sources was expected in these latter landscape units. Rat tissues have been widely studied as bioindicators of environmental pollution, and positive correlations between environmental and tissue lead concentrations have been widely reported (Way & Schroder, Reference Way and Schroder1982; Ceruti et al., Reference Ceruti, Ghisleni, Ferretti, Cammarata, Sonzogni and Scanziani2002; Nakayama et al., Reference Nakayama, Ikenaka, Hamada, Muzandu, Choongo, Yabe, Umemura and Ishizuka2013; Bortey-Sam et al., Reference Bortey-Sam, Nakayama, Ikenaka, Akoto, Baidoo, Mizukawa and Ishizuka2016).
In the city of Buenos Aires, a clear spatial segregation was described for rat species, R. rattus and R. norvegicus (Cavia et al., Reference Cavia, Cueto and Suárez2009). This may restrict the extrapolation of our results, because it is difficult to differentiate whether differences in lead concentration in parasites and hosts are due to the effect of differences in environmental lead levels between landscape units, or to the effect of behavioural differences between rat species. Rattus rattus is a better climber and builds nests out of artificial materials, allowing this species to be dominant in residential areas, while the abundance of R. norvegicus is correlated with the presence of plant cover and water in the environment, enabling them to be successful in shanty towns (Cavia et al., Reference Cavia, Cueto and Suárez2009; Feng & Himsworth, Reference Feng and Himsworth2014). This could lead to differences between rodent species in the rates of contact with different types of lead pollution sources. However, despite the behavioural differences between both host species, we believe that our results did reflect the conditions of lead pollution in each landscape unit. Higher lead levels were detected in rats and parasites from residential neighbourhoods compared to shanty towns, in agreement with the greater presence of lead sources, as mentioned previously.
Parasites are widely recognized as indicators of anthropogenic pollution. In some cases, the presence or absence of parasite species is associated with chemical contamination of the environment, while other parasites have the ability to accumulate trace metals from the environment in their tissues (Sures, Reference Sures2004; Vidal-Martínez & Wunderlich, Reference Vidal-Martínez and Wunderlich2017). According to Thielen et al. (Reference Thielen, Zimmermann, Baska, Taraschewski and Sures2004), parasites without a digestive tract, such as cestodes, are able to concentrate more metals than host tissues. In the present study, the concentration of lead in H. diminuta was significantly higher than in rat livers, and the bioaccumulation factor obtained in the residential neighbourhoods revealed that H. diminuta can accumulate lead up to five times the level accumulated by the host´s liver. This value is lower than that obtained by Sures et al. (Reference Sures, Scheible, Bashtar and Taraschewski2003) and Al-Quraishy et al. (Reference Al-Quraishy, Gewik and Abdel-Baki2014) in natural conditions for the rat/H. diminuta system. It has been noted that parasites accumulate heavy metals much faster than their host, but a maximum is reached approximately after 4 weeks. Therefore, the bioaccumulation factor would tend to be smaller in chronic exposure situations (Oyoo-Okoth et al., Reference Oyoo-Okoth, Admiraal, Osano, Kraak, Were-Kogogo, Gichuki, Ngure, Makwali and Ogwai2012; Čadková et al., Reference Čadková, Miholova, Száková, Valek, Jankovska and Langrova2014). Additionally, methodological differences in tissue digestion could explain the results between different studies. Here, the whole biomass of parasites was digested for lead determination, while other researchers have taken only a sample of parasites. It has been mentioned that metals do not have a homogeneous distribution inside the parasite (Riggs et al., Reference Riggs, Lemly and Esch1987; Vijayalakshmi et al., Reference Vijayalakshmi, Ramalingam and Satyaprema2003; Horáková et al., Reference Horáková, Čadková, Száková and Jankovská2017). This could affect the value and accuracy of bioaccumulation factor determination.
In this study, no effect of parasitism with H. diminuta was found on the lead concentration of the host liver. In agreement with the results of Sures et al. (Reference Sures, Scheible, Bashtar and Taraschewski2003) for the same host–parasite model, and Jankovská et al. (Reference Jankovská, Miholová, Bejček, Vadlejch, Šulc, Száková and Langrová2010a) for red foxes (Vulpes vulpes) infected with Mesocestoides spp., no difference in lead concentration was observed between parasitized and non-parasitized rats. However, other studies have reported an effect of parasitism on heavy-metal uptake of the host. Jankovská et al. (Reference Jankovská, Vadlejch, Száková, Miholová, Kunc, Knížková and Langrová2010b) found significant differences between parasitized and non-parasitized animals in an experimental study of lead accumulation in a sheep–cestode system (Moniezia expansa/Ovis aries). According to Čadková et al. (Reference Čadková, Miholova, Száková, Valek, Jankovska and Langrova2014), the toxicokinetics in a host body depends on the lead concentration to which it is exposed. However, and consistent with our results, for similar lead exposure levels no significant differences were found in lead concentration between parasitized and non-parasitized animals. This is reasonable because it is possible that cestodes take the lead attached to bile, which had been absorbed by the host previously and then excreted into the duodenum via the hepatic cycle (Sures et al., Reference Sures, Scheible, Bashtar and Taraschewski2003; Čadková et al., Reference Čadková, Miholova, Száková, Valek, Jankovska and Langrova2014).
Considering that information describing heavy-metal pollution within the city of Buenos Aires is scarce, the results of this study allow us to update the data about the degree of lead contamination. The rats captured in the two landscape units were exposed to biologically available lead, showing higher concentrations in the residential neighbourhoods than in shanty towns. Considering that rats and H. diminuta are distributed worldwide, this monitoring system for lead pollution could be an effective tool for application in other urban ecosystems throughout the world.
Acknowledgements
We are especially grateful to the team of Laboratorio de Ecología de Roedores Urbanos for their assistance during the field sampling, and to Dr Gerardo Cueto for her his help in the statistical analysis. We would also like to thank Emiliano Muschetto, Amir Dyzenchauz, Maria Jose Guida, Victoria Vadell and two anonymous reviewers for reading and providing useful comments about drafts of the manuscript.
Financial support
Financial support was provided by Universidad de Buenos Aires, Gobierno de la Ciudad de Buenos Aires and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of relevant national and institutional guidelines on the care and use of animals (National Law 14346).