1. Introduction
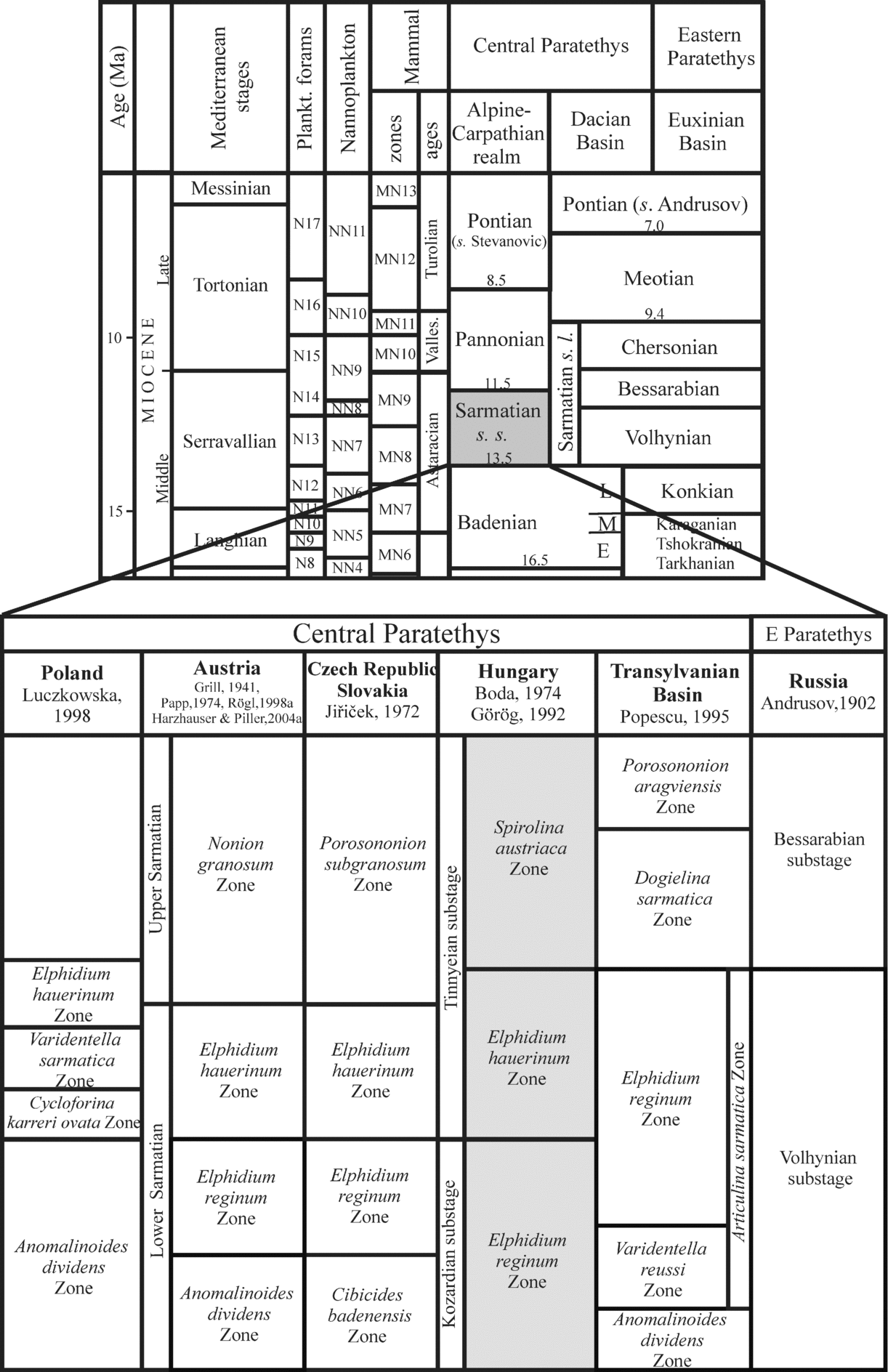
The Paratethys was an epicontinental sea that developed as a relict of the ancient Tethys Ocean. It existed between Early Oligocene and late Middle Miocene times. The uplift of the Dinarids during the Middle Miocene caused a distinct change in the oceanographic and biotic evolution of the Paratethys. This geodynamic process interrupted or limited the connections between the Paratethys and the Mediterranean (Rögl & Steininger, Reference Rögl and Steininger1983; Rögl, Reference Rögl1999; Steininger & Wessely, Reference Steininger and Wessely2000) and was the main cause of distinct palaeobiological developments in these bioprovinces. This separate biogeographic evolution has necessitated the establishment for the Paratethys of a regional time-scale differing from the standard Mediterranean chronostratigraphic stage system (Fig. 1).
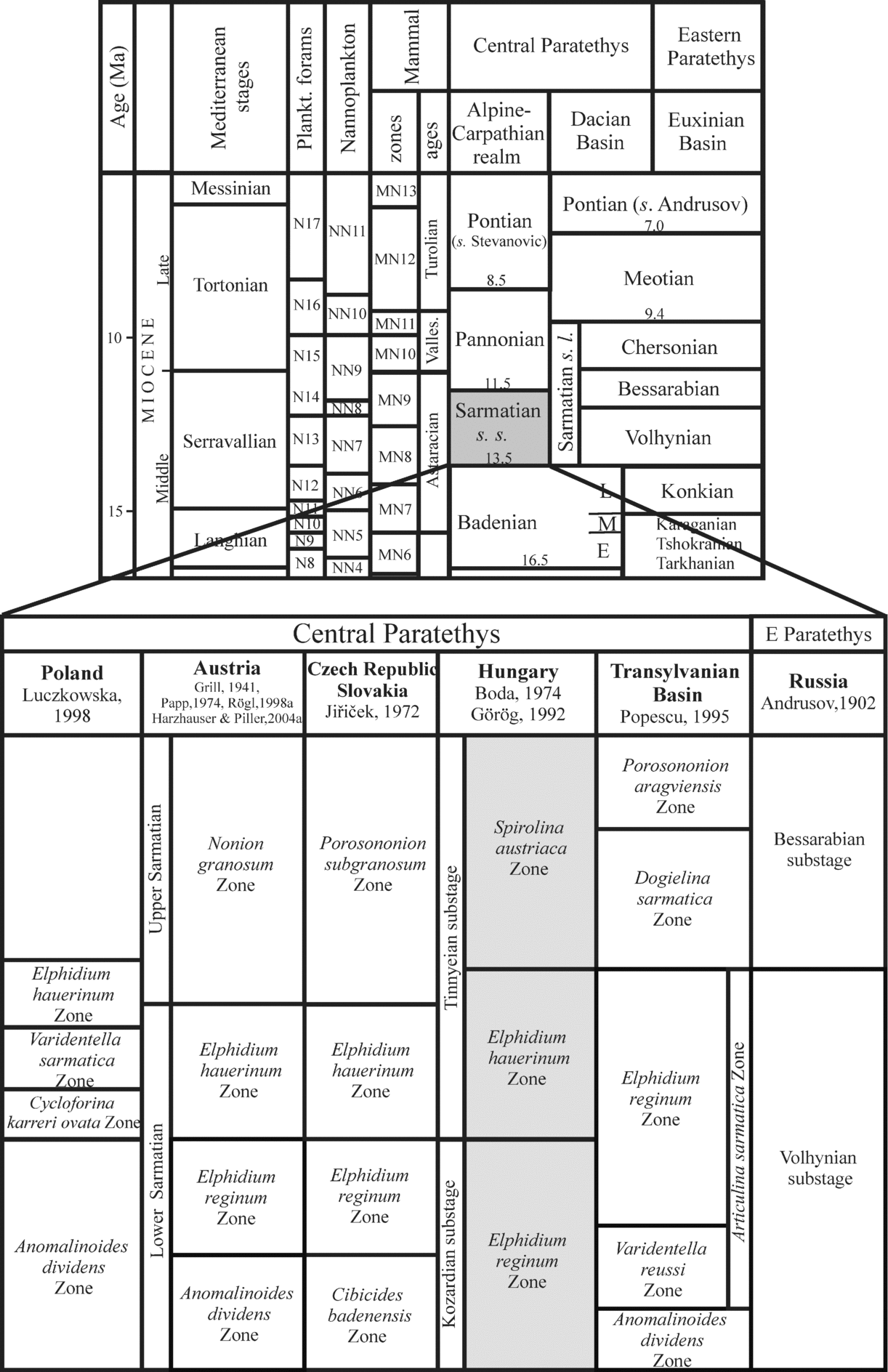
Figure 1. Stratigraphic correlation of the Mediterranean and Paratethys provinces (modified after Mátyás et al. Reference Mátyás, Burns, Müller and Magyar1996) and the Sarmatian biostratigraphic zones of the Paratethys (modified after Görög, Reference Görög1992).
In the Pannonian Basin of the Central Paratethys, the changes in palaeoenvironmental conditions caused by the above-mentioned tectonic instability resulted in a characteristic evolution of the flora and fauna at the base of the Sarmatian s. str. (Suess, Reference Suess1866). The Sarmatian corresponds to the Late Serravallian of the Mediterranean time scale and covers the time span between 12.8 Ma and 11.5 Ma (Rögl, Reference Rögl, Rögl, Rupp and Ctyroka1998a; Harzhauser & Piller, Reference Harzhauser and Piller2004a). At the Badenian/Sarmatian boundary, several marine stenohaline groups of organisms disappeared (radiolarians, corals, scaphopods, cephalopods, polyplacophorans, brachiopods and echinoids), and among other groups it was mostly the euryhaline forms that persisted into the Sarmatian Central Paratethys. Currently there is still discussion focused on the environmental factors that changed in the Sarmatian sea causing the biotic modifications. The Sarmatian fauna was classically regarded as a brackish-water community (e.g. Papp, Reference Papp1956; Boda, Reference Boda1959; Fordinál, Zágorsek & Zlinská, Reference Fordinál, Zágorsek and Zlinská2006; Vrsaljko et al. Reference Vrsaljko, Pavelić, Miknić, Brkić, Kováčić, Hećimović, Hajek-Tadesse, Avanić and Kurtanjek2006) with gradually decreasing salinity. However, several authors have suggested recently that the Sarmatian sea was in fact more or less marine, even with hypersaline episodes, and explained the diversity fall by significant changes in water chemistry, especially high alkalinity (e.g. Pisera, Reference Pisera, Franseen, Esteban, Ward and Rouchy1996; Filipescu, Popa & Wanek, Reference Filipescu, Popa and Wanek1999; Harzhauser & Kowalke, Reference Harzhauser and Kowalke2002; Piller & Harzhauser, Reference Piller and Harzhauser2005; Harzhauser, Piller & Latal, Reference Harzhauser, Piller and Latal2007).
Published geochemical data on the Sarmatian of the Central Paratethys are scarce (Mátyás et al. Reference Mátyás, Burns, Müller and Magyar1996; Latal, Piller & Harzhauser, Reference Latal, Piller and Harzhauser2004; Janz & Vennemann, Reference Janz and Vennemann2005; Harzhauser, Piller & Latal, Reference Harzhauser, Piller and Latal2007). Moreover, these analyses were made in different outcrops of the Vienna, Pannonian and Transylvanian basins that represent only parts of the whole Sarmatian succession.
The aim of the present work is to identify the major palaeoenvironmental changes that occurred during the Sarmatian with the help of palaeontological and geochemical methods from an entire Sarmatian section. The palaeontological and geochemical investigations were carried out on skeletal material of benthic invertebrates (foraminifera, ostracods and gastropods) from two boreholes, complemented by geochemical analyses of rodent teeth. Changes in water temperature and salinity in brackish and marine environments were traced by combining Mg/Ca and oxygen isotope ratios (Epstein et al. Reference Epstein, Buchsbaum, Lowenstam and Urey1953; Dodd & Crisp, Reference Dodd and Crisp1982; Grossman & Ku, Reference Grossmann and Ku1986; Schwalb, Burns & Kelts, Reference Schwalb, Burns and Kelts1999; Vander Putten et al. Reference Vander Putten, Dehairs, Keppens and Baeyens2000; Lubinski, Polyak & Forman, Reference Lubinski, Polyak and Forman2001; Takesue & van Geen, Reference Takesue and van Geen2004; Peros et al. Reference Peros, Reinhardt, Schwarcz and Davis2007). Other environmental conditions (productivity, oxygen level in bottom waters, water depth) were reconstructed using variations of the carbon isotope ratio and qualitative and quantitative palaeontological analyses (e.g. Hartmann, Reference Hartmann and Gruner1975; Murray, Reference Murray1991; van Eijden & Ganssen, Reference Eijden and Ganssen1995; Langer, Reference Langer1995).
2. Geological setting and stratigraphy
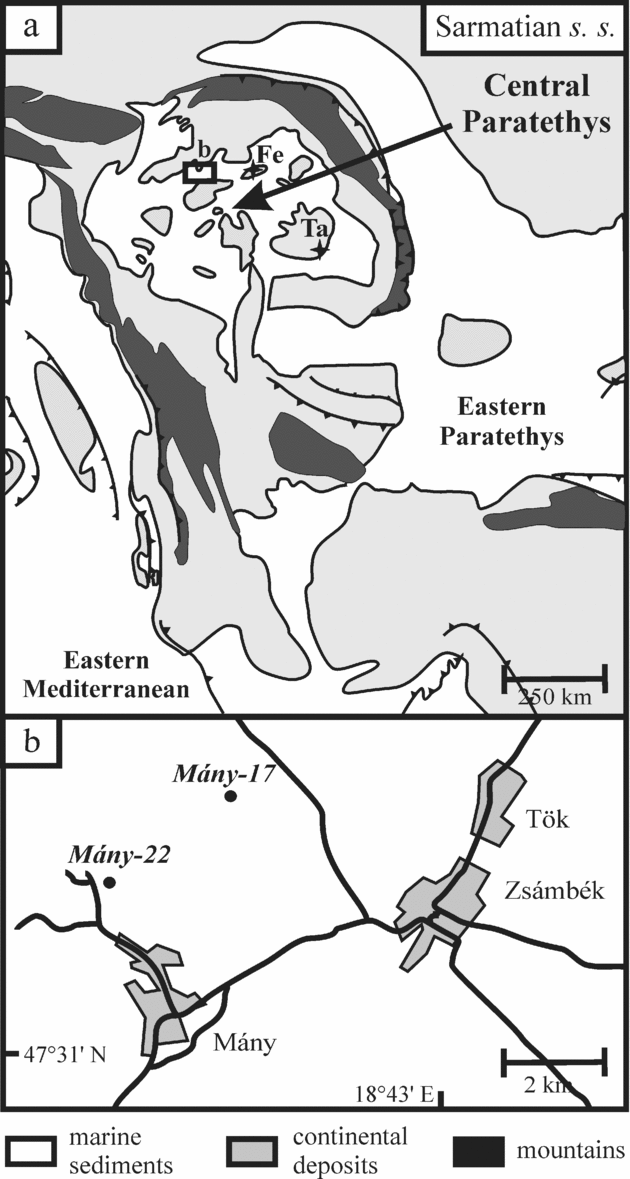
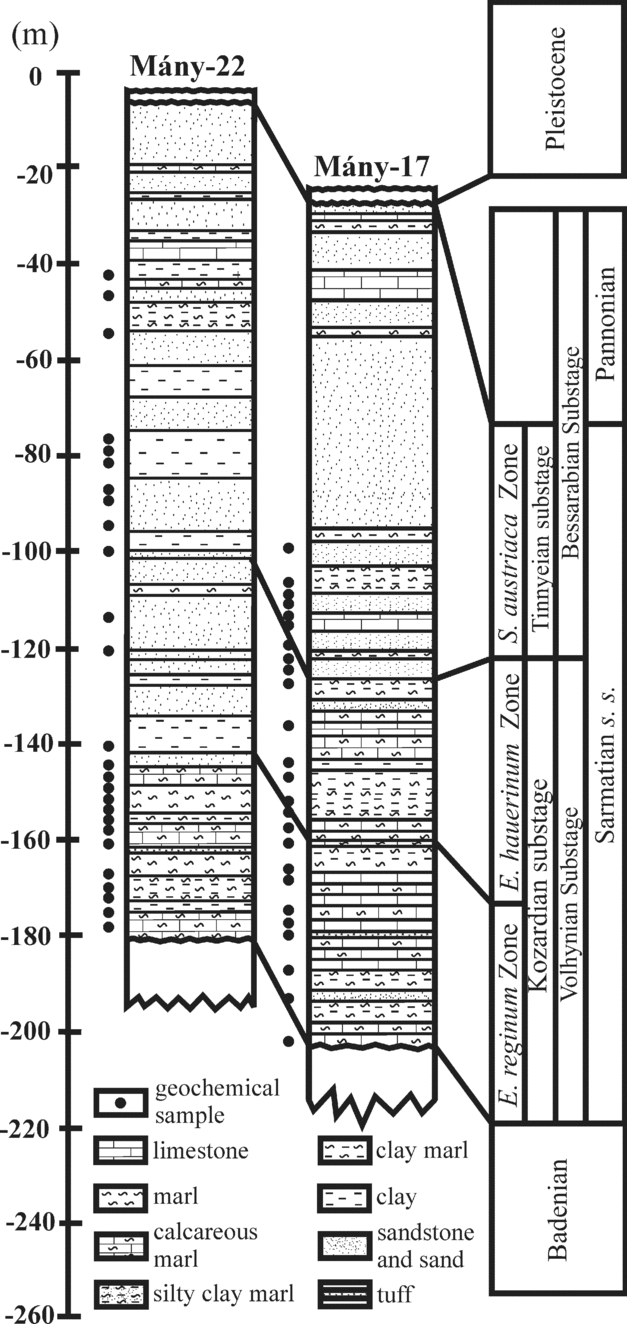
The Zsámbék Basin is located in the central part of the Pannonian Basin near Budapest (Fig. 2). The studied boreholes (Mány-17 and Mány-22) penetrated Sarmatian deposits that are underlain by Badenian strata of similar lithology, but with sharp changes in biofacies as attested by a strong decrease in the diversity of the macro- and microfauna. The Sarmatian succession is overlain by Pleistocene sediments (Fig. 3).
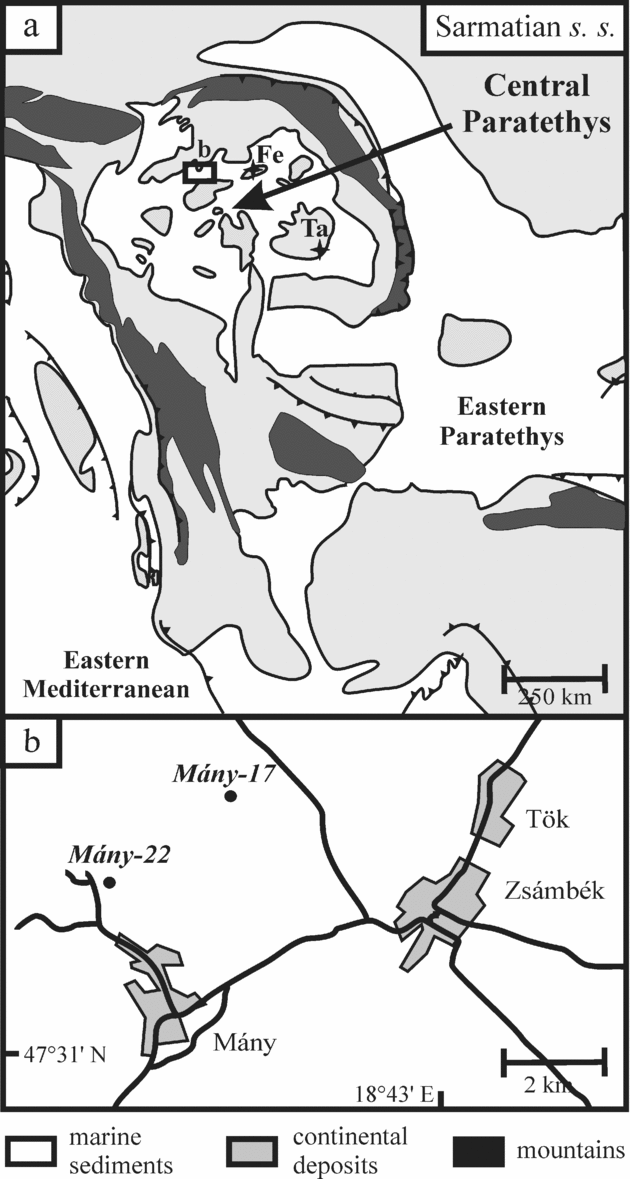
Figure 2. (a) Palaeogeographic map of the Central Paratethys (modified after Rögl, Reference Rögl1998b; Popov et al. Reference Popov, Rögl, Rozanov, Steininger, Shcherba and Kovac2004) with the location of the studied Zsámbék Basin; Felsőtárkány (Fe) and Tăşad (Ta) outcrops. The inset map (b) shows the position of the boreholes in the basin.
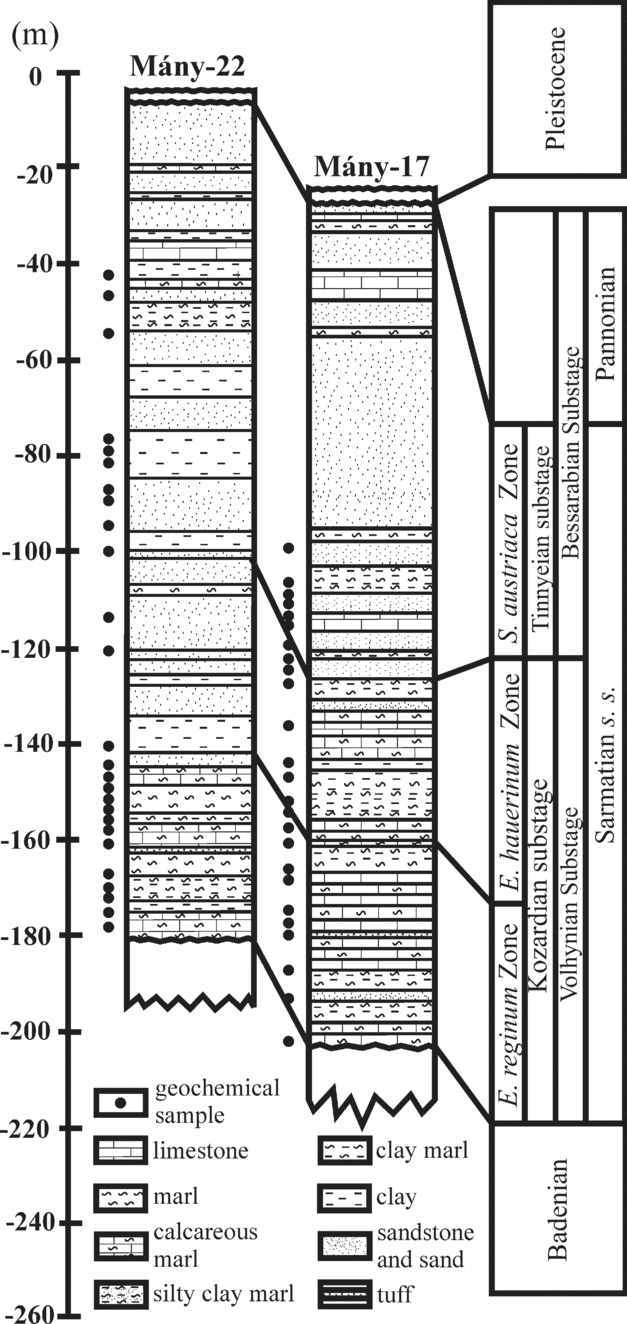
Figure 3. Lithostratigraphic logs of the studied boreholes (modified after Görög, Reference Görög1992).
The lithology of the Sarmatian layers is heterogeneous. In the lower part, there are mainly grey to greenish-grey mollusc-bearing clays, clayey marls with intercalations of sandstones and calcareous marls. Moreover, the clayey marls contain diatomites associated with dacitic tuff and bentonite intercalations. This series can be placed in the Kozard Formation (Hámor, Reference Hámor and Császár1997). The upper part of the Sarmatian succession belongs to the Tinnye Formation and consists of clayey marls with carbonized plant remains, calcareous sandstones and oolitic limestones (Hámor & Ivancsics, Reference Hámor, Ivancsics and Császár1997; Cornée et al. Reference Cornée, Moissette, Saint Martin, Kázmér, Tóth, Görög, Dulai and Müller2009). Sandy intercalations occur between these layers (Fig. 3). These sedimentological data indicate that during the Sarmatian this region was most likely covered by a shallow sea with numerous islands (Magyar, Geary & Müller, Reference Magyar, Geary and Müller1999).
In each borehole a threefold benthic foraminiferal subdivision was proposed by Görög (Reference Görög1992): Elphidium reginum Zone, Elphidium hauerinum Zone and Spirolina austriaca Zone. These zones have been correlated to the regional substages based on mollusc faunas (Boda, Reference Boda1974) (Fig. 1).
3. Material and methods
Ninety-eight samples from two boreholes (Mány-17 and Mány-22) were studied for their faunal content and fifty-two samples analysed for their trace element and stable isotope compositions. For each core sample, about 100 g of air-dried sediment were soaked in a dilute solution of hydrogen peroxide and then washed over a column of sieves of diminishing mesh sizes to extract the carbonate skeletal fauna (foraminifera, gastropods and ostracods) for both investigations.
Phosphatic rodent teeth were extracted from the Upper Sarmatian non-marine clay series of Gödür-kert (3/2 sample) and Felnémet roadcut (2/3, 2/7 samples) at Felsőtárkány (NE Hungary) and from the Lower Sarmatian green clay beds of Tăşad (Ta sample; E Romania; Fig. 2) (Hír et al. Reference Hír, Kókay, Venczel, Gál and Kessler2001; Hír, Reference Hír2004).
3.a. Palaeontological analyses
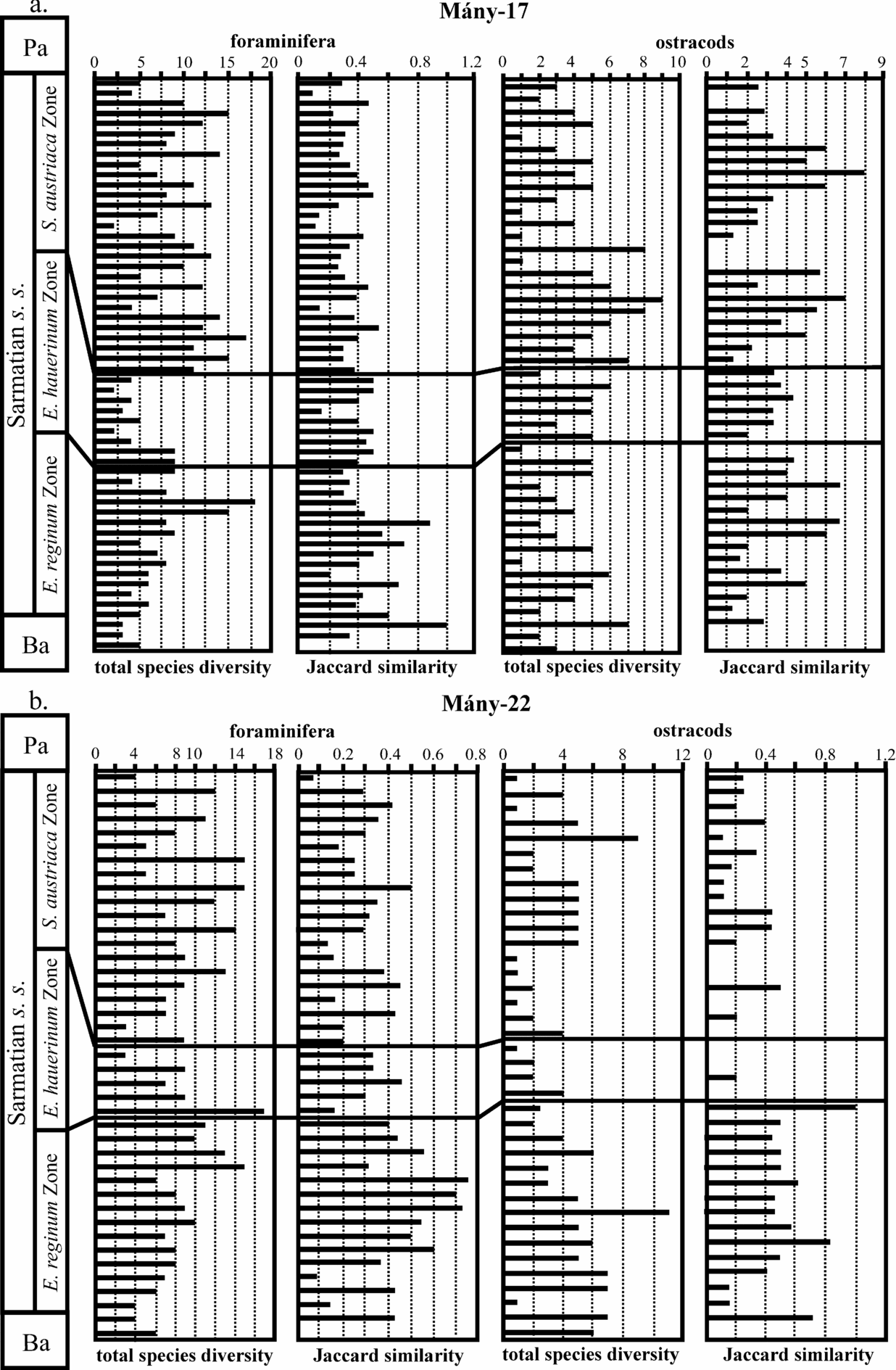
The taxonomic study and quantitative analysis of the benthic foraminifera were made by Görög (Reference Görög1992). A detailed systematic description of the ostracod fauna from the same boreholes was prepared by Tóth (Reference Tóth2008). In this paper, we have attempted a simultaneous palaeoecological interpretation of the foraminifera and ostracods based on qualitative (taxonomic composition of the fauna) and quantitative analyses. These microfaunas are characterized by the total diversity and the Jaccard's Coefficient of Community (R) emend. Ruban & Tyszka (Reference Ruban and Tyszka2005), which are two indices of changes in faunal associations (Fig. 4). Total diversity means the total number of species. R reflects the similarity of two fossil assemblages from different stratigraphic intervals: R = C/[(N1 + N 2) − C], where C is the number of taxa present in both intervals, and N1 and N2 are the number of taxa in the lower and upper interval, respectively.

Figure 4. Variations in foraminifer and ostracod diversity and Jaccard's index in the Zsámbék Basin during the Sarmatian. Ba – Badenian; Pa – Pannonian.
3.b. Geochemical analyses
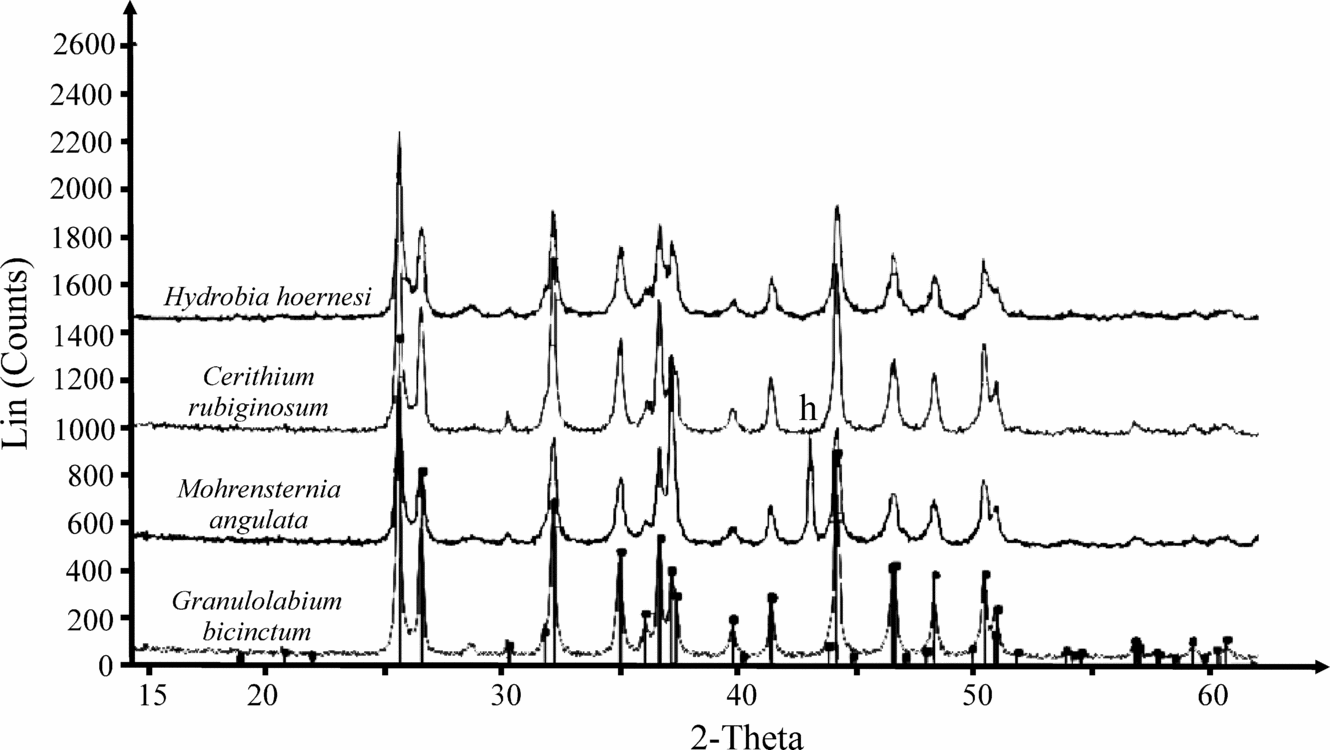
In all samples, the calcitic shells of foraminifera and ostracods and aragonitic shells of gastropods preserved their original crystal structure (proved by X-ray diffraction technique) (Fig. 5). The shells were cleaned three times with deionized water in an ultrasonic bath to remove the sedimentary matrix. The resulting sediment-free shells were then picked under a stereomicroscope. Oxygen and carbon stable isotope compositions and trace element compositions were measured on bulk carbonate skeletons of gastropods because of the small size of their shells, as well as the relatively low intrashell variability of the studied taxa (Latal, Piller & Harzhauser, Reference Latal, Piller and Harzhauser2004). Whole gastropod shells were therefore crushed in an agate mortar until a fine powder was obtained.
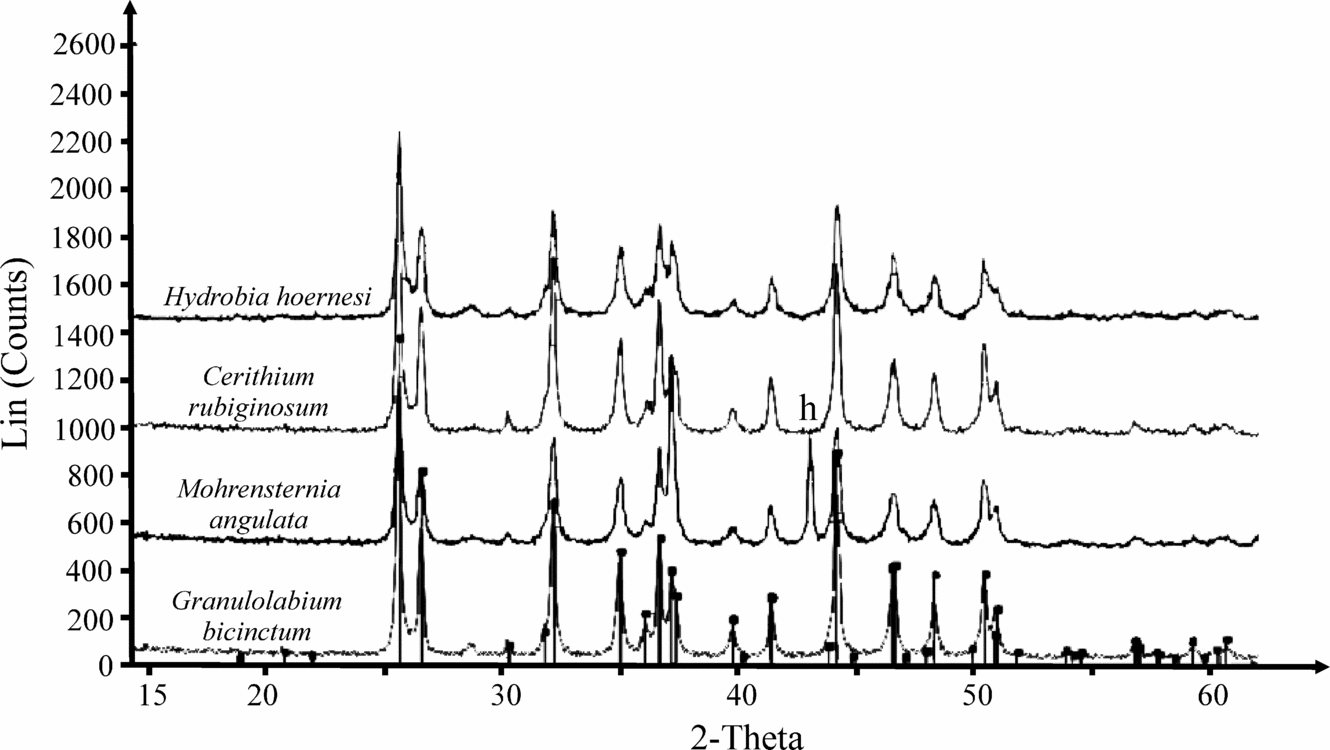
Figure 5. X-ray diffractogram of shells of gastropod species used for geochemical analyses. All peaks of aragonite except peak of aluminium (h) deriving from the sample holder.
3.b.1. Trace elements in carbonates
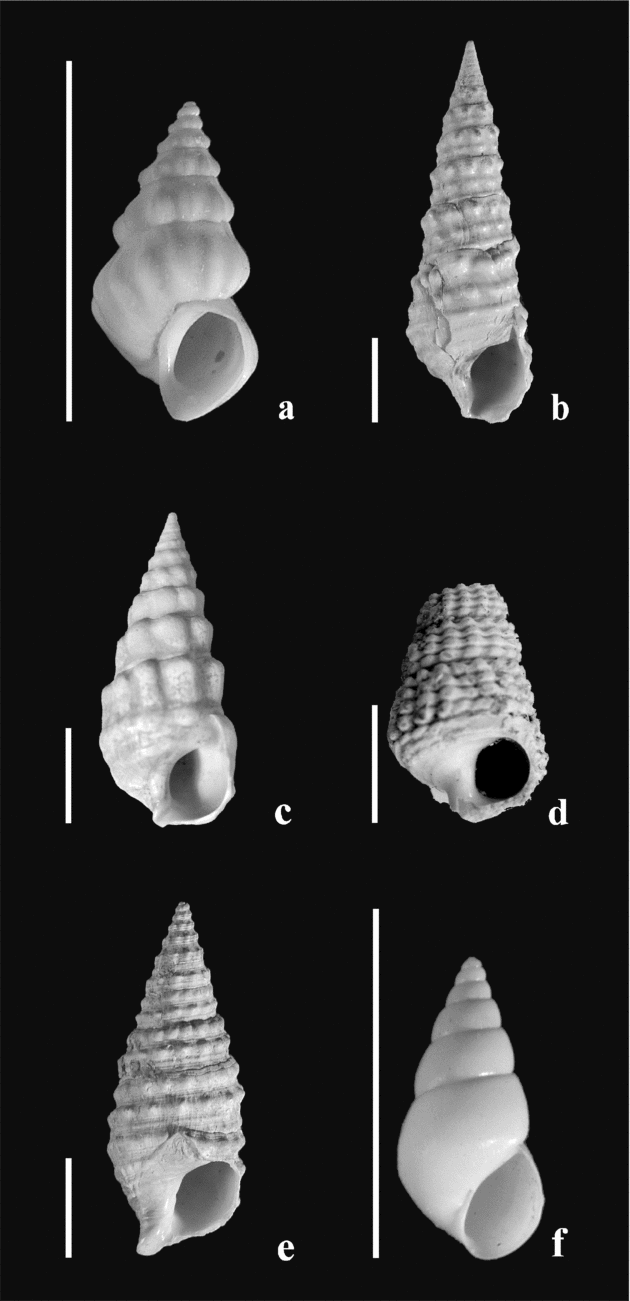
Trace element analyses have been performed on twenty-three samples of gastropods (Mohrensternia inflata, Granulolabium bicinctum, Potamides disjunctus, Hydrobia hoernesi and Cerithium rubiginosum; Fig. 6; see online Appendix Table A1 at http://journals.cambridge.org/geo). Mg and Ca concentrations in about 5 mg of powdered samples were measured by ICP–MS using the masses 24Mg and 44Ca. Indium was used as an internal standard. Distilled HNO3 (4.5 M) was used for the digestion of these calcium carbonate shells. Overall reproducibility is estimated at ±2% (1σ) for Mg/Ca measurements.
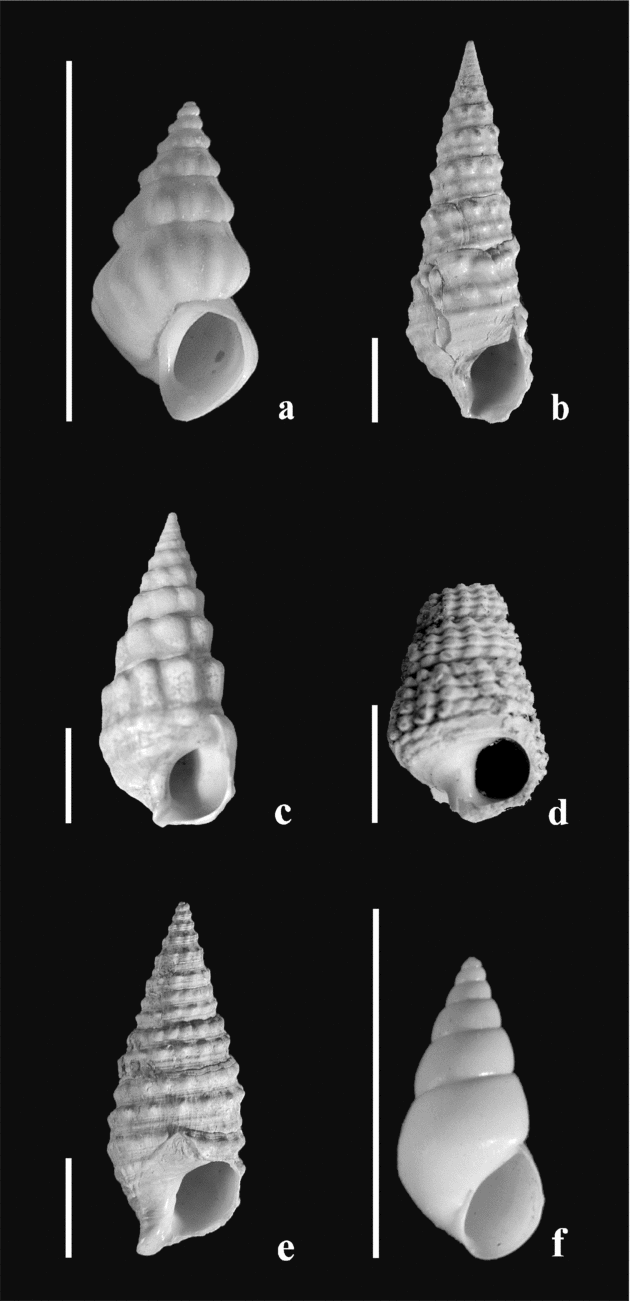
Figure 6. Gastropod species used for geochemical analyses. Scale bar = 5 mm. (a) Mohrensternia angulata (Eichwald). Mány-17 borehole, depth 144.6–147 m. (b) Granulolabium bicinctum (Brocchi). Mány-17 borehole, depth 92–92.3 m. (c) Granulolabium bicinctum (Brocchi). Mány-17 borehole, depth 128.6–130 m. (d) Potamides sp. Mány-17 borehole, depth 144.1–147 m. (e) Cerithium rubiginosum Eichwald. Mány-17 borehole, depth 111–111.2 m. (f) Hydrobia hoernesi Friedberg. Mány-17 borehole, depth 81.5–85.3 m.
3.b.2. Carbon and oxygen isotope compositions of carbonates
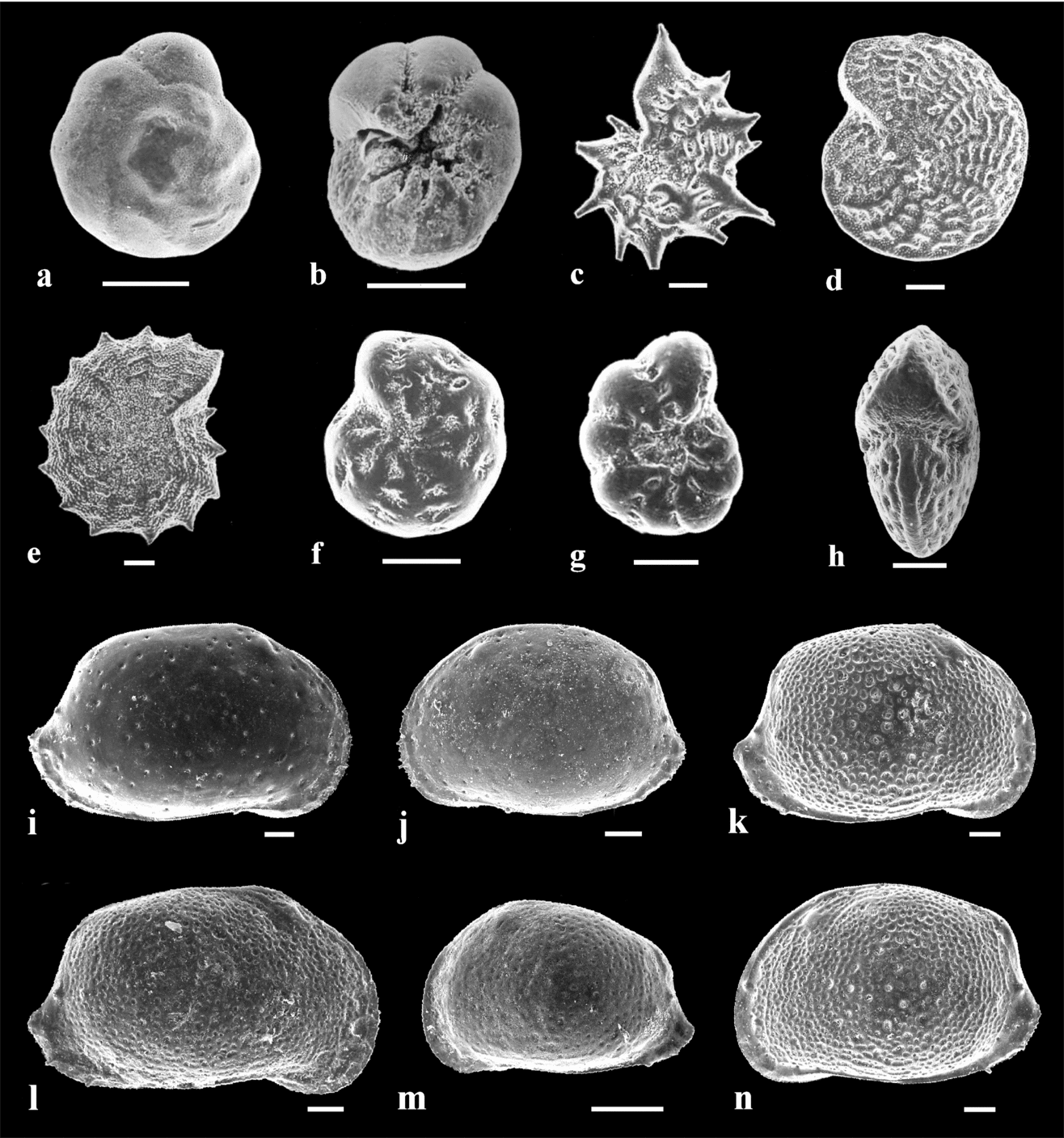
One hundred and seven carbon and oxygen stable isotope compositions were determined for four species of foraminifera (Elphidium aculeatum, E. hauerinum, E. macellum and Ammonia beccarii; Fig. 7), two ostracod species (Aurila mehesi and A. notata; Fig. 7) and five gastropod species (Mohrensternia angulata, Granulolabium bicinctum, Potamides disjunctus, Hydrobia hoernesi and Cerithium rubiginosum; Fig. 6). Stable isotope ratios were determined by using an auto sampler MultiPrep™ system coupled to a dual-inlet GV Isoprime™ isotope ratio mass spectrometer (IRMS). For each sample, about 300 μg of calcium carbonate were reacted with oversaturated phosphoric acid at 90 °C (7–14 valves of Aurila, 15–20 shells of Elphidium and 28–44 shells of Ammonia were used). Isotopic compositions are quoted in δ-notation in per mille relative to V–PDB (see online Appendix Tables A1, A2). All sample measurements were triplicated and adjusted to the international reference NIST NBS19. External reproducibility was ±0.1 ‰ for δ18O values and ±0.05 for δ13C values (1σ).
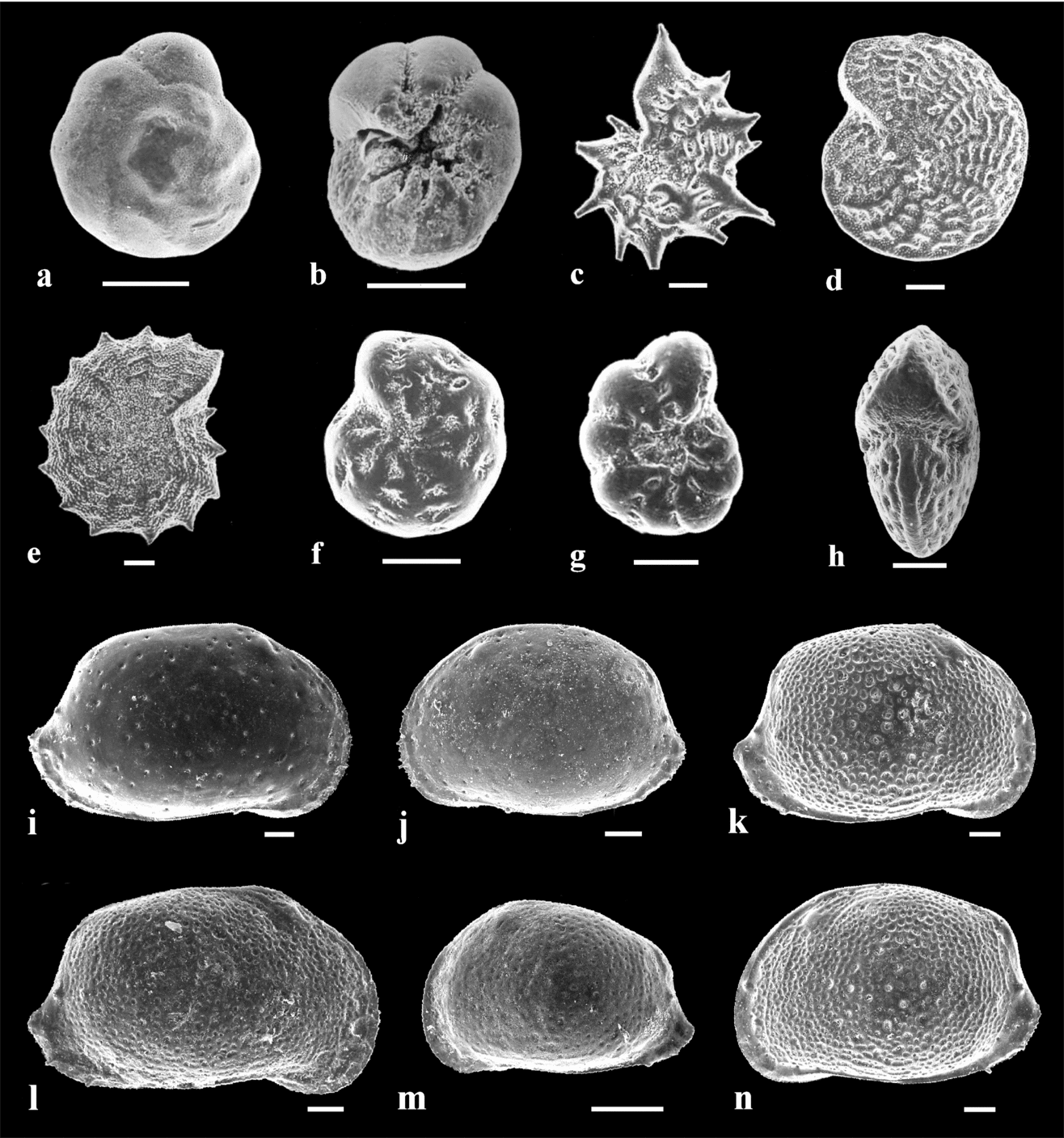
Figure 7. Foraminifer and ostracod species used for geochemical analyses. Scale bar = 100 μm (a) Ammonia beccarii (Linné). Dorsal side. Perbál-5 borehole, depth 98–104 m. (b) Ammonia beccarii (Linné). Ventral side. Perbál-5 borehole, depth 98–104 m. (c) Elphidium aculeatum (d'Orbigny). Side view of a juvenile specimen. Mány-17 borehole, depth 152.8–153 m. (d) Elphidium macellum (Fichtel & Moll). Side view. Mány-22 borehole, depth 170–173 m. (e) Elphidium aculeatum (d'Orbigny). Side view of an adult specimen. Mány-17 borehole, depth 152.8–153 m. (f, g) Elphidium hauerinum (d'Orbigny). Side view. Perbál-5 borehole, depth 128.8–134.4 m. (h) Elphidium macellum (Fichtel & Moll). Apertural view. Mány-22 borehole, depth 170–173 m. (i) Aurila mehesi (Zalányi). RV. Mány-17 borehole, depth 168.7–171.2 m. (j) Aurila mehesi (Zalányi). LV. Mány-17 borehole, depth 168.7–171.2 m. (k) Aurila notata (Reuss). RV♀. Mány-22 borehole, depth 45–52.5 m. (l) Aurila notata (Reuss). RV♂. Mány-22 borehole, depth 70–72 m. (m) Aurila notata (Reuss). LV, juvenile specimen. Mány-22 borehole, depth 70–72 m. (n) Aurila notata (Reuss). LV♀. Mány-22 borehole, depth 45–52.5 m.
3.b.3. Oxygen isotope compositions of phosphates
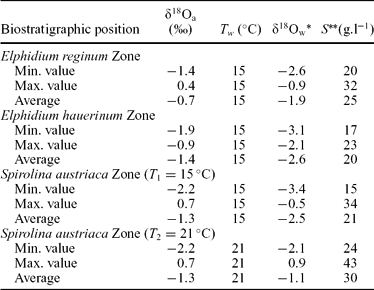
Four oxygen isotope compositions were measured on the incisors of rodents (Table 1). Three or four teeth were used for each measurement. Phosphate from biogenic apatites was isolated as Ag3PO4 crystals, following a protocol originally worked out by Crowson et al. (Reference Crowson, Showers, Wright and Hoering1991) and slightly modified by Lécuyer et al. (Reference Lécuyer, Grandjean, O'Neil, Cappetta and Martineau1993). The 18O/16O ratios are measured after reaction of silver phosphate with graphite to form CO2 (O'Neil et al. Reference O'Neil, Roe, Reinhard and Blake1994; Lécuyer et al. Reference Lécuyer, Grandjean, Barrat, Nolvak, Emig, Paris and Robardet1998). CO2 samples were analysed at the University of Lyon with a GV PRISM II stable isotope ratio mass spectrometer using dual inlet systems with automated cold fingers. Isotopic compositions are quoted in the standard δ-notation relative to V-SMOW. The reproducibility of measurements carried out on tooth enamel samples is better than 0.2 ‰ (1σ). Silver phosphate precipitated from standard NBS120c (natural Miocene phosphorite from Florida) was repeatedly analysed (δ18O = 21.75 ± 0.20; n = 4) along with the silver phosphate samples derived from the rodent teeth.
Table 1. Phosphate oxygen isotope compositions of rodent teeth (δ18Op) and estimates of local meteoric water composition and air temperature

*Annual mean oxygen isotope compositions of meteoric waters were calculated using the fractionation equation established by Navarro et al. (Reference Navarro, Lécuyer, Montuire, Langlois and Martineau2004):
**Annual mean air temperatures were estimated according to von Grafenstein et al.'s (Reference Grafenstein, Erlenkeuser, Müller, Trimborn and Alefs1996) equation:
4. Palaeontological results (see online Appendix Tables A1, A2)
The palaeoecological study of the foraminifera is mainly adapted from Görög (Reference Görög1992). These results are complemented with and compared to the interpretation of the ostracod fauna. The Sarmatian beds from the studied boreholes contain a great abundance of well-preserved ostracods that most likely represents the original biocenosis because juvenile/small and adult/large forms coexist, indicating that no sorting occurred. The low carapace/valve ratio reflects a slow sedimentation rate (Oertli, Reference Oertli1971). Ostracods have already been applied successfully to palaeoecological reconstructions because they occur in all aquatic environments and are very sensitive to small changes in salinity (e.g. Morkhoven, Reference Morkhoven1962; Hartmann, Reference Hartmann and Gruner1975).
4.a. Elphidium reginum Zone
At the Badenian/Sarmatian boundary, the faunal diversity strongly decreased, accompanied by a sharp species turnover. Among more than one hundred species of Badenian ostracods, only seven survived into the Sarmatian (Callistocythere egregia, Hemicyprideis dacica dacica, Hemicytheria omphalodes, Senesia vadaszi, Loxoconcha ex. gr. punctatella, Loxocorniculum hastatum and Xestoleberis fuscata) (Tóth, Reference Tóth2008). Only two Mediterranean species occur in the Sarmatian fauna of the Zsámbék Basin, while several endemic species appeared (Amnicythere sp., A. tenuis, Callistocythere incostata, Cnestocythere aff. truncata, Euxinocythere praebosqueti, E. diafana, E. naca, Cytheridea hungarica, Miocyprideis sarmatica, Aurila merita, A. mehesi, Loxoconcha kochi and L. porosa). This faunal pattern argues in favour of a very limited connection between the Paratethys and the Mediterranean at the Badenian/Sarmatian boundary. In the Elphidium reginum Zone, Aurila mehesi, Cytheridea hungarica and Senesia vadaszi are the most common among the fifteen species documented. The abundance of Aurila mehesi and Cytheridea hungarica probably depends on the substrate lithology, the latter genus preferring sandy bottoms (Hartmann, Reference Hartmann and Gruner1975). The ostracod community indicates epineritic, infralittoral to upper circalittoral zones with well-ventilated waters at depths less than 80 m (Hartmann, Reference Hartmann and Gruner1975). Modern Xestoleberis, Hemicytheria, Aurila and Loxoconcha species live mainly on algae or seagrasses in the littoral zone (Puri, Bonaduce & Gervasio, Reference Puri, Bonaduce, Gervasio and Neale1969). Many ostracod specimens show a significant reduction of ornamentation that also suggests a stressed environment (Hartmann, Reference Hartmann and Gruner1975).
Among the foraminifera, the abundance of Hauerinidae in the lowermost strata indicates shallow, warm-temperate waters. Above the Hauerina-rich layers, the dominance of large elphidiids points to deeper-water settings (Görög, Reference Görög1992). The specimens of Cibicides that mainly lived attached on plants or on hard substrates are also very frequent. In the uppermost strata of the Elphidium reginum Zone, a biofacies containing Cibicides, Articulina and Nodophthalmidium indicates near-shore settings and occurs together with Bolivina which prefers a deeper environment (Görög, Reference Görög1992).
The total species diversity, the slight diversification towards the upper part of the Elphidium reginum Zone (mainly for the foraminifera) and the values of the R index suggest an increase in the stability of the environmental factors (Fig. 4).
4.b. Elphidium hauerinum Zone
At the boundary between the Elphidium reginum and Elphidium hauerinum zones, a significant change is observed in the composition of the ostracod and foraminifer assemblages, without distinct lithological variations or depositional hiatus. The microfauna is less diversified in this zone than in the lower one. Kollmann (Reference Kollmann1958), Jiřiček (Reference Jiřiček and Brestenská1974) and Zelenka (Reference Zelenka, Whatley and Maybury1990) reached a similar conclusion for the ostracod fauna of the Vienna Basin. Eight species vanished and only three taxa (Amnicythere sp., Aurila notata and Loxoconcha porosa) appeared in this zone. In the lowermost beds of the Elphidium hauerinum Zone, the so-called small-sized ostracods (Amnicythere, Euxinocythere, Callistocythere, Loxoconcha, Xestoleberis) became conspicuous elements. Above all, very thin-shelled and unusually small specimens of Xestoleberis are very frequent in these layers. The thin, smooth valves and the small size suggest poorly oxygenated and/or cold water (Hartmann, Reference Hartmann and Gruner1975). The latter condition is unlikely because the presence of Callistocythere indicates warm waters (Morkhoven, Reference Morkhoven1962). Above these beds, the large and more ornamented Aurila notata and Hemicytheria omphalodes are dominant. Their distribution depends on the substrate type: Aurila prefers mixed sediments, while Hemicytheria has a preference for sandy bottoms (Cernajsek, Reference Cernajsek1972), a pattern agreeing with our observations.
In the lowermost beds of the Elphidium hauerinum Zone, infaunal foraminifera (Bolivina, Bulimina, Buliminella, Caucasina, Cassidulina, non-keeled elphidiids) become dominant beside the scanty miliolids and the elongated, uncoiled Articulina-type assemblage. These infaunal groups indicate colder (more temperate) and deeper waters and are generally associated with fine-grained, organic-rich sediments (Murray, Reference Murray1991; Görög, Reference Görög1992). In the upper part of the zone, the fauna is characterized by the dominance of euryhaline infaunal genera such as Ammonia and Nonion. The presence of Ammonia in great abundance indicates shallow (less than 50 m), warm-temperate waters.
The decreasing trend of the total species diversity of ostracods and foraminifera (mainly in the Mány-22 borehole) indicates a greater instability of the environment towards the end of this zone. The values of the Jaccard's index are lower in the Elphidium hauerinum Zone than in the Elphidium reginum Zone, suggesting a higher instability of the fauna in the middle zone (Fig. 4).
4.c. Spirolina austriaca Zone
At the boundary between the underlying Elphidium hauerinum Zone and the Spirolina austriaca Zone, a pronounced diversity increase is observed in the ostracod and foraminifer assemblages. Seven ostracod species appear (Cnestocythere aff. truncata, Euxinocythere naca, E. praebosqueti, Cyamocytheridea derii, C. leptostigma leptostigma, Miocyprideis janoscheki and Loxoconcha kochi). In this upper zone Aurila notata is generally most frequent, except in a few layers where Cyamocytheridea leptostigma leptostigma is prevalent. A few Badenian species (e.g. Cyamocytheridea derii, C. leptostigma leptostigma, Miocyprideis janoscheki) occur in this zone, while they are absent in the lower Sarmatian layers as well as in the foraminiferal fauna (e.g. Spirolina austriaca, Schlumbergerina fabularoides) (Görög, Reference Görög1992; Tóth, Reference Tóth2008). The appearance of Cnestocythere in these beds is noteworthy because this genus is known exclusively from normal marine environments (Morkhoven, Reference Morkhoven1963). The ostracod fauna indicates shallow (epineritic, infra- to circalittoral) settings with almost normal marine conditions.
The foraminiferal assemblages show a similar overall trend. The fauna is more diverse, and some species appear, mainly among the Hauerinidae which normally live in greater numbers in shallow and warm to warm-temperate waters of the infralittoral zone at depths between 30 and 50 m. The mass occurrences of a finely agglutinated species, Schlumbergerina fabularoides, indicate shallow waters with a sandy substrate at depths of about 10–30 m (although this species may have been transported a short distance downslope into deeper parts of the sedimentary basin). Moreover, the great abundance of Triloculina and Milionella and the presence of irregularly coiled specimens of Hauerinidae suggest the development at times of hypersaline conditions (Murray, Reference Murray1991). Spirolina austriaca, a very frequent species in this zone, probably favours normal to elevated salinities, because the present-day Spirolina pertusus is very abundant in hypersaline (40–70 ‰) areas (Haig, Reference Haig1988). This is also supported by the presence of evaporites in the same stratigraphic level a few kilometres south of the studied area (Jámbor, Reference Jámbor1974).
Significant diversifications and renewals of the ostracod and foraminifer faunas are indicated by higher values of the total species diversity in the Spirolina austriaca Zone than in the underlying one, but the low Jaccard's indices (except for the ostracods in Mány-17) suggest a high faunal instability that is probably caused by variations in salinity (close to normal marine to hypersaline) and in temperature conditions of these shallow waters (Fig. 4).
5. Geochemical results (see online Appendix Tables A3, A4)
Mg over Ca ratios have been analysed along with stable isotope compositions (δ13C and δ18O) on the same material for gastropods (n = 22). Data obtained from Mány-17 and Mány-22 boreholes are compiled together in Figure 8. Stable isotope ratios have been determined for all foraminifera (Ammonia, n = 13; Elphidium, n = 46) and for all ostracod species (n = 27), but Mg/Ca ratios were not measured on these samples due to an insufficient amount of material.
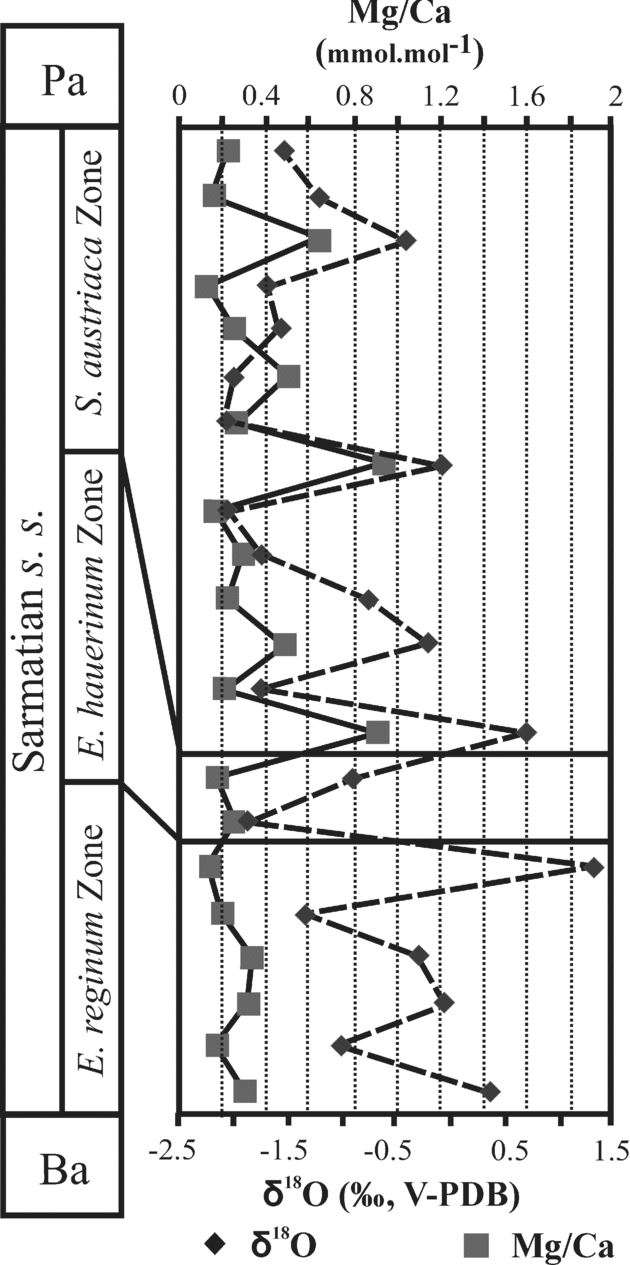
Figure 8. Comparison of Mg/Ca and δ18O data of gastropods from the Zsámbék Basin. Ba – Badenian; Pa – Pannonian.
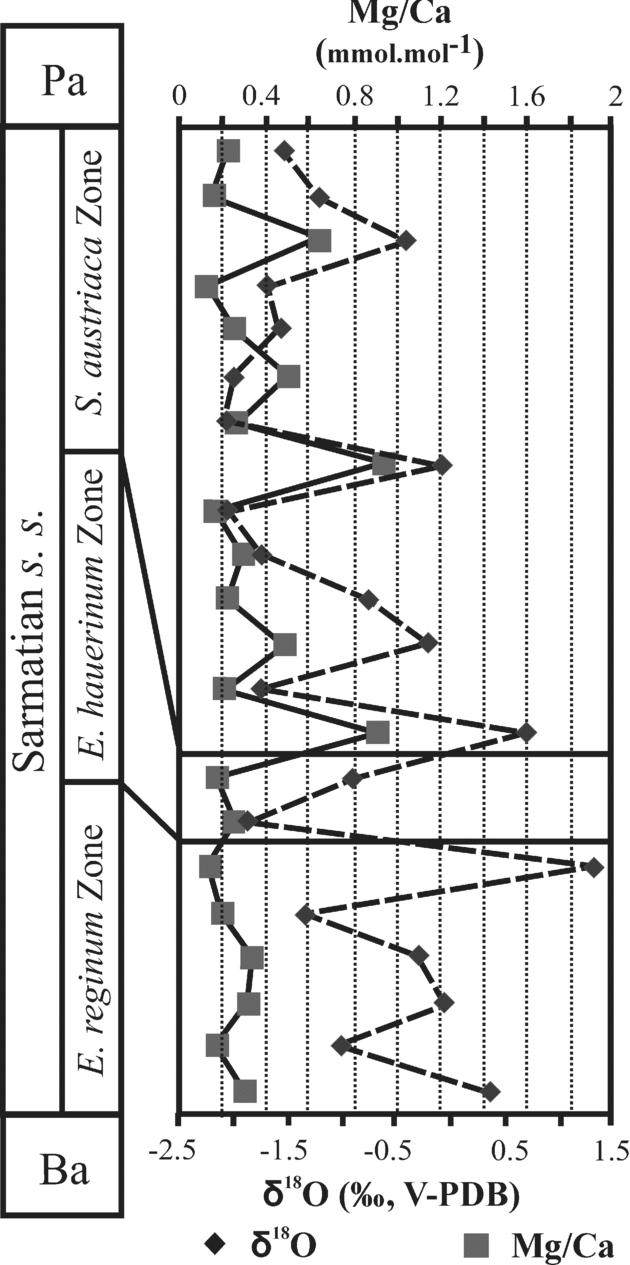
The Mg/Ca ratios of the gastropod skeletons belonging to the Elphidium reginum and Elphidium hauerinum zones exhibit a narrower range of values (between 0.14 and 0.34 mmol mol−1), in contrast to the great fluctuations of Mg/Ca ratio in the upper zone (from 0.13 to 0.91 mmol mol−1). δ18O values range from −2.1 to 1.3 ‰. Mg/Ca ratios and δ18O values are not correlated (r2 = 0.2) (Fig. 8).
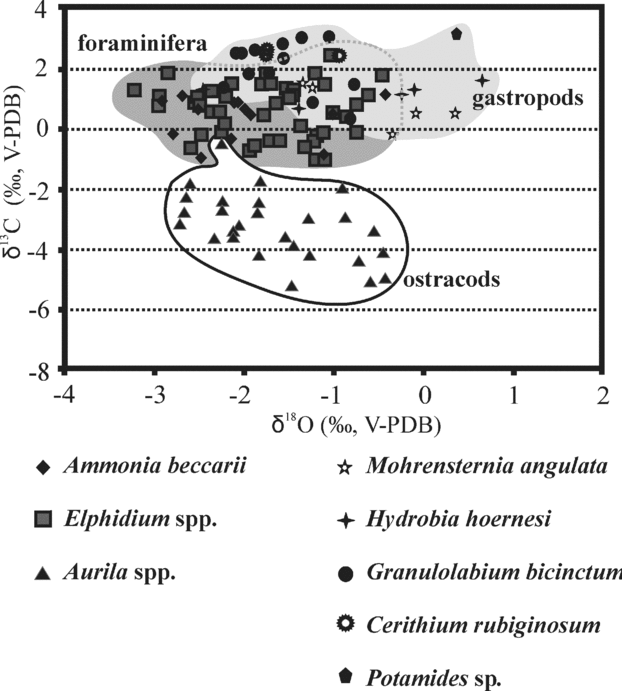
Both carbon and oxygen isotope compositions of foraminifera (Ammonia and Elphidium) and ostracods lie in comparable ranges for the Mány-17 and Mány-22 boreholes. No significant differences are observed between δ18O values of foraminifera and ostracods, all ranging from –3.5 ‰ to –0.5 ‰ (Fig. 9). A progressive increase in δ18O values from about −3 ‰ up to −0.5 ‰ towards the end of the Elphidium reginum Zone is best illustrated by the foraminifera in the Mány-22 borehole, from which numerous samples were analysed with a high stratigraphic resolution. Foraminifera from the same borehole combined with ostracods from borehole Mány-17 show that the oxygen isotope compositions decrease in the Elphidium hauerinum zone and at the beginning of the Spirolina austriaca zone (Fig. 10).
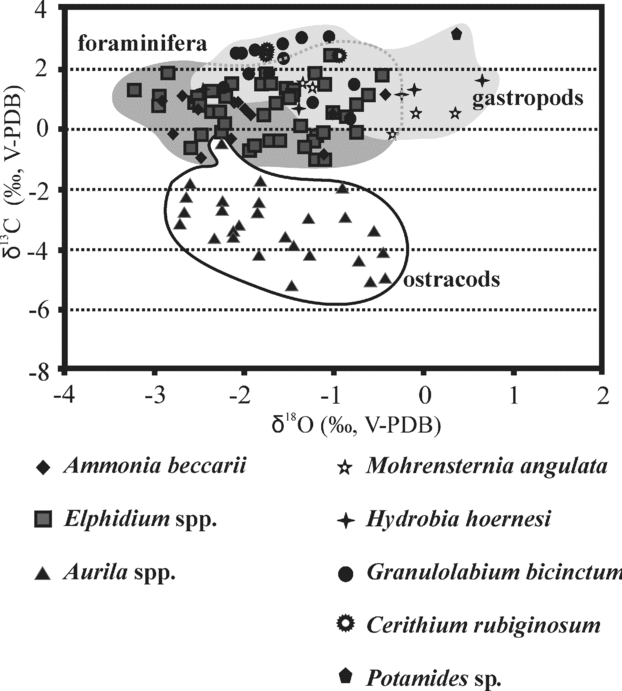
Figure 9. Oxygen isotope and carbon isotope variations of foraminifera (Elphidium spp., Ammonia beccarii), ostracods (Aurila spp.) and gastropods (Mohrensternia angulata, Hydrobia hoernesi, Granulolabium bicinctum, Cerithium rubiginosum, Potamides sp.) from different boreholes of the Zsámbék Basin.
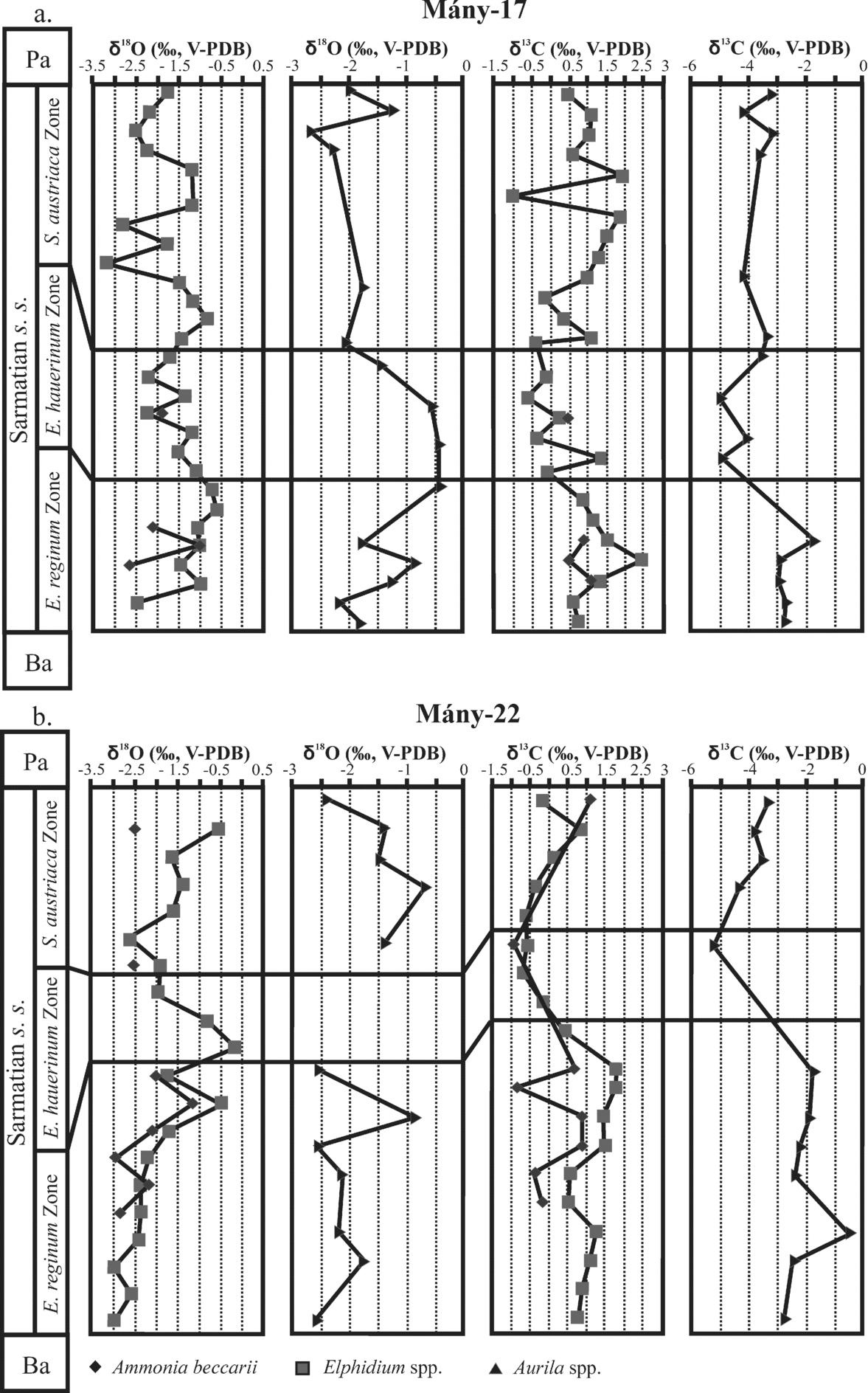
Figure 10. Oxygen and carbon isotope compositions of foraminifera and ostracods from the Zsámbék Basin. Ba – Badenian; Pa – Pannonian.
Ostracods in both boreholes as well as foraminifera from the Mány-22 borehole clearly show an abrupt decrease of δ13C values at the Elphidum reginum/Elphidium hauerinum Zone boundary, despite large differences in absolute values, ostracods having much lower δ13C values than foraminifera and gastropods (Figs 9, 10). In the upper zone, the carbonate skeletons of fossils are characterized by higher δ13C values than in the middle zone, but they have lower carbon isotope compositions than in the lower zone (Fig. 10).
Oxygen isotope compositions range from 17.7 ‰ to 19.8 ‰ for four samples of Sarmatian rodent teeth from Felsőtárkány and Tăşad (Table 1). The δ18O value from the Lower Sarmatian teeth is 17.7 ‰, while the average of the oxygen isotope compositions from the Upper Sarmatian teeth is 18.4 ‰.
6. Discussion
6.a. Estimation of water temperature and salinity based on phosphate and carbonate geochemical data
The oxygen isotope composition of freshwater inputs to the Central Paratethys is assumed to be directly derived from local meteoric waters whose δ18O value is estimated from the rodent teeth compositions (Table 1). Using the fractionation equation established by Navarro et al. (Reference Navarro, Lécuyer, Montuire, Langlois and Martineau2004) for modern rodents, a δ18O value of −5.7 ‰ for the Early Sarmatian and a δ18O range of −5.5 to −2.2 ‰ for the Late Sarmatian are computed for the freshwater end-members (Table 1). The Early Sarmatian data coincide well with the δ18O value of meteoric water estimated from the c. 13.5 Ma large mammal teeth of the Steinheim Basin (Tütken et al. Reference Tütken, Vennemann, Janz and Heizmann2006). Since correlations have also been established between the weighted oxygen isotope composition of precipitation and mean annual air temperature (MAT) at middle to high latitudes (von Grafenstein et al. Reference Grafenstein, Erlenkeuser, Müller, Trimborn and Alefs1996), the combination of both relations leads to an estimated MAT between 15 and 21 °C (Table 1). We emphasize that this method was applied to rodent teeth assuming that the present-day relationship between δ18O in precipitation and air temperature is valid for the Sarmatian. This air temperature estimate is similar to other estimates based on palaeoflora from the same outcrops (Erdei et al. Reference Erdei, Hably, Kázmér, Utescher and Bruch2007) and more generally from the Pannonian Basin (Jiménez-Moreno et al. Reference Jiménez-Moreno, Rodríguez, Pardo-Igúzquiza, Fauquette, Suc and Müller2005).
The next step is to consider that this mean annual air temperature could parallel the mean temperature of surface waters in the Central Paratethys. According to this assumption, δ18O values of seawater can be computed by using the aragonite–water fractionation equation determined by Grossman & Ku (Reference Grossmann and Ku1986) from the temperature and the isotopic compositions of shallow-water gastropod shells that are known to precipitate their carbonate skeleton in oxygen isotope equilibrium with seawater (Grossman & Ku, Reference Grossmann and Ku1986; Lécuyer, Reynard & Martineau, Reference Lécuyer, Reynard and Martineau2004) (Table 2). Assuming that the shallow Sarmatian Sea was homogeneous in temperature we obtain the δ18O values of the seawater (Table 2).
Table 2. Oxygen isotope compositions of aquatic gastropods (δ18Oa) and estimates of seawater composition and salinity
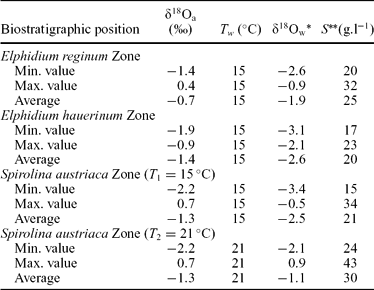
*δ18O values of seawater was computed using the aragonite–water fractionation equation determined by Grossman & Ku (Reference Grossmann and Ku1986):
**Salinity of seawater was estimated using a mass balance equation:
The oxygen isotope composition of the marine end-member can be considered as close to −0.5 ‰, according to the δ18O values of shark teeth from Badenian beds of the Pannonian and Vienna basins (Kocsis et al. Reference Kocsis, Vennemann, Hegner, Fontignie and Tütken2009). The corresponding salinity is about 34 ‰ for a period preceding the onset of the major stage of Antarctic ice growth (Zachos et al. Reference Zachos, Pagani, Sloan, Thomas and Billups2001). These δ18O and salinity values for both water end-members (freshwater with δ18O values of −5.6 ‰) are used to reconstruct the changes in salinities that occurred in the Sarmatian Central Paratethys.
In the Elphidium reginum and Elphidium hauerinum zones, the near constant Mg/Ca ratios of gastropods suggest a stable water temperature (Fig. 8). The δ18O values of gastropods, ranging from −1.4 to 0.4 ‰, correspond to salinities from 20 to 32 ‰, the maximum values being recorded at the end of the lower zone. The middle zone, with similar Mg/Ca ratios, is characterized by δ18O values of gastropods ranging from −1.9 to −0.9 ‰, without any marked temporal trend. These δ18O values correspond to salinities between 17 and 23 ‰ (Table 2). The Spirolina austriaca Zone is characterized by various Mg/Ca ratios reflecting fluctuations in water temperature (Fig. 8). They correlate well with the large range in temperatures (15–21 °C) calculated from the phosphate data. The δ18O values of the gastropods, fluctuating over time, range from −2.2 to 0.7 ‰, and they were used to compute salinity values between 15 and 43 ‰ (Table 2).
6.b. Integrated interpretation of the palaeontological and geochemical records
6.b.1. Elphidium reginum Zone
The ostracod, foraminifer and mollusc faunas of the Lower Sarmatian beds are characterized by their low diversity, great abundance of euryhaline taxa and almost complete lack of stenohaline forms, contrary to the highly diversified, normal marine Badenian faunas. The microfauna suggests shallow, warm-temperate, well-ventilated seawater (littoral zone, maximum 80 m deep) with rich algae and/or seagrass vegetation on the bottom. The change in foraminiferal fauna (appearance of large elphidiids above the Hauerina-rich layers) indicates increasing water depths in this zone. At the end of the Elphidium reginum Zone the appearance of infaunal Bolivina also indicates deeper conditions. The presence and abundance of partially redeposited foraminifera of the Cibicides and Articulina-type assemblages suggest the expansion of the area covered by shallow waters. The quantitative analyses of the microfauna (total species diversity and R) indicate an increase in the stability of this previously unstable environment towards the end of this zone (Fig. 4). This phenomenon and the positive shifts in the δ18O and δ13C profiles are presumably indicative of a seawater incursion into the semi-open basin system of the Central Paratethys at the end of the Elphidium reginum Zone (Fig. 10). The Mg/Ca profile of the gastropod shells suggests more or less stable and constant bottom-water temperature conditions during the Early Sarmatian in the Zsámbék Basin (Fig. 8). As there is no correlation between the δ18O and Mg/Ca profiles of gastropod shells, we can exclude the influence of temperature in the oxygen isotope trends. The δ18O profiles are thus likely controlled by changes in salinity.
In the Elphidium reginum Zone a transgressive event can be observed as part of a 3rd order transgressive systems tract (TST) corresponding roughly to the TB 2.6 global cycle of Haq, Hardenbol & Vail (Reference Haq, Hardenbol, Vail, Wilgus, Hastings, Kendall, Posamentier, Ross and van Wagoner1988). Based on sequence stratigraphy, palaeontological and sedimentological analyses of the Sarmatian deposits, evidence of this phenomenon was also shown by Harzhauser & Piller (Reference Harzhauser and Piller2004b) and Kováć et al. (Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004) in the Vienna Basin, by Schreilechner & Sachsenhofer (Reference Schreilechner and Sachsenhofer2007) in the Styrian Basin, by Krézsek & Filipescu (Reference Krézsek and Filipescu2005) and Filipescu, Silye & Krézsek (Reference Filipescu, Silye and Krézsek2005) in the Transylvanian Basin and by Vrsaljko et al. (Reference Vrsaljko, Pavelić, Miknić, Brkić, Kováčić, Hećimović, Hajek-Tadesse, Avanić and Kurtanjek2006) in Croatia. This transgressive pulse culminated in a seawater incursion at the end of this zone. This hypothesis seems to be supported by the predominance of planktonic marine diatoms and silicoflagellates in the Lower Sarmatian diatomite intercalations of the Zsámbék Basin (Hajós, Reference Hajós1986).
From the geochemical analyses of the rodent teeth and the gastropod shells, temperatures of about 15 °C and salinities of 20–32 ‰ were estimated for the Early Sarmatian seawater (Tables 1, 2).
6.b.2. Elphidium hauerinum Zone
At the boundary between the Elphidium reginum and Elphidium hauerinum zones, the diversity of the microfauna decreased greatly. In the lowermost beds of the Elphidium hauerinum Zone, the small-sized, thin and smooth-shelled ostracods and the infaunal foraminifera (Bolivina, Bulimina, Buliminella, Caucasina, Cassidulina and non-keeled elphidiids) indicate colder (temperate) and deeper, less oxygenated waters than in the lower zone. The carbon isotope profiles of foraminifera, ostracod and gastropod shells also suggest less oxygenated, organic-rich sediments, because the negative shift in the δ13C values is caused by the oxidative processes in the surrounding organic-rich sediments (Fig. 10). The decomposition of organic matter releases 12C-enriched CO2 to the bottom water in which the biogenic carbonate precipitated. The presence of such organic-rich sediments can be explained by the maximum input of organic matter correlated with a relative sea-level rise. A similar event was demonstrated by Harzhauser & Piller (Reference Harzhauser and Piller2004b) and Kovać et al. (Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004) in the Vienna Basin. They consider this event as corresponding to a 3rd order HST (highstand systems tract).
Above these beds, the upper part of the Elphidium hauerinum Zone is characterized by large and more ornamented ostracods. Among the foraminifera the euryhaline genera Ammonia and Nonion become dominant. The presence of Ammonia in great abundance indicates shallow (less than 50 m deep), warm-temperate waters. The microfauna of the upper part of the Elphidium hauerinum Zone indicates a relative-sea-level fall which is confirmed by the results of Harzhauser & Piller (Reference Harzhauser and Piller2004b) and Kovać et al. (Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004), who demonstrated evidence of a LST (lowstand systems tract).
The decreasing trend of the total species diversity, and the changes in Jaccard's indices (R) of the ostracods and foraminifera suggest a higher instability in this zone than in the lower one (Fig. 4). These characters can be explained by the relative sea-level changes because the Mg/Ca profile indicates nearly constant bottom-water temperatures with the same values as in the lower zone (Fig. 8). The estimated salinity for this zone is 17–23 ‰ (Table 2).
6.b.3. Spirolina austriaca Zone
In this zone the microfauna is more diversified than in the previous one. Several Badenian foraminifer and ostracod species which are missing from the lower Sarmatian beds occur here. A similar phenomenon was documented by changes in gastropod faunas from the Vienna Basin (Harzhauser & Kowalke, Reference Harzhauser and Kowalke2002). Based on the quantitative analyses of the microfauna, significant diversification and renewal events of the ostracod and foraminifer faunas are indicated by higher values of the total species diversity in the Spirolina austriaca Zone than in the Elphidium hauerinum Zone (Fig. 4). These results correlate well with those of Steininger & Wessely (Reference Steininger and Wessely2000), who demonstrated that a narrow marine connection existed between the Eastern Mediterranean and the Eastern Paratethys and that a seaway connected the Eastern and the Central Paratethys at the end of the Sarmatian. The existence of these marine gateways is also supported by the results of Iljina (Reference Iljina1998) from the Eastern Paratethys.
The ecological interpretation of the ostracod and foraminifer faunas, together with the lithological and sedimentological features, indicate warm, well-ventilated shallow lagoon and marsh environments, which occasionally became hypersaline. The δ18O values and Mg/Ca ratios are very scattered, indicating fluctuations in bottom-water temperatures and salinity (Figs 8, 10). The estimated temperatures are 15–21 °C and the salinity 15–43 ‰ (Table 2).
However, the Sarmatian fauna from the studied boreholes in the Zsámbék Basin clearly indicates episodic marine incursions associated with a sea-level rise during the deposition of the upper zone. The occurrence of shallow lagoon and intertidal marsh facies in the basin can be explained by re-deposition from the margins. Similar events were described by Kováć et al. (Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004) from the Vienna Basin. Due to unstable shallow-water environmental conditions, there is no evidence for seawater incursions in stable isotope data, and for the transgressive event in changes of the lithology (e.g. the presence of the oolithic limestones).
7. Conclusions
The studied Sarmatian successions have been subdivided into three parts on the basis of earlier investigations of the foraminiferal fauna (Görög, Reference Görög1992) and the current study of the ostracod assemblages. These findings are compared with geochemical data to reconstruct the depositional history of the Zsámbék Basin and to explain the main changes occurring at the zone boundaries.
The lowermost Sarmatian beds of the Elphidium reginum Zone are characterized by low diversity faunal assemblages and the almost complete absence of stenohaline forms. Evidence for a transgressive event is then shown by the foraminiferal and isotopic data, with relatively stable bottom-water temperatures and periodic siliceous phytoplankton blooms. Similar trends were documented from other Paratethyan basins (Rögl, Steininger & Müller, Reference Rögl, Steininger and Müller1978; Steininger & Wessely, Reference Steininger and Wessely2000; Harzhauser & Piller, Reference Harzhauser and Piller2004b; Kováč et al. Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004; Krézsek & Filipescu, Reference Krézsek and Filipescu2005; Schütz et al. Reference Schütz, Harzhauser, Rögl, Ćorić and Galovic2007).
The lower part of the middle Sarmatian Elphidium hauerinum Zone displays a decrease in the taxonomic diversity of the foraminifera and ostracods. The microfaunal composition and the carbon isotope profiles indicate a sea-level highstand with relatively poorly oxygenated bottom waters. This environment changes later into well-ventilated lowstand conditions in the upper part of this zone. This regressive event is also supported by the results of Harzhauser & Piller (Reference Harzhauser and Piller2004b) and Kovać et al. (Reference Kováč, Baráth, Harzhauser, Hlavatý and Hudáčková2004).
At the boundary between the Elphidium hauerinum and Spirolina austriaca zones, the microfaunal diversity is again increased by the re-appearance of Badenian species which had vanished completely at the base of the Sarmatian. This is explained by a restored connection between the Paratethys and the Mediterranean. The renewal of the microfauna and the salinity estimated using geochemical data indicate close to normal marine conditions, with local development of slightly hypersaline environments. These phenomena can be explained by episodic marine incursions and evaporative processes. The Spirolina austriaca Zone is also characterized by fluctuating bottom-water temperatures.
Acknowledgements
We are grateful to János Hír for providing the rodent teeth and to Jean-Jacques Cornée for helpful discussions during the preparation of this paper. The authors thank François Fourel and François Martineau (both at University of Lyon, UMR 5125) for their technical assistance during the oxygen and carbon isotope analysis of carbonate and phosphate samples. Trace element analyses were carried out at the Ecole Normale Supérieure of Lyon with the help of Philippe Telouk and Chantal Douchet. The Hantken Foundation is acknowledged for support. This is contribution UMR 5125-09.XXX, MTA–MTM Paleo contribution No. 91.