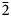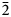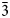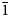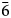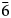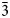Introduction
This paper is part of the series of articles containing descriptions of new arsenate minerals from the Arsenatnaya fumarole of the Tolbachik volcano at the Kamchatka Peninsula, Far-Eastern Region, Russia. Fourteen new arsenate species from this fumarole have already been characterised: yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016b), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018b), arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019a), pair of isostructural minerals edtollite K2NaCu5Fe3+O2(AsO4)4 and alumoedtollite K2NaCu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Ksenofontov, Pautov, Sidorov, Britvin, Vigasina and Pushcharovsky2019b), and anatolyite Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Pekov et al., Reference Pekov, Lykova, Yapaskurt, Belakovskiy, Turchkova, Britvin, Sidorov and Scheidl2019c).
In this paper we characterise the new mineral zubkovaite, Ca3Cu3(AsO4)4, (Cyrillic: зубковаит) named in honour of the Russian crystallographer and crystal chemist Natalia Vital'evna Zubkova (born 1976), Associate Professor at the Faculty of Geology of Lomonosov Moscow State University, a specialist in the structural mineralogy. She has studied crystal structures of more than 160 minerals and is a co-author of descriptions of 101 new mineral species approved by the International Mineralogical Association (IMA). Dr. Zubkova has made a significant contribution to the mineralogy of arsenates and the mineralogy of the fumarolic formation. Structures of 34 natural arsenates (including 16 copper-bearing species) have been first solved by Dr. Zubkova, or with her participation as a co-author. She has studied the crystal structures of 64 minerals from fumaroles of the Tolbachik and Bezymyannyi volcanoes at Kamchatka.
Both new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (IMA2018–008). The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue number 96202.
Occurrence, mineral associations and morphology
The Arsenatnaya fumarole is located at the summit of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption (55°41′N, 160°14′E, 1200 m asl). This scoria cone is a monogenetic volcano formed in 1975 (Fedotov and Markhinin, Reference Fedotov and Markhinin1983). Arsenatnaya is one of the largest fumaroles at Tolbachik, and is the most interesting for its unique mineralogy. The general description of this active, hot fumarole including the characterisation of zonation in distribution of mineral assemblages is given by Pekov et al. (Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a).
The holotype specimen of zubkovaite was found by us in July 2015 at the northern area of the Arsenatnaya fumarole, at the depth of 1.5 m under day surface. The temperature in this area, measured using a chromel–alumel thermocouple at the time of collecting, was ~400°C. Additional samples were collected in July 2018 from another mineral association at the depth of 1 m under day surface. We consider that zubkovaite was deposited directly from volcanic gas as a sublimate or, more likely, crystallised as a result of the interaction between fumarolic gas and basalt scoria at the temperatures not lower than 400°C. Basalt seems the most probable source of Ca which has low volatility in volcanic gases (Symonds and Reed, Reference Symonds and Reed1993).
Zubkovaite was found in two mineral assemblages in the polymineralic zone of Arsenatnaya (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a). In the holotype material, it is associated closely with anhydrite, svabite, hematite, johillerite, tilasite, fluorophlogopite, As-bearing potassic feldspar and aphthitalite. Zubkovaite occurs here as coarse long-prismatic, sometimes lath-like crystals up to 0.01 mm × 0.01 mm × 0.2 mm, typically slightly split and cavernous. They are combined in radiating clusters, isolated or forming interrupted crusts up to 1 cm × 1.5 cm in area and up to 0.3 mm thick on the surface of basalt scoria altered by fumarolic gas, and typically partially replaced by aggregates of fluorophlogopite, potassic feldspar and hematite. The new mineral commonly forms intimate intergrowths with svabite and aggregates of these arsenates are coated by anhydrite crusts (Figs 1, 2 and 3).

Fig. 1. Blue clusters and interrupted crusts of zubkovaite (intergrown with svabite: see Fig. 3) covered by aqua–transparent, colourless anhydrite coatings and associated with iron-black hematite and minor blue–violet johillerite. The holotype specimen, catalogue number 96202. Field of view width: 5.8 mm. Photo: I.V. Pekov & A.V. Kasatkin.

Fig. 2. Radiating aggregate of coarse long prismatic crystals of zubkovaite with massive anhydrite crust (in the left part of the photograph). The holotype specimen, catalogue number 96202. Scanning electron microscopy (SEM) secondary electron (SE) image.

Fig. 3. Clusters of zubkovaite (1) and svabite (2) coated by anhydrite (3); anhydrite crusts (which look like veinlets in the section) are also observed around separate svabite individuals and zubkovaite aggregates: (a) polished cross-section; (b) its magnified fragment. The holotype specimen, catalogue number 96202. SEM back-scattered electron images. Field of view width: (a) 1.5 mm; (b) 0.35 mm.
In the samples collected in 2018 zubkovaite was found in association with aphthitalite, johillerite, nickenichite, bradaczekite, hatertite, tilasite, hematite, cassiterite and As-bearing potassic feldspar. The new mineral forms aggregates (up to 0.5 mm across) consisting of coarse, distorted, sometimes skeletal, short prismatic crystals (Fig. 4) or grains irregular in shape up to 0.05 mm in size. They overgrow bradaczekite and johillerite crystal clusters and usually are embedded in aphthitalite crusts.

Fig. 4. Aggregate of coarse short prismatic crystals of zubkovaite. SEM (SE) image.
Physical properties and optical characteristics
Zubkovaite is transparent, bright sky-blue (the holotype), turquoise-coloured or light bluish-green. Streak is light bluish. Lustre is vitreous. The mineral is brittle. One direction of imperfect cleavage was observed under the microscope. Parting was not observed. The fracture is uneven. The Mohs’ hardness is ~3. Density calculated using the empirical formula is 4.161 g cm–3.
Zubkovaite is optically biaxial (–), α = 1.747(5), β = 1.774(5) and γ = 1.792(5) (589 nm). 2Vmeas = 75(10)° (estimated by the conoscopical figure on the section perpendicular to the optical axis) and 2Vcalc = 77°. Dispersion of optical axes is weak with r > v. Pleochroism is distinct: Z (blue) > Y (pale blue) > X (very pale bluish, almost colourless).
Raman spectroscopy
The Raman spectrum of the holotype specimen of zubkovaite (Fig. 5) was obtained using an EnSpectr R532 spectrometer with a green laser (532 nm) at room temperature. The output power of the laser beam was ~7 mW. The spectrum was processed using the EnSpectr expert mode program in the range 100 to 4000 cm–1 with a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~5 μm. The spectrum was obtained for a randomly oriented crystal.

Fig. 5. The Raman spectrum of zubkovaite; bands corresponding to admixed anhydrite are marked with an asterisk.
The strongest bands in the region 750–950 cm–1 correspond to As5+–O stretching vibrations of AsO43– anions. Bands in the region 380–550 cm–1 can be assigned to bending vibrations of AsO4 tetrahedra and Cu2+–O stretching vibrations. Bands with frequencies lower than 350 cm–1 correspond to Ca–O stretching vibrations and lattice modes. The absence of bands with frequencies higher than 950 cm–1 (excepting ones belonging to admixed anhydrite: marked with asterisk in Fig. 5) indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in zubkovaite.
Chemical composition
The chemical composition of zubkovaite was determined using a Jeol 733 electron microprobe instrument (wavelength-dispersive spectroscopy mode, acceleration voltage of 20 kV, a beam current of 20 nA and a 3 μm beam diameter). The following standards were used: wollastonite (Ca), Cu (Cu), InAs (As) and ZnS (S). Contents of other elements with atomic numbers higher than carbon are below detection limits.
The chemical composition of the holotype sample of zubkovaite (average of 7 spot analyses, in wt.%; ranges are in parentheses) is: CaO 19.22 (18.71–19.74), CuO 27.37 (27.13–27.73), As2O5 52.54 (51.26–53.17), SO3 0.67 (0.00–2.14), total 99.80.
The empirical formula calculated on the basis of 16 O atoms per formula unit is Ca2.96Cu2.97(As3.945S0.07)Σ4.015O16. The simplified formula is Ca3Cu3(AsO4)4 (Z = 2), which requires CaO 19.41, CuO 27.54, As2O5 53.05, total 100.00 wt.%.
X-ray crystallography and crystal structure
Powder X-ray diffraction data of zubkovaite (Table 1) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with cylindrical image plate detector (radius 127.4 mm) using Debye–Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA, and exposure of 12 min. Angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The monoclinic unit-cell parameters calculated from the powder data are: a = 16.841 (9), b = 5.044(1), c = 9.110(5) Å, β = 117.33(4)° and V = 687.5(5) Å3.
Table 1. Powder X-ray diffraction data (d in Å) of zubkovaite.

*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold
Single-crystal X-ray studies of zubkovaite (the crystal was separated from the holotype sample) were carried out using a STOE StadiVari diffractometer equipped with a Dectris PILATUS 300K pixel detector. The measured crystal was small (0.01 mm × 0.01 mm × 0.03 mm) and irregular in shape (distorted prismatic). The crystal structure was solved by direct methods and refined with the use of SHELX-97 software package (Sheldrick, Reference Sheldrick2015) to R = 0.0719. The crystal data and the experimental details are given in Table 2, coordinates and displacement parameters of atoms in Table 3, selected interatomic distances in Table 4 and bond-valence calculations in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 2. Crystal data, data collection information and structure refinement details for zubkovaite.

Table 3. Atom coordinates (x, y, z) and thermal displacement parameters (U, Å2) for zubkovaite.

Table 4. Selected interatomic distances (Å) in the crystal structure of zubkovaite.

Table 5. Bond-valence calculations* for zubkovaite.

*Bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991); **slight overcharge is probably caused by partial substitution of As5+ for S6+ (see Table 1).
The crystal structure of zubkovaite is shown in Fig. 6a. The mineral is monoclinic, space group C2; the studied crystal demonstrates twinning by merohedry Class I (Nespolo and Ferraris, Reference Nespolo and Ferraris2000), with twin domains ratio 65/35. The structure is based on trimers of Cu2+-centred polyhedra. Each trimer consists of one Cu(1)-centred distorted square CuO4 [the Cu(1)–O distances lie in the range of 1.919(18)–1.987(15) Å] in the core and two Cu(2)-centred distorted square pyramids CuO5 [the Cu(2)–O distances lie in the range of 1.903(15)–2.157(16) Å] (Fig. 7a). Two crystallographically independent As atoms play different roles: distorted As5+(2)O4 tetrahedra link neighbouring trimers into ribbons running along the b axis (Fig. 8) whereas distorted As5+(1)O4 tetrahedra link adjacent ribbons into heteropolyhedral layers coplanar to (![]() $\bar 2$01) (Fig. 9a). Restraints were applied to the As(1)–O(8) and As(2)–O(5) distances at the final stage of refinement. Ca cations are located in the interlayer space (Fig. 6a).
$\bar 2$01) (Fig. 9a). Restraints were applied to the As(1)–O(8) and As(2)–O(5) distances at the final stage of refinement. Ca cations are located in the interlayer space (Fig. 6a).

Fig. 6. Crystal structures of zubkovaite (a) and synthetic Ca3Cu3(AsO4)4 (b, drawn after Osterloh and Müller–Buschbaum, Reference Osterloh and Müller-Buschbaum1994). The unit cells are outlined.

Fig. 7. Trimers of Cu–centred polyhedra in the crystal structures of zubkovaite (a) and synthetic Ca3Cu3(AsO4)4 (b, drawn after Osterloh and Müller–Buschbaum, Reference Osterloh and Müller-Buschbaum1994).

Fig. 8. Ribbon formed by the trimers of Cu-centred polyhedra linked via As(2)O4 tetrahedra in the crystal structure of zubkovaite. For legend see Fig. 6.

Fig. 9. Heteropolyhedral Cu–As–O layers in the crystal structures of zubkovaite (a) and synthetic Ca3Cu3(AsO4)4 (b, drawn after Osterloh and Müller–Buschbaum, Reference Osterloh and Müller-Buschbaum1994). For legend see Fig. 6.
Discussion
Among minerals, zubkovaite is unique in terms of structure. Its synthetic chemical analogue Ca3Cu3(AsO4)4 studied by Osterloh and Müller-Buschbaum (Reference Osterloh and Müller-Buschbaum1994) is structurally similar but not identical to the mineral. Both are monoclinic with close unit-cell dimensions but the mineral adopts space group C2 whereas the synthetic phase crystallises in space group P21/a and has a somewhat denser structure (Table 6). The general motifs of both structures are close, however, details of atomic arrangement in zubkovaite and synthetic Ca3Cu3(AsO4)4 are different, which results in the differences in geometry of the trimers (Fig. 7) and an overall high degree of distortion of the structure of zubkovaite in comparison with that of synthetic Ca3Cu3(AsO4)4 (Figs 6 and 9). Probably this causes merohedral microtwinning of the mineral.
Table 6. Comparative data for zubkovaite and synthetic Ca3Cu3(AsO4)4.

* From JCPDS–ICDD #82-982 – powder diffraction file from the International Centre for Diffraction Data, http://www.icdd.com/
Zubkovaite and its synthetic chemical analogue reported by Osterloh and Müller-Buschbaum (Reference Osterloh and Müller-Buschbaum1994) can be considered as two modifications of Ca3Cu3(AsO4)4 related closely structurally. However, they may be distinguished clearly using powder X-ray diffraction data. The measured and calculated powder X-ray diffraction patterns of zubkovaite are very close to one another and differ from that of synthetic Ca3Cu3(AsO4)4 in both positions (d values) and intensities of many reflections (Tables 1 and 6). This difference is the most demonstrative in the position of one of the strongest reflections located in low-angle region, namely ![]() $\bar 2$01: d meas = 7.44 and d calc = 7.46 Å for zubkovaite and d calc = 7.335 Å for synthetic Ca3Cu3(AsO4)4. The refinement of the crystal structure of zubkovaite based on the structure model for synthetic Ca3Cu3(AsO4)4 given by Osterloh and Müller-Buschbaum (Reference Osterloh and Müller-Buschbaum1994) proved to be unstable most likely due to the high degree of the structural distortion, which made it impossible for a structure to be solved in a centric space group.
$\bar 2$01: d meas = 7.44 and d calc = 7.46 Å for zubkovaite and d calc = 7.335 Å for synthetic Ca3Cu3(AsO4)4. The refinement of the crystal structure of zubkovaite based on the structure model for synthetic Ca3Cu3(AsO4)4 given by Osterloh and Müller-Buschbaum (Reference Osterloh and Müller-Buschbaum1994) proved to be unstable most likely due to the high degree of the structural distortion, which made it impossible for a structure to be solved in a centric space group.
Acknowledgements
We are grateful to Vasiliy O. Yapaskurt for assistance in obtaining SEM (SE) images. We thank Peter Leverett and two anonymous referees for valuable comments. This study was supported by the Russian Foundation for Basic Research, grant no. 17-05-00179. The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.33