Introduction
Visual processing is context-specific, such that the neural response to a visual stimulus depends on nearby stimuli or backgrounds. One well-studied example is known as surround suppression, wherein the response to a visual stimulus is reduced by similar nearby stimuli (Cavanaugh et al. Reference Cavanaugh, Bair and Movshon2002; Webb et al. Reference Webb, Dhruv, Solomon, Tailby and Lennie2005; Angelucci & Bressloff, Reference Angelucci and Bressloff2006; Nurminen & Angelucci, Reference Nurminen and Angelucci2014). The perceived luminance contrast of a stimulus is also reduced by surrounding stimuli with similar features (e.g. orientation, direction of motion; Yu et al. Reference Yu, Klein and Levi2001). This surround suppression effect is believed to be important for visually detecting edges and determining the salience of image features during camouflage.
Patients with schizophrenia (SZ) show a number of visual processing abnormalities including a reduced influence of surrounding context. It has been suggested that studying these impairments may provide insight into the neural underpinnings of this disorder; by precisely characterizing visual deficits in SZ, one may be able to attribute them to specific neural mechanisms (Butler et al. Reference Butler, Silverstein and Dakin2008; Phillips & Silverstein, Reference Phillips and Silverstein2013; Yoon et al. Reference Yoon, Sheremata, Rokem and Silver2013; Notredame et al. Reference Notredame, Pins, Deneve and Jardri2014). A number of recent studies have shown weaker surround suppression among SZ patients compared with healthy controls (HCs; Dakin et al. Reference Dakin, Carlin and Hemsley2005; Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006; Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Robol et al. Reference Robol, Tibber, Anderson, Bobin, Carlin, Shergill and Dakin2013; Schallmo et al. Reference Schallmo, Sponheim and Olman2013; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013; Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013a , Reference Yang, Tadin, Glasser, Hong, Blake and Park b ; but see Chen et al. Reference Chen, Norton and Ongur2008; Barch et al. Reference Barch, Carter, Dakin, Gold, Luck, MacDonald, Ragland, Silverstein and Strauss2012). However, it is not clear whether this deficit is specific to SZ, or if it is also observed in other psychiatric conditions such as bipolar affective disorder (BP; Dakin et al. Reference Dakin, Carlin and Hemsley2005; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013a ). Further, it is not known to what extent persons with genetic liability for such disorders, such as first-degree biological relatives of SZ patients (SZrel; Schallmo et al. Reference Schallmo, Sponheim and Olman2013) or of patients with BP (BPrel), show similar deficits. Finally, the extent to which diminished surround suppression in SZ depends on the similarity between targets and surrounding stimuli is not well established (Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013).
The current study examines suppression of visual contrast perception by surrounding stimuli among SZ and BP patients, SZrel, BPrel and HC subjects, to determine whether deficits in surround suppression are present in each group. We varied the configuration of surrounding stimuli to examine how such deficits depend on the similarity between target and surround. Understanding whether diminished surround suppression is diagnostically specific to SZ or reflects mechanisms underlying genetic liability will help clarify the role of basic visual functions in severe psychopathology.
Method
Participants
The following subjects were recruited: 32 out-patients with SZ, one out-patient with schizo-affective disorder–depressed type (grouped with SZ for analysis), 23 BP out-patients, seven out-patients with schizo-affective disorder–bipolar type (grouped with BP), 28 unaffected first-degree biological relatives of SZ patients (SZrel), 11 relatives of BP patients (BPrel), 10 relatives of patients with schizo-affective disorder–bipolar type (grouped with BPrel) and 45 HCs. Diagnoses were made from structured interview [Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR; APA, 2000); Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Reference First1997); Psychosis Module from the Diagnostic Interview for Genetic Studies (Nurnberger et al. Reference Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, Harkavy-Friedman, Severe, Malaspina and Reich1994)] and clinical data reviewed in a consensus process by a doctorate-level clinical psychologist or advanced doctoral students. During consensus diagnosis, one SZrel and one BPrel were found to have diagnoses of SZ and BP, respectively. These were retained as relatives; repeating our analyses without them yielded an equivalent pattern of results. Additionally, we repeated our analyses excluding schizo-affective disorder–bipolar-type patients, to explore the effect of grouping them with BP patients. Results were equivalent; analyses and results for the larger group are reported.
Exclusion criteria used during recruitment were identical to those previously reported (Schallmo et al. Reference Schallmo, Sponheim and Olman2013). All subjects had normal or corrected-to-normal visual acuity. HC subjects had no history of SZ, BP or other psychotic diagnoses for themselves and their first-degree biological relatives. Subjects provided written informed consent and were compensated $15/h. This protocol was approved by the Institutional Review Boards of the University of Minnesota and the Minneapolis VA Medical Center.
Subjects reported their parents’ level of education using a seven-point scale. Intelligence quotient (IQ) was estimated using the Wechsler Adult Intelligence Scale (Jeyakumar et al. Reference Jeyakumar, Warriner, Raval and Ahmad2004). The following behavioral measures were used to assess symptom levels: Brief Psychiatric Rating Scale (BPRS; Overall & Donald, Reference Overall and Donald1962), Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1982), Scale of the Assessment of Positive Symptoms (SAPS; Andreasen, Reference Andreasen1984), Schizotypal Personality Questionnaire (SPQ; Raine, Reference Raine1991) and Sensory Gating Inventory (SGI; Hetrick et al. Reference Hetrick, Erickson and Smith2012). Medication levels were converted to chlorpromazine (CPZ), lithium (Li) and imipramine (Imip.) equivalent doses (Andreasen et al. Reference Andreasen, Pressler, Nopoulos, Miller and Ho2010).
Stimuli
Stimuli were generated using MATLAB and PsychToolbox (Brainard, Reference Brainard1997; Pelli, Reference Pelli1997) on a MacMini running OSX, and displayed on a 19-inch LCD monitor that subtended 35.1 × 26.7 degrees of visual angle at a viewing distance of 61 cm. Display luminance was linearized using custom software. Mean luminance was 84 cd/m2. The stimuli comprised two circular patches of sinusoidal luminance modulation (gratings hereafter) 1° in diameter, with a spatial frequency of two cycles/°, presented at 2° eccentricity to the left and right of a central fixation mark (blue square eight pixels across) along the horizontal meridian. In most conditions, an annular stimulus surrounding one circle was also presented, with an outer diameter of 3°. Gratings drifted at 3.75 cycles/s. The orientation of the circular gratings and direction of stimulus motion were randomly assigned on each trial from four orientations (0–135°), each with two relative directions of motion.
Different stimulus conditions were defined by the presence and configuration of an annular sine wave grating stimulus surrounding one of the circular gratings (Fig. 1). We refer to the circular stimulus presented with the surround as the target, and the stimulus presented alone as the reference. Note that target and reference were always presented peripherally. We included different surround conditions to examine whether greater similarity between target and surround would evoke stronger perceived contrast suppression, as predicted (Yu et al. Reference Yu, Klein and Levi2001; Cavanaugh et al. Reference Cavanaugh, Bair and Movshon2002). These surround conditions were designed to probe whether low-level differences in visual stimulus features (e.g. orientation, motion direction) would differentially affect surround suppression in certain subject groups. A suppression deficit specific to conditions with more similar targets and surrounds may suggest impairment in feature-selective suppression in the early visual cortex, while an impairment across conditions might reflect deficiencies in more broadly tuned mechanisms (Angelucci & Bressloff, Reference Angelucci and Bressloff2006; Nurminen & Angelucci, Reference Nurminen and Angelucci2014).
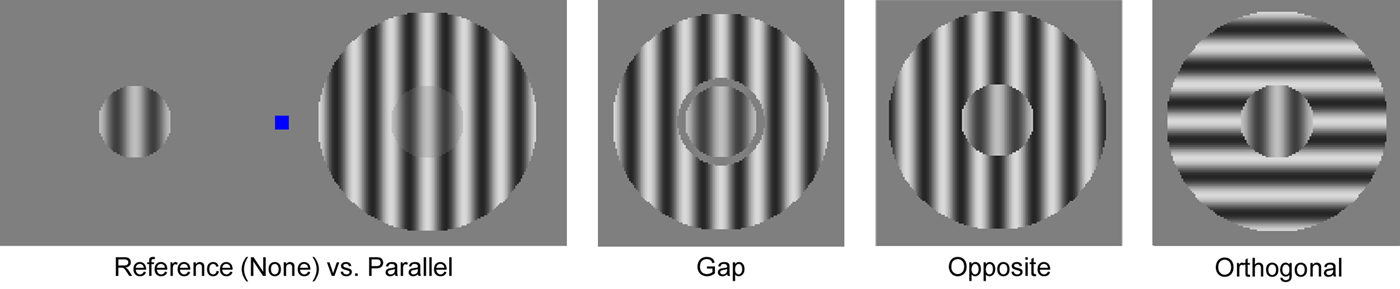
Fig. 1. Surround suppression stimuli. The left panel illustrates the stimulus layout, with the reference (left, no surround, identical to the None condition) and Parallel condition stimuli (right) offset horizontally from a blue central fixation square. Target and reference stimuli are shown at 50% contrast, surrounds at 70%. For the color figure, see the online version of the paper.
In the Parallel condition, target and surround had the same orientation and spatial phase. The inner diameter of the surround was 1°, so target and surround were abutting. The Gap condition was identical to the Parallel condition, except that a 0.1° mean luminance gap separated target and surround (edges not blurred), to mitigate brightness induction (Yu et al. Reference Yu, Klein and Levi2001). The Opposite condition was identical to the Parallel condition, except target and surround drifted in opposite directions. In Fig. 1, target and surround are illustrated with opposite spatial phase to convey that the opposite drift direction disrupts their relative phase relationship. In the Orthogonal condition, target and surround stimuli were oriented orthogonally (90°). Finally, there was a None condition in which no surround was presented. Surrounds were displayed at 70% Michelson contrast, and target contrast was 50%. The position of the target and surround v. reference stimuli (left or right of fixation) was random in each trial.
Paradigm
Subjects fixed their eyes on the central square and used their peripheral vision to compare target and reference contrast. In each trial, stimuli were presented for 300 ms; afterward subjects indicated which circular stimulus (left or right; i.e. target or reference) was higher contrast by pressing the corresponding arrow key. Response time was unlimited. The fixation mark was displayed for 400 ms between trials. A total of 25 trials were presented for each condition in a random intermixed order, which composed one run. Each subject completed at least four runs. Prior to the experiment, subjects completed several practice trials during which they viewed static and drifting stimuli. They were instructed to attend to the peripheral stimuli while maintaining fixation, and to compare the perceived contrast of the target and reference, but not the surround. Total experiment duration including practice was approximately 10 min.
This task was designed to measure the perceived contrast of the target stimulus. For the first 85 subjects, the contrast of the reference was adjusted across trials using a one-up, one-down staircase method to determine the point of subjective equality between the perception of target and reference contrast. This method converges on the reference contrast reported as higher 50% of the time (Garcia-Perez, Reference Garcia-Perez1998), which we refer to as the perceived contrast. For the remaining 72 subjects, reference contrast was adjusted across trials using the Psi adaptive staircase procedure (Prins & Kingdom, Reference Prins and Kingdom2009) to determine the point of subjective equality. This method (not readily available when the task was designed) provides a more efficient estimate of psychometric function slope, which quantifies the noise associated with estimates of perceived contrast (Kingdom & Prins, Reference Kingdom and Prins2010). The proportion of subjects that completed each version of the task did not differ between groups (χ 2 4 = 4.60, p = 0.3). Perceived contrast was measured separately in each run for each surround condition. Reference contrast varied between 14 and 74%, starting at 40 or 60% (on alternating runs).
Data analysis
For the one-up, one-down staircase, perceived contrast was defined as the average contrast from the last six trials in each condition. For the Psi staircase, perceived contrast and corresponding psychometric function slopes were calculated by fitting a logistic function to the staircase responses using a maximum likelihood criterion (Prins & Kingdom, Reference Prins and Kingdom2009). Guess rate and lapse rate were both set to 4%. Psi staircases with perceived contrast values <0% or >100% were excluded (196 out of 1140). We saw no significant difference in perceived contrast values between the two staircase methods (one-way ANOVA, F 1,145 = 0.13, p = 0.7); data from both were subsequently combined.
Change in perceived contrast was quantified for each condition, run and subject as the difference between perceived and veridical target contrast (50%), with negative values indicating suppression. To quantify the relative effect of different surround configurations, contextual modulation indices were calculated by subtracting the change in perceived contrast from the Parallel condition from values in the Gap, Orthogonal and Opposite conditions for each run in every subject.
A number of datasets showed around +20% perceived contrast (i.e. enhancement) for all or most surround conditions. This contradicts the well-established pattern of surround suppression (Yu et al. Reference Yu, Klein and Levi2001). We believe that these subjects failed to follow task instructions and erroneously compared the reference contrast with the surround contrast (70% in all conditions). As the actual target contrast was 50%, such a pattern of responses would resemble 20% enhancement of perceived target contrast. Data sets that met the following criteria were analysed as a separate enhancement group: change in perceived contrast in three or more surround-present conditions was ⩾+10% (averaged across runs), and change in perceived contrast was not ⩽−10% in any condition. We found that 10 SZ, 11 BP, three SZrel, four BPrel and seven HC subjects showed such enhancement.
The proportion of subjects exhibiting enhancement did not significantly differ across diagnosis groups (χ 2 4 = 8.27, p = 0.082). For enhancement subjects, perceived contrast did not vary across surround conditions (excluding the None condition; two-way ANOVA, 5 groups × 4 conditions; F 3,30 = 0.93, p = 0.4) or groups (F 4,30 = 1.78, p = 0.16), and there was no significant group × condition interaction (F 16,120 = 1.49, p = 0.11). Overall, enhancement group data are inconsistent with feature-selective surround modulation (Yu et al. Reference Yu, Klein and Levi2001; Cavanaugh et al. Reference Cavanaugh, Bair and Movshon2002), but instead suggest that subjects were responding to surrounding stimulus contrast (70%), rather than perceived target contrast (about 50%). Therefore, enhancement group data were excluded from further analyses.
Ethical standards
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
We sought to determine how surrounding stimulus configuration affected perception of target contrast among SZ and BP patients, their unaffected first-degree biological relatives and HCs (demographics reported in Table 1). Fig. 2 shows the change in perceived contrast for each condition in each group, following exclusion of subjects who did not comply with task instructions (see Method). Stimulus conditions are arranged in Fig. 2, with greatest target-surround feature similarity (Parallel) on the left, to least (Orthogonal) on the right, followed by the None condition in which no surround was present. Weaker surround suppression was expected for less-similar surrounding stimuli (Yu et al. Reference Yu, Klein and Levi2001; Cavanaugh et al. Reference Cavanaugh, Bair and Movshon2002).

Fig. 2. Surround suppression task results. (a) Data from healthy control (HC), schizophrenia (SZ) and bipolar affective disorder (BP) groups. (b) Data from HCs, first-degree biological relatives of SZ patients and first-degree biological relatives of BP patients. Surround conditions are shown across the x-axis. Change in perceived contrast relative to the 50% contrast target is plotted on the y-axis; negative values indicate suppression. Values are means, with vertical bars representing standard errors of the mean. Across all conditions, suppression is significantly weaker for SZ and BP v. HC, but stronger for BP than SZ groups. Ortho., Orthogonal. For the color figure, see the online version of the paper.
Table 1. Subject demographic information

Data from subjects retained in the final analyses are presented as mean (standard deviation) unless otherwise indicated.
SZ, schizophrenia; SZrel, first-degree biological relatives of SZ patients; BP, bipolar affective disorder; BPrel, first-degree biological relatives of BP patients; HC, healthy controls; IQ, intelligence quotient; BPRS, Brief Psychiatric Rating Scale; SPQ, Schizotypal Personality Questionnaire; SGI, Sensory Gating Inventory; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale of the Assessment of Positive Symptoms; CPZ, chlorpromazine; Imip., imipramine.
a IQ scores in SZ were lower than those of BPrel subjects (Tukey's honestly significant difference test, p < 0.05), but no other differences between groups were significant.
b BPRS scores were significantly higher in SZ patients than in all other groups. Scores for BP subjects were higher than in HCs, but not significantly higher than BPrel subjects.
c SPQ scores were significantly higher in SZ and BP than in HC subjects. SZ scores were higher than for SZrel subjects, but scores were not significantly different for SZ v. BP, or BP v. BPrel.
d SGI scores were significantly higher for SZ, BP and BPrel groups compared with HCs, while other between-group differences were not significant.
We first compared changes in perceived contrast across all surround conditions and subject groups (two-way ANOVA, 5 conditions × 5 groups); a significant main effect of condition (F 4,117 = 97.0, p < 0.001) indicated that surround configuration significantly affected target contrast perception, as expected. Collapsing across groups, changes in perceived contrast differed significantly in post-hoc tests between each surround condition, with more negative values when target and surround were more similar [Parallel < Gap < Opposite < Orthogonal < None; Tukey's honestly significant difference test (HSD), q 236–239 > 3.89, p < 0.05]. This matches the expected form of configuration-dependent surround suppression (Yu et al. Reference Yu, Klein and Levi2001; Cavanaugh et al. Reference Cavanaugh, Bair and Movshon2002): greater feature similarity (e.g. orientation and direction of motion) evoked greater suppression of perceived target contrast. We also observed a main effect of subject group (F 4,117 = 2.70, p = 0.034); post-hoc tests showed significantly weaker suppression of perceived contrast in SZ subjects than in all other groups (across all conditions), while BP subjects showed weaker suppression than HC, BPrel and SZrel groups, but stronger than SZ subjects (Tukey's HSD, q 31–56 > 4.35, p < 0.05; Fig. 2 a). Effect sizes were fairly small (HC v. SZ, q 56 = 11.6, Cohen's d = 0.42; HC v. BP, q 52 = 6.08, d = 0.26; BP v. SZ, q 37 = 4.35, d = 0.20). No difference in contrast perception was observed between HC, SZrel and BPrel groups (Fig. 2 b). This indicates that surround suppression is greatly diminished during contrast perception among SZ subjects (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013b ), reduced among BP subjects (but less so than in SZ subjects), and equally strong among relatives with a genetic risk for SZ or BP as in HC subjects.
We next sought to determine whether suppression deficits among patients were more evident in certain conditions. Although the group × condition interaction for perceived contrast was not significant (F 16,465 = 1.35, p = 0.16), we computed contextual modulation indices as the difference in perceived contrast between the Parallel and Gap, Orthogonal, or Opposite conditions (Fig. 3), to further examine how surround similarity affected the strength of suppression. Indices differed across conditions as expected (two-way ANOVA, 3 conditions × 5 groups; F 2,115 = 76.6, p < 0.001). However, we saw no significant effect of group (F 4,115 = 0.25, p = 0.9), and no interaction between group and condition (F 8,221 = 1.25, p = 0.2). These results indicate that different surround configurations evoked similar changes in contrast perception across all subject groups.

Fig. 3. Contextual modulation indices. (a) Data from healthy control (HC), schizophrenia (SZ) and bipolar affective disorder (BP) groups. (b) Data from HCs, first-degree biological relatives of SZ patients (SZrel) and first-degree biological relatives of BP patients (BPrel). Indices were calculated as the difference in perceived contrast between the Parallel condition and each of the three conditions on the x-axis. Values are means, with vertical bars representing standard errors of the mean. Ortho., Orthogonal. For the color figure, see the online version of the paper.
We examined relationships between task performance and demographic data to determine whether such factors might influence the magnitude of surround suppression. Although gender composition and IQ scores differed significantly across groups (Table 1), we still observed a significant difference in suppression of perceived contrast across groups with gender and IQ included as covariates (F 4,82 = 3.67, p = 0.009). No significant correlations were observed between changes in perceived contrast and age, education, parents’ education, symptomatology scores (BPRS, SANS, SAPS, SPQ and SGI), or medication levels (CPZ, Li or Imip. equivalents). Only two subjects reported daily use of benzodiazepines (one BP, one SZrel), and excluding these subjects yielded equivalent results. For both SZ and BP subjects, symptom dimension scores from the BPRS, SANS and SAPS (Wilson & Sponheim, Reference Wilson and Sponheim2014) and factor scores for the SGI (Hetrick et al. Reference Hetrick, Erickson and Smith2012) were uncorrelated with surround suppression levels. There was no difference in suppression between HC, SZrel and BPrel subjects with low SPQ scores (⩽1, n = 23) and those with high scores (⩾12, n = 22; t 37 = 0.48, p = 0.6). In contrast, weaker surround modulation has been reported among non-clinical schizotypal subjects (Uhlhaas et al. Reference Uhlhaas, Silverstein, Phillips and Lovell2004), which may reflect differences in study sample composition. We did observe significant negative correlations between estimated IQ and perceived contrast changes for all subjects in the Parallel, Gap and Opposite conditions (Pearson's r 105–107 < −0.34, p < 0.016, Bonferroni corrected). Subjects with higher IQ showed stronger surround suppression, consistent with a recent study in healthy subjects (Melnick et al. Reference Melnick, Harrison, Park, Bennetto and Tadin2013). However, as there was a significant difference in suppression between groups with IQ as a covariate, IQ scores alone cannot account for our observation of diminished surround suppression among SZ and BP subjects.
Finally, we examined whether surround suppression deficits might be attributed to non-visual factors such as off-task performance (e.g. lapses of attention, lack of effort; see Barch et al. Reference Barch, Carter, Dakin, Gold, Luck, MacDonald, Ragland, Silverstein and Strauss2012; Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013). We conducted two additional analyses, the first of which compared the standard deviations of perceived contrast measurements for each subject acquired in four separate runs. If a particular group had a higher level of off-task performance, then this would lead to less stable estimates of perceived contrast and greater variability across runs. However, variability in perceived contrast measurements did not differ significantly between groups (two-way ANOVA, 5 conditions × 5 groups; F 4,117 = 0.39, p = 0.8). We also examined psychometric function slopes from the Psi staircase version of our task (see Method). Greater slope values indicate a more reliable perceptual transition at the point of subjective equality. We expected that greater off-task performance would be associated with smaller (less reliable) slopes. We found no evidence for any difference in slopes across groups (two-way ANOVA, 5 conditions × 5 groups; F 4,52 = 0.66, p = 0.6). These results are not consistent with group differences in perceived contrast being attributable to off-task performance.
Discussion
The aim of this study was twofold. First, we investigated whether a putative deficit in surround suppression is only evident in SZ or is also observed in BP and unaffected first-degree biological relatives of SZ and BP patients. Second, we examined how such deficits might depend on similarity between target and surrounding stimuli. We observed overall weaker surround suppression among SZ patients, and to a lesser extent those with BP, compared with their unaffected relatives who showed no difference from HC subjects. The magnitude of deficits among patients did not depend strongly on the configuration of surrounding stimuli. Diminished surround suppression is fairly well documented in SZ, having been observed by a number of groups using different paradigms and stimuli (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006; Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Robol et al. Reference Robol, Tibber, Anderson, Bobin, Carlin, Shergill and Dakin2013; Schallmo et al. Reference Schallmo, Sponheim and Olman2013; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013; Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013b ). Here we have clearly demonstrated that BP patients also show weaker surround suppression (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013a ), albeit to a lesser degree than those with SZ. Our results are also the first observation that the magnitude of surround suppression during contrast perception is not tightly linked to genetic liability for SZ or BP, as SZrel and BPrel showed equivalent suppression to HC subjects.
Our results align with a growing literature showing impaired visual context processing in SZ (Butler et al. Reference Butler, Silverstein and Dakin2008; Phillips & Silverstein, Reference Phillips and Silverstein2013; Yoon et al. Reference Yoon, Sheremata, Rokem and Silver2013; Notredame et al. Reference Notredame, Pins, Deneve and Jardri2014). Some of these deficits may be specific to peripheral vision, such as abnormal temporal processing (Chen et al. Reference Chen, Norton and Stromeyer2014) and spatial crowding (Kraehenmann et al. Reference Kraehenmann, Vollenweider, Seifritz and Kometer2012; but see Robol et al. Reference Robol, Tibber, Anderson, Bobin, Carlin, Shergill and Dakin2013). Conversely, weaker surround suppression has been observed in SZ using both foveal (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006; Barch et al. Reference Barch, Carter, Dakin, Gold, Luck, MacDonald, Ragland, Silverstein and Strauss2012) and peripheral stimuli (Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013b ), suggesting a deficit that extends across the visual field. However, a more precise understanding of the neural mechanism(s) underlying weaker suppression in SZ has remained elusive. For example, it is not yet clear to what extent this deficit is selective for similarity (e.g. parallel orientation) between target and surrounding stimuli, as few studies have examined multiple stimulus configurations (Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013).
Overall, we found little evidence to support a specific deficit in suppression for particular surround configurations; no group × condition interaction was observed for changes in perceived contrast, and contextual modulation indices did not vary significantly across groups. The most parsimonious explanation for these results is that SZ patients show a broad deficit in the strength of surround suppression that is not selective for surrounding stimulus features. This proposal contrasts with previous findings showing weaker surround suppression in SZ for parallel but not orthogonal stimuli (Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013). However, those two studies employed larger (2.2 or 3.3° wide) lower spatial frequency (both 1.1 cycles/°) annular gratings that were presented more peripherally (3.3 or 6.2° eccentricity) than our stimuli (1° diameter circular gratings, two cycles/°, 2° eccentricity). One might therefore attribute this discrepancy to differences in surround suppression across the visual field. Suppression may be stronger and less selective at greater eccentricities (Xing & Heeger, Reference Xing and Heeger2000; but see Williams et al. Reference Williams, Singh and Smith2003), which could reflect differences in the relative contribution of selective and non-selective suppression mechanisms (Angelucci & Bressloff, Reference Angelucci and Bressloff2006; see below). Variability in the balance between these mechanisms, which could be differentially affected by SZ, may explain why others using more peripheral stimuli have found orientation-specific impairments in SZ (Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013), while the deficit we observed in this study was clearly non-selective.
Beyond reducing surround suppression, SZ may impair overall perception of contrast and/or motion. Specifically, a deficit in magnocellular contrast sensitivity has been proposed (Butler et al. Reference Butler, Zemon, Schecter, Saperstein, Hoptman, Lim, Revheim, Silipo and Javitt2005; Martinez et al. Reference Martinez, Hillyard, Dias, Hagler, Butler, Guilfoyle, Jalbrzikowski, Silipo and Javitt2008, Reference Martinez, Hillyard, Bickel, Dias, Butler and Javitt2012), though this has been disputed (Skottun & Skoyles, Reference Skottun and Skoyles2007). Deficits in motion perception observed in SZ (Chen et al. Reference Chen, Bidwell and Holzman2005; Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006; Chen et al. Reference Chen, Norton and Ongur2008) but not in BP or SZrel (Chen et al. Reference Chen, Bidwell and Holzman2005) may also depend on impaired functioning in the magnocellular pathway and/or motion-selective cortical areas such as visual area MT (middle temporal). Reports conflict regarding the effect of surrounding context during motion perception in SZ and BP (Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006; Chen et al. Reference Chen, Norton and Ongur2008; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013a , Reference Yang, Tadin, Glasser, Hong, Blake and Park b ). In contrast perception, previous studies examining surround suppression in SZ used static (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Tibber et al. Reference Tibber, Anderson, Bobin, Antonova, Seabright, Wright, Carlin, Shergill and Dakin2013; Yang et al. Reference Yang, Tadin, Glasser, Hong, Blake and Park2013b ) or contrast-reversing stimuli (Yoon et al. Reference Yoon, Rokem, Silver, Minzenberg, Ursu, Ragland and Carter2009; Seymour et al. Reference Seymour, Stein, Sanders, Guggenmos, Theophil and Sterzer2013), while the current study used drifting target and surround gratings. Thus, motion perception deficits (Tadin et al. Reference Tadin, Kim, Doop, Gibson, Lappin, Blake and Park2006) might have enhanced the group differences we observed. Further study is warranted to clarify how specific neural pathways (e.g. magnocellular v. parvocellular) contribute to perceptual surround suppression in both HC and SZ subjects.
Surround suppression is believed to be driven by multiple neural mechanisms whose anatomical substrates include feed-forward, recurrent, lateral and feedback connections within and between early visual cortical areas (e.g. primary visual cortex, V1; Angelucci & Bressloff, Reference Angelucci and Bressloff2006; Nurminen & Angelucci, Reference Nurminen and Angelucci2014). Previous work suggests that these separate processes include an early stage that is insensitive to the configuration of surrounding stimuli, and a later stage that is more sharply tuned (Webb et al. Reference Webb, Dhruv, Solomon, Tailby and Lennie2005). This early stage produces a baseline level of surround suppression, and may operate within the lateral geniculate nucleus of the thalamus (LGN), or the input layers of V1 (Webb et al. Reference Webb, Dhruv, Solomon, Tailby and Lennie2005). Broadly tuned early suppression may serve as a mechanism for visual gain control (Nurminen & Angelucci, Reference Nurminen and Angelucci2014); recent work in SZ has suggested impaired gain control in this disorder (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Butler et al. Reference Butler, Silverstein and Dakin2008; Phillips & Silverstein, Reference Phillips and Silverstein2013). On the other hand, the later stage evokes stronger suppression for more similar surrounds, consistent with cortical mechanisms that are more strongly selective for visual stimulus features such as orientation. Thus, our observation of weaker suppression among SZ and BP patients across surround configurations appears consistent with a deficit in the putative early stage. This agrees with reduced gain control (Dakin et al. Reference Dakin, Carlin and Hemsley2005; Butler et al. Reference Butler, Silverstein and Dakin2008; Phillips & Silverstein, Reference Phillips and Silverstein2013), and may suggest impaired neural suppression within the LGN or V1.
Abnormal inhibition by the neurotransmitter γ-amino butyric acid (GABA) has been reported in SZ (Lewis et al. Reference Lewis, Hashimoto and Volk2005; Hashimoto et al. Reference Hashimoto, Bazmi, Mirnics, Wu, Sampson and Lewis2008; Yoon et al. Reference Yoon, Maddock, Rokem, Silver, Minzenberg, Ragland and Carter2010; Rokem et al. Reference Rokem, Yoon, Ooms, Maddock, Minzenberg and Silver2011; Kelemen et al. Reference Kelemen, Kiss, Benedek and Kéri2013). One study measured lower GABA concentrations in the visual cortex among SZ v. HC subjects using magnetic resonance spectroscopy, and found that lower GABA correlated with weaker surround suppression (Yoon et al. Reference Yoon, Maddock, Rokem, Silver, Minzenberg, Ragland and Carter2010). If surround suppression deficits do indeed depend on GABA, then our results may point to the unique impairment of a particular subtype of GABA neurons among SZ and BP patients. The role of GABAergic inhibition during surround suppression is not yet fully understood (Ozeki et al. Reference Ozeki, Finn, Schaffer, Miller and Ferster2009). However, recent work indicates that early- and late-stage suppression may involve different subtypes of GABAergic neurons in V1. The activity of parvalbumin-positive (PV+) neurons appears consistent with early untuned suppression, while somatostatin-positive (SOM+) inhibition more closely matches the later sharply tuned component described above (Ma et al. Reference Ma, Liu, Li, Huang, Zhang and Tao2010; Adesnik et al. Reference Adesnik, Bruns, Taniguchi, Huang and Scanziani2012). Thus, our observation of weaker surround suppression across conditions in SZ and BP may suggest a deficit in an early untuned suppression mechanism, which may be consistent with impaired PV+ GABAergic functioning. Including a variety of stimulus conditions designed to probe the neural mechanisms underlying surround suppression (Nurminen & Angelucci, Reference Nurminen and Angelucci2014) may benefit future studies of the role of GABA in visual abnormalities in SZ.
Dakin et al. (Reference Dakin, Carlin and Hemsley2005) reported weaker surround suppression in patients with SZ, but normal suppression among a psychiatric control group, compared with HCs. Diagnoses varied among the 13 psychiatric controls in their study, including BP as well as personality disorder, post-traumatic stress disorder and treatment-resistant mood disorders (the number of subjects with each diagnosis was not provided). Yang et al. (Reference Yang, Tadin, Glasser, Hong, Blake and Park2013a ) also examined surround suppression among a group of 16 BP patients. They observed suppression that was weaker than in HCs but stronger than in SZ patients; however, these group differences were not significant. Studying a larger group of subjects (n = 19) in the current study allowed us to observe a significant deficit in surround suppression among BP subjects that was also significantly attenuated compared with SZ subjects. However, we were not able to examine patients with schizo-affective disorder as a separate group, due to a small sample size (n = 5 following exclusion). Future work may consider whether surround suppression among schizo-affective patients falls on a continuum between SZ and BP.
Reports of impaired visual processing in both SZ and BP are not without precedent; one group found equivalent deficits among SZ and BP subjects in a shine-through Vernier masking task (Chkonia et al. Reference Chkonia, Roinishvili, Reichard, Wurch, Puhlmann, Grimsen, Herzog and Brand2012). This differs from other studies showing normal masking in BP (Goghari & Sponheim, Reference Goghari and Sponheim2008; Sponheim et al. Reference Sponheim, Sass, Noukki and Hegeman2013; Jahshan et al. Reference Jahshan, Wynn, McCleery, Glahn, Altshuler and Green2014), which may reflect differences in Vernier v. object configuration discrimination. Additionally, in a rapid serial visual presentation task (Jahshan et al. Reference Jahshan, Wynn, McCleery, Glahn, Altshuler and Green2014), BP subjects showed better performance than SZ subjects but worse than HCs during letter identification at intervals expected to evoke an attentional blink effect. Our observation of a moderate deficit in surround suppression in BP might reflect an impairment in visual context processing shared among patients with SZ and BP (but see also Chen et al. Reference Chen, Bidwell and Holzman2005; Kéri et al. Reference Kéri, Kelemen, Benedek and Janka2005).
Previous work has also examined visual processing in unaffected relatives of patients to assess how a genetic risk for mental illness might contribute to task performance (Kéri et al. Reference Kéri, Kelemen, Benedek and Janka2001; Must et al. Reference Must, Janka, Benedek and Kéri2004; Chkonia et al. Reference Chkonia, Roinishvili, Makhatadze, Tsverava, Stroux, Neumann, Herzog and Brand2010; Schallmo et al. Reference Schallmo, Sponheim and Olman2013; Sponheim et al. Reference Sponheim, Sass, Noukki and Hegeman2013). We have recently reported normal performance among SZrels during visual contour detection, as well as normal flanker suppression (Schallmo et al. Reference Schallmo, Sponheim and Olman2013); the current study builds upon this work by showing that context processing among SZrels (and BPrels) is also not impaired during contrast perception. Conversely, studies of backward masking have reported impairments among SZrels (Kéri et al. Reference Kéri, Kelemen, Benedek and Janka2001; Must et al. Reference Must, Janka, Benedek and Kéri2004; Chkonia et al. Reference Chkonia, Roinishvili, Makhatadze, Tsverava, Stroux, Neumann, Herzog and Brand2010; Sponheim et al. Reference Sponheim, Sass, Noukki and Hegeman2013). A distinction between temporal and spatial masking may explain the discrepancy between our observation of normal surround suppression among relatives and previously reported impairments in backward masking. Normal performance in SZrels and BPrels suggests that deficient surround suppression reflects the clinical expression of these disorders, rather than marking genetic liability.
Acknowledgements
This work was supported by the National Institute of Health (C.A.O., grant number R21 NS075525; institutional training grant number T32 GM0847); the National Science Foundation (M.P.S., grant number GRF 00006595); the University of Minnesota Graduate School (M.P.S., Doctoral Dissertation Fellowship); and the Veterans Health Administration (S.R.S., grant number CSMRF I01CX000227-01). The authors thank Heidi Weber, Kalia Thao, Katelynn McConnell, Nicolaas VanMeerten and Timothy Lano for assistance during data collection and Cheng Qiu for comments on the manuscript.
Declaration of Interest
None.






