I. INTRODUCTION
Lead is a naturally occurring metal found in the earth’s crust and is a ubiquitous environmental contaminant. The properties of this element, including corrosion resistance, high density, softness, and malleability, have caused it to be used in a wide range of applications such as the production of ammunition, X-ray shielding, solder, and batteries. Historically, lead compounds have been widely used as pigments in paint and as antiknock agents in gasoline. The use in house paint was banned in the U.S. in 1978 and the use as a gasoline additive was phased out over 1973 to 1996. Other applications have also been reduced amid growing concerns over, and mounting evidence of, adverse health effects associated with lead exposure.
The elimination of leaded gasoline in the U.S. is considered a triumph for public health. Blood lead levels (BLLs), which are used to assess recent lead exposure, dropped from an average of 12.8 μg per deciliter (μg/dL) to 2.8 μg/dL between 1976 and 1991 (ATSDR, 1999). Since the banning of lead in soldered food cans and household piping, levels of exposure via food and water have also been greatly reduced. Despite this success, many potentially significant sources of exposure still exist. These include occupations such as smelting and refining, the presence of deteriorating paint and dust in homes built before 1978, contaminated soil, and use of lead-containing ceramics, cosmetics, or traditional medicines.
The recall of vast numbers of contaminated toys beginning in 2007 brought lead back into the public health spotlight. Of the more than 18 million toys recalled, most were imported from China and contained excessive levels of lead in their paint coatings (Weidenhamer, Reference Weidenhamer2009). Contaminated toys are of concern because they are handled frequently by children and exposure from normal hand-to-mouth activity during play can lead to increased BLLs. Children are especially vulnerable to lead toxicity, since their bodies absorb 40% to 50% of ingested lead, while adults absorb less than 15% (Ziegler et al., Reference Ziegler, Edwards and Jensen1978). Contaminated toys, thus, constitute a source of highly preventable lead exposure. The Consumer Product Safety Improvement Act (CPSIA) of 2008 was introduced in direct response to the toy recalls. It requires third party testing of all products intended for use by children including items such as cribs, bibs, and inexpensive metal jewelry, as well as toys. The Act set an initial limit of 600 parts per million (ppm), i.e., 600 mg/kg, by weight for total lead and lead in paint, with further reductions of the threshold planned (110th Congress, 2008).
II. HEALTH EFFECTS AND CLINICAL MEASUREMENTS
Adverse health effects have been associated with lead exposure for centuries. The ancient Romans knew that lead
TABLE I. Lowest observed effect levels (LOEL) of blood lead (BPb) in children. Adapted from ATSDR (1988).
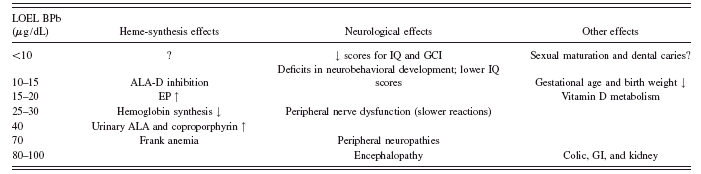
could cause serious illness, madness, and death but did not understand the consequences of chronic low-level exposure from sources such as lead pipes and leaded wine (Lewis, Reference Lewis1985). Lead affects most systems in the body including the central and peripheral nervous systems as well as the renal, cardiovascular, and reproductive systems. The effects of acute exposure can include lead encephalopathy, anemia, peripheral neuropathy, and renal failure. Lower-level chronic exposure is associated with more subtle effects including hypertension, dental caries, osteoporosis, and reproductive dysfunction. Children are particularly susceptible to the adverse effects of lead on the central nervous system because the brain develops rapidly during childhood. Exposures at any time in utero through childhood are associated with behavioral problems and deficits in IQ (ATSDR, 1999). The characteristic effects of lead observed in children, for various BLLs, are listed in Table I.
Blood lead analysis is the preferred method for screening and diagnostic purposes given that a single blood lead measurement reflects exposure over recent months (Parsons et al., Reference Parsons, Geraghty and Verostek2001). Because children are so vulnerable to lead poisoning and are at high risk of exposure, many states require that healthcare providers test all children at 12 months and 24 months of age. This screening schedule is recommended by the Centers for Disease Control and Prevention (CDC), because children’s BLLs increase most rapidly at 6 to 12 months and peak at 18 to 24 months (Centers for Disease Control and Prevention, 1991). The level of concern, or elevated blood lead level (EBLL), i.e., the level indicative of non-normal exposure, is also established by the CDC. The value was initially set at 60 μg/dL in the 1950s and has been reduced successively to the current value of 10 μg/dL set in 1991. Although adverse cognitive outcomes such as lower intelligence and slower development have long been associated with lead exposure, the increasing evidence for these and other health effects at lower BLLs, combined with the availability of improved analytical measurement techniques, caused the threshold to be lowered. Figure 1 depicts the threshold reduction timeline along with the evolution of analytical techniques. The level of concern is often misinterpreted as a threshold below which children are “safe” and above which they are “lead-poisoned.” Research conducted in the past decade appears to indicate that in fact no safe threshold exists.
The analytical instrumentation and methods for the measurement of blood lead have improved significantly over the past several decades. Blood lead concentration was initially determined via a time-consuming colorimetric method that required at least 7 mL of venous blood, a very difficult amount to obtain from a child’s small vein. Delves cup flame atomic absorption spectrometry was introduced in 1970; required a sample volume of less than 100 μL; and was the precursor of graphite furnace (electrothermal atomization) absorption spectrometry (GFAAS), which is commonly used today. Modern GFAAS, which often includes Zeeman background correction for nonspecific absorption, was introduced by the early 1980s; it offers a method detection limit (MDL) of 1 μg/dL, fewer interferences, automation, and limited sample preparation (Parsons and Slavin, Reference Parsons and Slavin1993). Inductively coupled plasma mass spectrometry (ICP-MS) is expensive to conduct but is more sensitive than GFAAS offering an MDL of 0.05 μg/dL for blood lead (Palmer et al., Reference Palmer, Lewis, Geraghty, Barbosa and Parsons2006). It is interesting to note that around 1970, only ten clinical laboratories were certified by New York State to measure the lead in blood. Today, more than 75 laboratories are certified.
Anodic stripping voltammetry (ASV) is an electrochemical method for the determination of blood lead that was introduced in the early 1970s, and benchtop instruments attain detection limits as low as 1.5 μg/dL (Roda et al., Reference Roda, Greenland, Bornschein and Hammond1988). Today, the development of screen-printed electrode technology has resulted in handheld ASV instruments designed for blood lead. Currently, handheld ASV is used primarily for point-of-care screening with the LeadCare and LeadCare II blood lead analyzers. These instruments are portable, require a capillary blood sample of only 50 μL, and produce results in minutes (ESA Magellan Biosciences, 2008). Disposable,

Figure 1. (Color online) Blood lead levels defined as elevated by the CDC and evolution of analytical methods for measurement of lead concentration in blood (1960s to present).
single-use sensors and automatic calibration enable use by nonspecialized laboratory personnel in physicians’ offices or clinics. Many users of this test method participate in proficiency testing (PT) to assess their performance relative to NIST-traceable methods. However, LeadCare II is Clinical Laboratory Improvement Amendment (CLIA)-waived in the U.S. and PT is not generally required.
The trend toward more sensitive and reliable methods, and improved laboratory performance, has enabled the study of toxicological effects at lower BLLs and routine screening at lower defined levels of concern. Early methods, such as the erythrocyte protoporphyrin (EP) test, an indirect assessment for the lead in blood, were replaced with more sensitive, direct blood lead methods, as the blood lead limit was lowered to 25 and then 10 μg/dL (Parsons and Slavin, Reference Parsons and Slavin1993). Thus, the currently acceptable methods are ASV, GFAAS, and ICP-MS.
The lead concentration in body fluids such as blood and urine can also be determined by X-ray fluorescence (XRF). Lead in circulating blood is removed by the kidneys and is excreted in urine. While a urine specimen is easily and noninvasively obtained, it is less reliable than a blood specimen for determining lead exposure due to the biological variability of urine (Parsons et al., Reference Parsons, Geraghty and Verostek2001). A prototype energy dispersive (ED) XRF instrument utilizing doubly curved crystal (DCC) optics to produce an intense highly monochromatic X-ray beam source was recently constructed by X-Ray Optical Systems. The new technology, called high definition spectroscopy (HDS), has been used to develop instrumentation for determining the lead content in body fluids, biological tissues, and consumer products. A small volume (less than 2 mL) of body fluid is measured in a disposable sample cup. The MDL for lead is estimated at 45 μg/L, or 4.5 μg/dL, for a 1600 s measurement. It is anticipated that future instruments will offer MDLs of 5 μg/L in 300 s (Gibson et al., Reference Gibson, Chen and Li2008). Other toxic elements such as mercury and arsenic and bioessential metals including copper, iron, and zinc are potentially measurable as well.
Long-term lead exposure can be assessed by measuring the lead content of bone using XRF. When absorbed by the body, the lead is taken up into soft tissues and into the bone, where it can be stored for decades. The bone compartment contains 90% to 95% of the body’s lead burden and the measurement of lead in the bone is thus indicative of long-term or cumulative exposure (Schroeder and Tipton, Reference Schroeder and Tipton1968). K-shell XRF (or L-shell to a lesser degree) has been used to measure lead in vivo, often in the tibia. The most widely used configuration includes a C109d excitation source with a high-purity germanium detector arranged in a backscatter geometry, with the subject’s lower leg immobilized at a short distance. The XRF measurements are noninvasive; take less than half an hour; and expose the subject to less radiation than does a typical chest X-ray. However, this technique is currently used at only a few universities and research centers internationally and has suffered from a lack of well-characterized reference materials for calibration. An interlaboratory study was recently conducted to assess the agreement among KXRF laboratories (Parsons et al., Reference Parsons, Bellis, Hetter, Geraghty, Berglind, Ginde, Mata and Todd2008).
III. NEW EVIDENCE
Cognitive function, often measured as IQ or general cognitive index (GCI), has been widely studied in relation to postnatal lead exposure. Results from a study using data from the Third National Health and Nutrition Examination Survey (NHANES III) indicated an inverse relationship between the blood lead concentration and math and reading scores for children with BLLs less than 5 μg/dL (Lanphear et al., Reference Lanphear, Dietrich, Auinger and Cox2000). A prospective cohort study measuring IQ at 3 and 5 years of age reported a significant inverse relationship with the BLL and a 7.4 point reduction in IQ for a lifetime average BLL up to 10 μg/dL (Canfield et al., Reference Canfield, Henderson, Cory-Slechta, Cox, Jusko and Lanphear2003). Across all BLLs measured in the study, an increase of 1 μg/dL was associated with a 0.46 point loss in IQ, while for children with BLLs below 10 μg/dL, a 1.37 point loss was estimated (Canfield et al., Reference Canfield, Henderson, Cory-Slechta, Cox, Jusko and Lanphear2003). Similar results, also indicating an inverse nonlinear relationship between the BLL and IQ, were reported by Bellinger and Needleman (Reference Bellinger and Needleman2003). In light of the evidence for an association between BLLs below 10 or even 5 μg/dL and deficits in cognitive function, some public health experts have called for a reduction in the current level of concern to 5 μg/dL or below (Lanphear et al., Reference Lanphear, Dietrich, Auinger and Cox2000).
The CDC’s Fifth Edition of Preventing Lead Poisoning in Young Children included A Review of Evidence of Adverse Health Effects Associated with Blood Lead Levels <10 μg/dL in Children, which was prepared by a workgroup of the Advisory Committee on Lead Poisoning Prevention (Centers for Disease Control and Prevention, 2005). The group set out to determine whether the available evidence supports negative associations between health outcomes and BLLs <10 μg/dL, and whether the associations, if found, represent a causal effect of lead on health (Centers for Disease Control and Prevention, 2005). An exhaustive literature search was conducted for studies, published in English between 1990 and 2003, in which BLL, as measured by GFAAS or ASV, was found to be associated with IQ, GCI, cognitive function, other neurobehavioral measures, visual function, neurotransmitter metabolite levels, growth, sexual maturation, dental caries, blood pressure, renal function, or heme-synthesis biomarkers. Fifty relevant articles representing both longitudinal cohort and cross sectional study designs, including those studies cited above, were selected for review.
The workgroup concluded that available evidence supports an inverse association between the BLL and cognitive function in children and that the dose-response curve has a steeper slope at lower BLLs (Centers for Disease Control and Prevention, 2005). Despite the limited number of studies investigating BLL associations with other health outcomes, the workgroup was also able to conclude that consistent associations exist between BLLs <10 μg/dL and indicators of poorer health (Centers for Disease Control and Prevention, 2005). Additionally, the observed associations between higher BLLs in the range <10 μg/dL and cognitive function were believed to be caused, at least in part, by lead toxicity (Centers for Disease Control and Prevention, 2005). The existence of a definitive causal relationship could not be established by the workgroup, due to the uncertainty associated with potential residual confounding by socioeconomic factors, which are known to affect lead exposure and health outcomes.

Figure 2. (Color online) Evolution of CPSIA lead content regulations for children’s products (courtesy of X-Ray Optical Systems, Inc.).
In 2005, the CDC decided to maintain the level of concern at ≥10 μg/dL citing practical reasons. First, the small sample size of the available data precluded the determination of the true magnitude of the IQ effect. Second, because no threshold of effect has been established, the selection of a lower level of concern would be arbitrary and would provide a false sense of safety for BLLs below the level of concern (Centers for Disease Control and Prevention, 2005). Third, the risk of misclassification would increase if the threshold was lowered due to the uncertainty that is associated with laboratory analysis. Misclassification would likely cause confusion and undue stress on the part of parents and would diminish the utility of a single blood lead test.
Current regulatory requirements in the United States allow for a variability of ±4 μg/dL or 10% of the target value, whichever is greater (40% at 10 μg/dL), for blood lead proficiency tests (Parsons et al., Reference Parsons, Geraghty and Verostek2001). Thus, for a 10 μg/dL sample, a range of 6 to 14 μg/dL is considered an acceptable performance and a significant misclassification risk already exists. The contamination of capillary blood specimens from finger puncture techniques produces approximately 4% error with stringent precautions and must also be taken into consideration (Parsons et al., Reference Parsons, Reilly and Esernio-Jenssen1997). Although GFAAS, ICP-MS, and ASV methods can produce results sufficiently accurate and precise to determine BLLs <10 μg/dL, the feasibility of controlling contamination and indeterminate error to an extent sufficient to permit meaningful routine measurements for a level of concern set to <10 μg/dL is questionable. Also, because we lack effective intervention strategies that will further reduce BLLs that are already below 10 μg/dL, there would be little value in labeling these children as “lead-poisoned.” Instead of adopting a lower level of concern, the CDC recommends that efforts be focused on primary prevention, which will benefit any child, regardless of the BLL.
IV. ENVIRONMENTAL MEASUREMENTS
The control of residential lead paint hazards by screening and abatement is the most significant primary prevention strategy employed, but it is only one of many environmental exposure sources which are monitored. The Department of Housing and Urban Development (HUD) limits the lead content on the interior and exterior painted surfaces in dwellings to 1.0 mg/cm2 or 0.5% (5000 ppm) by weight (ATSDR, 1999). The Environmental Protection Agency (EPA) limits the lead in dust wipe samples to 40 μg/ft2 for bare floor and 250 μg/ft2 for window sills (EPA, 2001). Also regulated by the EPA are the lead in drinking water, at 15 μg/L, and the lead in ambient air, at 0.15 μg/m3 averaged over 3 months (ATSDR, 1999). The EPA recommends that the lead in soil not exceed 400 ppm by weight in play areas or 1200 ppm in nonplay areas (EPA, 2001). The Consumer Product Safety Commission (CPSC) previously limited the total lead in a toy to 600 ppm, but this value is currently 300 ppm and will drop to 100 ppm in 2011 (Figure 2). (110th Congress, 2008). The Food and Drug Administration (FDA) sets a variety of limits for the products that it regulates including candy, nutritional supplements, and tableware.
As indicated by the regulations specified above, there is no consensus on an acceptable level of environmental exposure or a unit of measure for lead analysis. For instance, it is acceptable for children to live in a house with paint containing up to 5000 ppm of lead, but the paint on their toys is limited to 90 ppm. The regulations for the lead in paint have largely arisen through practical rather than health considerations (Centers for Disease Control and Prevention, 1991). The limitations of early field-portable XRF analyzers were recognized to be significant. Such instruments had an imposed inconclusive range of 0.4 to 1.6 mg/cm2 in which laboratory testing was mandatory (EPA, 1995). Although the instrumentation has improved such that a laboratory analysis is no longer required, the threshold has not been revised downward. Additionally, the HUD regulations for the lead in paint allow measurement units of either mg/cm2 by XRF, or weight percent (ppm) by chemical methods, because XRF generally cannot give accurate results in parts per million for coatings due to large uncertainties. The results between methods can only be compared if the paint thickness and density are known or estimated, but these parameters are rarely known in advance for most practical purposes. A question remains about the toxicological relevance of lead per unit area measurements and thus about their potential value in application to the analysis of the lead content in toys.
The assessment of the lead content in toys is a difficult problem owing in part to the variability between like samples and to generally small surface areas. Typically, the determination of the lead content in toys is done with laboratory-based wet chemistry techniques. A scalpel or razor blade is used to scrape paint from the product, with care taken not to contaminate the sample with scrapings of the underlying substrate, and the paint sample is then weighed. In the case of an unpainted product, such as a homogeneous plastic toy, sample shavings are collected from the plastic itself. The sample is treated with nitric acid with either a hot block or microwave-assisted heating, and is then filtered and diluted for analysis. The determination of lead can be accomplished by flame atomic absorption spectrometry (FAAS), inductively coupled plasma optical emission spectrometry (ICP-OES), GFAAS, or ICP-MS. The MDLs for FAAS and ICP-OES are in the low ppm (<15 mg/kg) range, while GFAAS and ICP-MS are more sensitive with MDLs in the low μg/kg range. The CPSC’s testing laboratory recommends the use of any of these techniques for the determination of the lead in paint and states that the CPSC standard operating procedure using ICP-OES is sufficient for measurement at the 90 ppm limit (CPSC, 2009).
The primary disadvantages of the above techniques are the need for lengthy digestion steps and the destruction of the toy. Alternative methods requiring little or no sample preparation include laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), laser-induced breakdown spectroscopy (LIBS), and XRF. An early study of LIBS for the determination of the lead in paint reported an MDL on the order of 140 mg/kg making it inadequate for the lower CPSC limits, but recent results indicate that LIBS can be used as a screening method to identify contaminated toys (Marquardt et al., Reference Marquardt, Goode and Angel1996; Godoi et al., Reference Godoi, Santos, Nunes, Leme, Rufini, Agnelli, Trevizan and Krug2009). XRF analysis is rapid and nondestructive, so it has been well received in the face of demanding new testing requirements. Modern portable handheld EDXRF analyzers offer point-and-shoot convenience, results in less than 2 min, and a low cost per analysis. However, portable XRF is a screening method and its use typically requires that toys above the CPSC threshold be reanalyzed by traditional laboratory methods. HDS, a benchtop EDXRF technique, has the unique advantage that the lead content in both paint layers and the substrate can be determined during a 7-min measurement. The paint layer is measured first followed by the measurement of an uncoated portion of the substrate. The MDL for the lead in paint by HDS is in the 8 to 20 mg/kg range depending on the substrate (X-Ray Optical Systems, Inc., 2009).
V. EXPERIMENTAL RESULTS
Although FAAS and ICP-OES are routinely used for the determination of the lead in toys, few studies have attempted to compare the performance of these methods to that of XRF. Agreement between FAAS and XRF analyses has been reported for lead dust wipes and paint chips (Sterling et al., Reference Sterling, Lewis, Luke and Shadel2000; Ashley et al., Reference Ashley, Hunter, Tait, Dozier, Seaman and Berry1998). While some manufacturers of XRF instruments cite a “good agreement” for the lead in toys, few independent studies have been reported. In our laboratory, we have initiated studies to assess the performance of commonly used chemical methods (FAAS and ICP-OES) and XRF methods (handheld and HDS) to determine whether they are fit-for-purpose for the analysis of toys.
Archived toy samples previously tested for lead content by FAAS in 2007 and 2008 were reanalyzed with HDS and handheld XRF. The toy samples included those with plastic, wood, and metal substrates. XRF secondary paint calibration standards (provided by X-Ray Optical Systems) and certified reference material (NIST SRM 2582 powdered lead paint) were analyzed by all methods to assess the accuracy and repeatability. Certified reference material NMIJ CRM 8105a, a homogeneous ABS resin, was also analyzed by both XRF methods. The instruments used in the study included FAAS (Varian SpectraAA-10), ICP-OES (Perkin Elmer Optima 3300XL), benchtop EDXRF (XOS HD-1000), and handheld XRF (Thermo Scientific Niton XLt). (Use of trade names is for identification purposes only and does not imply recommendation or endorsement by the New York State Department of Health). The methods and results were detailed at DXC 2009, as was the procedure for the production of the secondary standards (McIntosh et al., Reference McIntosh, Orsini, Smith, Yang, Aldous and Parsons2009; Verishinin et al., Reference Verishinin, Cusack, Li, Gibson, McIntosh, Parsons, Altkorn and Chan2009). An additional assessment of newer handheld XRF instruments, the Niton XL3t and Innov-x Alpha, was completed at a later time.
Briefly, approximately 50 mg of paint or homogeneous material were scraped from each toy, treated with microwave-assisted heating in 10% v/v nitric acid, filtered and diluted to 12 mL, and the lead content was then determined with FAAS. The same procedure was used for the analysis of secondary paint calibration standards with an additional dilution before the analysis by ICP-OES. NIST SRM 2582 was analyzed for quality control with each batch of toy samples or calibration standards. The analysis by HDS was performed according to the manufacturer’s protocol. However, the XLt was used in bulk sample mode to obtain results in mg/kg units. It should be noted that, while the manufacturer does not recommend that the XLt be used in this mode for painted surfaces, some users will do so regardless. The goal was to determine the extent to which (if any) such a practice is valid. The measurement time for each painted sample was approximately 7 min (4 min on the coating; 3 min on the substrate) for the HD-1000 and 2 min for the XLt.
An assessment of the agreement between FAAS and the XRF methods for the toy analysis proved difficult due to differences in the sampling of the material. Composite sampling (when multiple components or colors of a toy or toys are combined into a single sample) is common when the chemical methods are used due to insufficient paint on a toy. The result then reflects the average lead concentration in the mixture, and unless each of the components is of known weight, it is impossible to determine the specific concentration per component. Figure 3 depicts such an example in which a composite sample of multiple paint colors from a set of action figures was analyzed by FAAS and the individual paint colors were analyzed with XRF. The latter revealed that the brown paint has an excessively high lead concentration, well above the previous CPSC limit of 600 ppm. However, due to the dilution effect arising from the inclusion of lead-free

Figure 3. (Color online) Measured lead concentrations for various paint colors on plastic substrate (mean±SD; n=5).
colors such as black and dark brown in the composite sample, the result by FAAS is close to the limit. Although this set of toys failed (780 mg/kg by FAAS), it could as easily have passed if the composite sample had combined paints in slightly different proportions. The CPSC test method now includes a note on composite sampling and suggests the use of a “safety factor” to prevent false negative results (CPSC, 2009).
The results for the XRF secondary paint calibration standards better represented the performance of the methods because they were not subject to the sampling issues discussed above. For a standard of 150 mg/kg, mean results (±SD) were 154(10), 144(7), 143(21), and not detected (ND), for five determinations each by FAAS, ICP-OES, HDS, and handheld XRF, respectively. The mean results for the methods other than handheld XRF were found to be in agreement at the 95% confidence level (ANOVA indicating no significant difference F=0.9957; p=0.3980). The inability of handheld XRF to detect lead in paint coatings when used in the bulk sample mode simply reflects the limitations of the algorithms when applied in this manner.
The repeatability and accuracy of the methods were assessed by the measurement of certified reference materials (Table II). Both handheld XRF and HDS performed reasonably well for the homogeneous material (ABS plastic).
Because handheld XRF technology has advanced significantly in the past few years, we performed a limited, preliminary assessment of two newer handheld instruments to determine whether their effective performance for toys has improved. These new analyzers have consumer products and lead paint calibrations. For 30-s measurements of 150 mg/kg XRF secondary paint standards (variable paint thickness and type of plastic substrate), the Alpha reported ND consistently, while the XL3t gave results ND to 1.1 μg/cm2, with ND results occurring for the thinner paint layers. These values can be compared to the alternate limit set by the CPSIA of 2 μg/cm2 lead in small (<1cm2) painted areas (110th Congress, 2008). While the latter instrument was somewhat able to quantify the lead in the paint, the extent to which results reported in μg/cm2 units is protective of children's health is unclear. For a 60-s measurement of CRM 8105a by both instruments, the results were 155 and 121 mg/kg (43% and 12% bias) indicating no major improvement in performance from prior models, although the shorter measurement time should be taken into account.
VI. CONCLUSION
Chronic exposure to lead continues to be a public health problem in the 21st century. Although many sources of exposure have been reduced or eliminated, potentially significant sources, including contaminated consumer products, remain. New evidence suggesting that no safe blood lead level exists for children necessitates that current clinical and environmental thresholds be reexamined. Improvements in analytical methods have enabled an understanding of the adverse health effects at low exposure levels and further
TABLE II. Assessment of repeatability and accuracy via determination of lead concentration (mg/kg) in certified reference materials (n=10).

improvements will be required to select and implement new lower thresholds for environmental exposure sources. The development of practice standards and appropriate certified reference materials will better facilitate the accurate, repeatable determination of lead in consumer products by XRF.
ACKNOWLEDGMENTS
The authors thank X-Ray Optical Systems (XOS) for the loan of instrumentation and XRF secondary paint calibration standards and also for graduate student support (K.M.). The authors are particularly grateful to Dr. Walter M. Gibson of XOS for guidance and helpful discussions and to the analytical staff from the Laboratory of Inorganic and Nuclear Chemistry at the Wadsworth Center.







