Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder associated with persistent deficits in social communication, and the presence of restrictive, repetitive patterns of behavior, interests, or activities that often persists throughout life. 1 In patients with ASD, psychiatric practitioners encounter many ASD-associated symptoms that are clinically challenging to treat. Such untreated or poorly treated or refractory behavioral problems hinder learning and socialization and further worsen treatment outcomes for these patients. Some of these ASD-associated symptoms and comorbid psychiatric disorder are irritability, anxiety, aggression, self-injurious behaviors (SIBs), hyperactivity, and sensory integration difficulties. The prevalence of ASD has been reported to be increasing; however, apparent increase has generated controversy as well, which is mainly the issues surrounding the heterogeneity in its symptom presentation, lack of biologic diagnostic markers, and the changing diagnostic criteria.Reference Rice, Baio, Van Naarden, Doernberg, Meaney and Kirby 2 In the latest data published by the US Centers for Disease Control (CDC) that tracked its prevalence in 11 US communities showed a 15% increase nationally. 3 According to this CDC estimate, 1.7% (1 in 59) children suffered from ASD as compared to 1.5% (1 in 68) children in 2016. As per this trend, it is possible by the time of publication of this article that this prevalence rate will further increase. At a minimum, these trends suggest that in future, there will be an even higher number of children and adolescents with ASD presenting to healthcare facilities seeking help to manage ASD and these troublesome ASD-associated symptoms and comorbid psychiatric conditions. The most common associated symptoms with ASD is chronic irritability.Reference Brereton, Tonge and Einfeld 4 The aggressive behaviors range from 15% to 18%Reference Matson and Rivet 5 to 56% in one large sample.Reference Kanne and Mazurek 6 Several studies report 30% of children with ASD will demonstrate self-injurious behaviors.Reference Soke, Rosenberg and Hamman 7 Forty percent will have at least one stereotypical behavior which is part of core symptom of ASD.Reference Matson, Dempsey and Fodstad 8 Other comorbid psychiatric condition often requiring treatment with ASD are the symptoms of attention deficit and hyperactivity disorder (ADHD), that is hyperactivity, inattention, and impulsivity which are seen in 30% to 50% of individuals with ASD.Reference Leitner 9 Clinicians use an array of different treatment approaches including pharmacological as well as behavioral to alleviate these ASD-associated symptoms and comorbid psychiatric conditions. Unfortunately, patients with ASD are often sensitive to side effects of the psychotropics, thus adding to the common frustration among the treating practitioners to whom finding the optimal medication choice(s) too often proves elusive and is matter of much trial and error.Reference Molteni, Nobile, Cattaneo, Radice and Clementi 10
Pharmacological Options of Treatment of ASD, Comorbid Psychiatric Disorders and ASD-Associated Symptoms
Antipsychotics are a versatile pharmacologic family of medications used to help treat irritability and aggression ASD-associated symptoms,Reference Fung, Mahajan and Nozzolillo 11 , Reference Barnard, Young, Pearson, Geddes and O’Brien 12 and there is mixed evidence for utilization of the serotonin reuptake inhibitors (SSRIs) in treating anxiety symptoms comorbid in ASD.Reference Vasa, Carroll and Nozzolillo 13 , Reference Steingard, Zimnitzky, Demaso, Bauman and Bucci 14 However, sometimes side effects from these medication classes can pose clinical challenges such as behavioral activation from the SSRIs, and metabolic syndrome, dyskinesias, and arrhythmias from antipsychotics such as risperidone and aripiprazole.Reference McPheeters, Warren and Sathe 15 Therefore, it is important to have additional pharmacologic options in the armory of the clinicians treating the patients with ASD. The aim of this review is to evaluate the evidence available for use of selective serotonin and norepinephrine reuptake inhibitors (SNRIs) in the treatment of the core symptoms, associated symptoms, and comorbid conditions of ASD. To our knowledge, there was one review article which solely looked at venlafaxine. This review article will look into the efficacy of medications in the SNRI group for treating core symptoms of ASD, ASD-associated symptoms, and comorbid psychiatric disorder.
SNRIs, as the name suggests, are a class of dual neurotransmitter reuptake inhibitors and include its prototype venlafaxine in addition to desvenlafaxine, duloxetine, milnacipran, and levomilnacipran. SNRIs have shown promising benefits in the treatment of mood and anxiety disorders as well as other neuropsychiatric disorders and pain conditions. Venlafaxine has been available on the US market since 1993 and is primarily used to treat major depressive disorder (MDD), generalized anxiety disorder (GAD), panic disorder (PD), and social phobia (SP).Reference Schatzberg 16 Since 2004, duloxetine has been primarily used to treat MDD, GAD, fibromyalgia, chronic musculoskeletal pain, and diabetic peripheral neuropathy.Reference Dhaliwal and Molla 17 Milnacipran has been used to treat fibromyalgiaReference English, Rey and Rufin 18 with off label use in MDD, chronic fatigue, and anxiety disorders.Reference Pae, Marks and Shah 19 Although it is an SNRI, milnacipran shows mild inhibiting action on the N-methyl-d-aspartate (NMDA) receptor,Reference English, Rey and Rufin 18 a glutamate receptor implicated in synaptic plasticity and memory function.Reference Li and Tsien 20 Tricyclic antidepressants or tricyclic antidepressants, which act on noradrenergic and serotonergic systems similarly to SNRIs, have shown benefits in improving irritability and obsessive–compulsive symptoms, but their use has been limited by significant side effects.Reference Hurwitz, Blackmore, Hazell, Williams and Woolfenden 21 Similarly, reboxetine is a norepinephrine reuptake inhibitor whose use may be helpful in improving depressive and ADHD symptoms comorbid with ASD, but requires close monitoring due to increased adverse events (such as insomnia and gastrointestinal side effects). Using mouse modeling to assess extracellular monoamine levels in the mouse prefrontal cortex and striatum, researchers have shown that use of acute and chronic venlafaxine dosing and also chronic desipramine administration maximally activated the prefrontal adrenergic and dopaminergic systems without affecting striatal dopaminergic systems.Reference Higashino, Ago and Umehara 22 This is due to inhibition of noradrenaline transporter-mediated dopamine uptake in prefrontal cortex without affecting striatal dopaminergic system (so no abuse potential). ADHD symptoms are commonly comorbid with ASD,Reference Leitner 9 and the above animal model data suggests that SNRIs like venlafaxine might potentially be effective in the treatment of ADHD symptoms in persons with ASD.
Neurobiological Rationale for the Use of an SNRI in ASD, ASD-Associated Symptoms, and Comorbid Psychiatric Disorder
Sensory integration (SI) difficulties and anxiety, while two different symptom clusters, show overlap in the neurobiological underpinnings of their disruptive roles in ASD. In ASD, SI and anxiety are associated with hyperactivityReference Green, Rudie and Colich 23 of the primary sensory processing areas, limbic areas (emotional response), orbital frontal cortex (OFC) (emotional processing), amygdala (fear conditioning, detection, and response to threats),Reference Zald 24 and hippocampus (encoding and strengthening memories of aversive events).Reference Stein, Wiedholz and Bassett 25 Similar observations were made in obsessive–compulsive disorder (OCD), initially postulated to result from disruptions to the cortico–basal ganglia–thalamo–cortical (CBGTC) loops,Reference Maia, Cooney and Peterson 26 but more recent evidence points to critical involvement of the lateral and medial orbitofrontal cortices, the dorsal anterior cingulate cortex (cognitive conflict, and error monitoring and detection), and amygdalo–cortical circuitry.Reference Milad and Rauch 27 In general, increased prefrontal activation is associated with decreased amygdala activation in response to threat-relevant stimuli.Reference Wright, Albarracin, Brown, Li, He and Liu 28 In ASD, concurrent hyperactivity in the amygdala and OFC are indicative of a poor regulatory system, whereby the OFC activates, but then fails to sufficiently downregulate, the amygdala.Reference Green, Hernandez, Tottenham, Krasileva, Bookheimer and Dapretto 29 , Reference Sladky, Höflich and Küblböck 30 Serotonin, which is involved in top-down control of limbic areas and neurocircuitry involved in fear,Reference Miller, Hodzic and Weintraub 31 facilitates OFC and anterior cingulate cortex that are involved in modulating serotonin 5-HT2 receptors in these regions.Reference Siever 32 Interestingly, aggression and agitation are associated with reduced levels of serotonin in these regions.Reference Miller, Hodzic and Weintraub 31 Sleep, attention, and sensory perception is consistent with the known functioning of the locus coeruleus and norepinephrine (LC/NE). In ASD, altered sensory perception is implicated, in which multiple faulty perceptions are distributed in all sensory modalities. Restricted repetitive behavior is a derivative of poor adaptive behavior and is thought to be primarily mediated by the LC/NE system. In ASD, there is a failure to adequately regulate the functioning of the central neural circuitry.Reference London 33 Noradrenergic input originating in the LC functions both as activating and deactivating of central neural circuits. Clonidine, a central presynaptic alpha-2-receptor agonist that regulates noradrenergic release, helps to modulate sleep and behavior disturbances in ASD.Reference Ming, Gordon, Kang and Wagner 34 Dysfunctions of the dopaminergic mesocorticolimbic circuit leads to executive function deficits and social deficits, while dysfunction of the nigrostriatal circuit can explain the stereotypical behaviors of ASD.Reference Pavăl 35 , Reference Kriete and Noelle 36 All SNRIs act directly on the serotonergic and noradrenergic systems, and also the dopamine system, either directly or indirectly.
All SNRIs inhibit reuptake of serotonin and norepinephrine and to a lesser extent, dopamine as well.Reference Ellingrod and Perry 37 Venlafaxine and its metabolite desvenlafaxine selectively inhibit serotonin (5-HT) reuptake at low doses, whereas at high doses, they inhibit both serotonin and NE reuptake.Reference Baxter, Charlson, Cheng, Shidhaye, Ferrari and Whiteford 38 SNRIs are tolerated well similarly to SSRIs in the pediatric population. A recent systematic review and meta-analysis involving 36 randomized controlled studies concludes that there is no significant difference in side effects found between the class of SSRIs and SNRIs in children and adolescents.Reference Locher, Koechlin and Zion 39
Methodology
An extensive literature search was done using EBSCO.host (database in Appendix 1) for publications from 1991 up to October 2019 with no restrictions as to the language of publication (languages other than English that were adequately translatable using Google translate were included). We employed the following search terms in this literature search: “Autism” or “Autism spectrum disorder” or “ASD” or “PDD” or “Pervasive developmental disorder” or “Asperger” combined with “SNRI” or “venlafaxine” or “desvenlafaxine” or “duloxetine” or “milnacipran” or “levomilnacipran.” Due to the low number of the research studies obtained via this search algorithm, a broad inclusion criterion was set where all types of studies in which any of the four SNRIs used to treat either ASD, ASD-associated symptoms, and comorbid psychiatric disorder. This search yielded 130 articles, in which 31 were duplicates, so the remaining 99 publications were screened for relevance. Out of these 99 publications, 93 were excluded after assessing titles and abstracts. The search-generated articles such as SNRI in suicide, MDD, ADHD, pregnancy, anxiety disorder, and so on, which had no relevance current review and were excluded. Also, studies in which SNRIs were not used for the purpose of treating ASD, ASD-associated symptoms, and comorbid psychiatric disorder were excluded.
Two of the authors (S.M.T. and M.N.) then assessed full-text versions of the remaining six articles (one study results were obtained from the published data on clincaltrial.gov) which were deemed appropriate to the current review. Of these six articles, there were three articles on venlafaxine, two articles on milnacipran, and one article on duloxetine. All of these six studies used DSM-IV criteria to diagnose ASD. No articles were found on desvenlafaxine or levomilnacipran. Each research publication was then independently critically appraised by two authors (M.N. and S.M.T.). Instruments including the Clinical Global Impression (CGI) Scale, GAF (Global Assessment of Functioning), Conner’s Adult ADHD Rating Scale (CAARS), Yale-Brown Obsessive Compulsive Scale (YBOCS), Aberrant Behavior Checklist (ABC), and Diagnostic Analysis of Nonverbal Accuracy (DANVA) were cited in these articles. Evidence derived from these six papers is summarized in this review and based on the evidence presented in these articles, conclusions are stated and discussed.

Results
Venlafaxine
The effective antidepressant dose of venlafaxine usually ranges from 75 to 300 mg/day. The retrospective clinical report on venlafaxine use in ASD with a pilot of 10 subjects (9 male and 1 female) was published by Hollander et al.Reference Hollander, Kaplan, Cartwright and Reichman 40 In this report, a flexible dosing range of venlafaxine from 6.25 to 50 mg/day was used. Eight of the subjects in this study were children or adolescents and two were adults and the mean age of the study group was 10.46 years. Patient age ranged from 3 to 21 years and the mean length of treatment was 4.77 ± 2.46 months. The mean final dose in mg was 24.37 ± 14.86 mg/day. Mean Clinical Global Impression (CGI) improvement was 2.15 ± 1.03. Six of 10 patients were rated as sustained responders, with CGI final improvement score of “much improved” (2) to “very much improved” (1). The other four patients were rated as nonresponders. The authors claimed that symptoms in all three DSM-IV core symptom categories of ASD improved on the SNRI medication: deficits in social communication, social interaction, and the presence of restricted, repetitive patterns of behavior. Also, comorbid ADHD symptoms improved.
Carminati et alReference Carminati, Deriaz and Bertschy 41 published a case series of three adolescents with ASD with follow up at 6, 18, and 36 months, in which patients were treated with a low dose of venlafaxine (18.75 mg). All three patients were on other concurrent medications including the antipsychotic zuclopenthixol with poor response. However, with addition of venlafaxine at low dose, all three patients demonstrated improvements within the first 3 weeks of treatment as ascertained by CGI score of 1 (very much improved) or 2 (much improved). All three patients exhibited significant improvements in frustration tolerance, self-injurious behaviors, aggression, and hyperactivity, but only fair improvement in social communication, and restrictive behaviors.Reference Carminati, Deriaz and Bertschy 41
Carminati et al,Reference Carminati, Gerber and Darbellay 42 conducted a randomized double-blind placebo-controlled trial to assess the efficacy of venlafaxine at a dose of 18.75 mg/day on the reduction of behavioral problems in ASD in intellectually impaired patients.Reference Carminati, Deriaz and Bertschy 41 This study compared two treatment groups: those taking zuclopenthixol and/or clonazepam and 18.75 mg venlafaxine (six patients) vs those taking zuclopenthixol and/or clonazepam plus placebo (seven patients). Inclusion criteria included patients with Aberrant Behavior Checklist (ABC) factor 1 (irritability and restlessness) score ≥18 and/or a factor 4 (hyperactivity/noncompliance) score ≥15. Patients who received venlafaxine and not the placebo less antipsychotics and sedative treatments showed overall clinical improvements at 2 and 8 weeks. This study found that irritability improved in the entire sample after 2 weeks (P = .023) and trended toward significance by the study endpoint (P = .061). The ADI-R (Autism Diagnostic Interview-Revised, for use in children and adults) scores did not differ significantly for social interaction (P = .65) or communication (P = .76). No significant reduction in hyperactivity or nonadherence was noted between the groups.Reference Carminati, Gerber and Darbellay 42
Duloxetine
The effective antidepressant dose of duloxetine usually ranges from 60 to 120 mg/day. NielderhoferReference Nielderhofer 43 presented a case series assessing duloxetine use for reduction of comorbid behavioral symptoms in ASD. In this series, two patients were treated with duloxetine 40 mg for 10 weeks. These patients had previously failed other medications as defined by lack of treatment response or significant side effects requiring stoppage of treatment as per the authors. The behaviors were measured on the ABC, and included: irritability (score before treatment of 13.8 vs after treatment of 11.8); hyperactivity (score before treatment of 19.7 vs after treatment of 16.4); inadequate eye contact (score before treatment of 8.3 vs after treatment of 7.8); and inappropriate speech (score before treatment of 6.2 vs after treatment of 4.3). The symptom checklist scores were as follows: drowsiness (before treatment score of 1.6 vs after treatment score of 3.5) and decreased activity (before treatment score of 2.8 vs after treatment score of 3.7). No severe side effects were noted. The study concluded that the efficacy of duloxetine does not exceed that of other antidepressants in alleviating these target symptoms.Reference Nielderhofer 43
Milnacipran
Like venlafaxine, the effective antidepressant dose of milnacipran usually ranges from 150 to 300 mg/day. An open label prospective study conducted in Japan by Mashiko et alReference Mashiko, Ishikawa and Itagaki 44 in 15 adults meeting DSM-IV criteria for Asperger disorder and ADHD symptoms were treated with milnacipran for 12 weeks. There were 7 men and 8 women with ages ranging from 19 to 49 years. Social function was assessed by GAF scores and ADHD symptoms were assessed with Conners’ Adult ADHD Rating Scales-Objective-Screening Version (CAARS-O-SV) before and 12 weeks after milnacipran therapy. Out of 15 patients, 5 received milnacipran monotherapy and 10 received combined milnacipran and methylphenidate therapy. The mean ± standard deviation (SD) doses of milnacipran and methylphenidate were 103.67 ± 58.17 mg/day (range, 15-225 mg/day; N = 15) and 16.53 ± 14.55 mg/day (range 8-40 mg/day; N = 10), respectively. The GAF score (mean ± SD) in the 15 patients improved significantly from 44.00 ± 8.49 before therapy (N = 15) to 65.00 ± 9.26 after milnacipran therapy (F = 33.89, df = 1, P < .001). CAARS-O-SV for inattention before milnacipran treatment was 73.33 ± 13.11 and after milnacipran treatment was 52.27 ± 7.97). There was no significant change in hyperactivity and impulsiveness symptoms combined (before milnacipran treatment 42.87 ± 8.47 vs after milnacipran therapy 41.26 ± 6.13).Reference Mashiko, Ishikawa and Itagaki 44
Hollander et alReference Hollander, Kaplan, Cartwright and Reichman 40 investigated milnacipran effects on several domains of symptoms comorbid with ASD. Final data of the study were published in February 2020 in clinicaltrials.gov. 45 In their 12-week randomized, double-blind placebo-controlled study, they administered up to 200 mg of milnacipran and 100 mg of placebo with parallel assignment in 10 subjects aged 18 to 65 years old with ASD. Medication and placebo were administered at intervals of 2, 4, 6, 8, 10, and 12 weeks. They measured the following primary outcomes: (1) change in CAARS scores converted to T Score and (2) change in ABC-Hyperactivity sub component. They also measured the following secondary out comes: (1) change in Autism severity on CGI scale, (2) change in repetitive behaviors of ASD using YBOCS-compulsion and rigidity subscale, and (3) analysis of nonverbal activity on DAVVA2.
Average age of the patient was 25.10 years. There was one patient who was less than 18 years old but all others were adults. There were three women and seven men in the study. All patients were Caucasian race. All 10 of them completed the study. On CAARS: (1) in inattention subscale, the change was from 68.6 to 53.4 in active medication group and from 71.8 to 57 in placebo group. (2) Hyperactivity and restlessness subscale change was 55 to 44.8 in medication group and 50.8 to 39.8 in placebo group. (3) On impulsivity subscale, change was 5.2 to 47.8 in medication group and 49.2 to 43.6 in placebo group. On ABC-Hyperactivity scale, change in scores from baseline to 12 weeks was from 19 to −10.4 in active medication group and from 9.8 to −4.2 in placebo group.
Secondary outcome measures were as follows: On CGI, minimal or no change was seen in five patients in active medication group and similar results noted in placebo group although one patient seemed to get worse overall. On CYBOCS change from baseline to 12 weeks was from 12.2 to 12.4 in active medication group and no change in placebo group. On DANVA2-AF, change from baseline to 12 weeks was from 16.5 to 18 in active medication group and from 18.5 to 18.8 in placebo group. Improvement was noted in inattention and hyperactivity subscales. However, no change was noted in impulsivity, stereotypy, emotional lability, social withdrawal, or inappropriate speech subscales.
Discussion
Associated symptoms and comorbid psychiatric disorder are common and frequent in children with autism spectrum disorders.Reference Simonoff, Pickles, Charman, Chandler, Loucas and Baird 46 Many different medications from different medication classes are shown to be effective in treating some of the associated symptoms and comorbid psychiatric conditions with ASD. A report based on U.S. studies as part of the Autism Registry Project of the Mental Health Research Network (MHRN) documented that psychotropic medications were more prevalent among insured children with ASD. The most common classes were stimulants, alpha-agonists or atomoxetine (30.2%), antipsychotics (20.5%), and antidepressants (17.8%).Reference Madden, Lakoma and Lynch 47 , Reference Golubchik, Sever and Weizman 48 A systematic review of seven studies (of which, two are large-sample studies of citalopram and fluoxetine) that examined the effectiveness of SSRIs in ASD patients did not find any therapeutic benefits for this class of medication for social communicational symptoms of ASD, but found evidence for benefits in treatment of repetitive behaviors associated with ASD.Reference Williams, Wheeler, Silove and Hazell 49 Interestingly, sulforaphane, an isothiocyanate naturally abundant in broccoli, which upregulates genes that protect aerobic cells against oxidative stress, inflammation, and DNA-damage (all of which are prominent and possibly mechanistic characteristics of ASD) demonstrated in a double-blind randomized trial, significant improvement in the behavioral symptoms of ASD.Reference Singh, Connors and Macklin 50 At present, aripiprazole and risperidone have good evidence for the treatment of irritability in ASD, and their uses for this are approved by the FDA.Reference Stepanova, Dowling, Phelps and Findling 51 Logically, it is important to investigate and expand alternative medications to manage the challenging associated symptoms, and comorbid psychiatric disorder in ASD. Risperidone and fluvoxamine provide fair evidence for treating challenging behaviors such as repetitive self-injurious or aggressive behavior and risperidone was the only medication with multiple trials showing its efficacy as elaborated in a review of its overall effectiveness with irritability and aggression. However, as is well known, risperidone, a second generation antipsychotic, has the notable serious side-effect profile of metabolic syndrome with long-term use.Reference Calarge, Acion, Kuperman, Tansey and Schlechte 52 In contrast, sleep problems can be fairly well treated with benign medications like melatonin although unlike risperidone it is not efficacious for treating aggression or sensory integration difficulties.Reference Ward, Nanjappa, Hinder and Roy 53 Sertraline has been shown to be effective in the treatment of anxiety associated with transitions and agitation in the ASD population.Reference Steingard, Zimnitzky, Demaso, Bauman and Bucci 14 Duloxetine shows good effectiveness controlling anxiety symptoms and it is the only medication approved for pediatric GAD.Reference Strawn, Prakash and Zhang 54 Having more medication options to treat safely is clinically desirable. However, the number of randomized controlled trials (RCT) available to evaluate the evidence for use of SNRIs in ASD and comorbidities is quite limited (Tables 1 and 2 summarize these data). Carrying out RCTs in children presents ongoing challenges due to recruitment problems that in turn create limited sample sizes with limited statistical power. However, it is surprising that to date, there remain inadequate numbers of RCTs on SNRIs used in adults with ASD. To generate robust evidence, it is important that more RCTs with large sample size with adequate statistical power be carried out.
Table 1. Summary of Six Reports on SNRI Use in ASD, ASD-Associated Symptoms, and Comorbid Psychiatric Disorder.

Abbreviations: A, adult; ABC, Aberrant Behavior Checklist; C, child; CAARS, Conners’ Adult ADHD Rating Scales-Objective-Screening Version (CAARS-O-SV); CGI, Clinical Global Impression; CS, case series; DSM, Diagnostic and Statistical Manual/edition; F, females; GAF, Global Assessment of Functioning; ICD, The World Health Organization International Statistical Classification of Diseases and Related Health Problems; ID, intellectual disability; M, males; N, neuroleptic; RCT, randomized controlled trial; RDBPCT, randomized double blind placebo-controlled trial; S, stimulant.
Table 2. Summary of Six Reports on SNRI Use in ASD, ASD-Associated Symptoms, and Comorbid Psychiatric Disorder: Instruments and Specific Symptom Response.
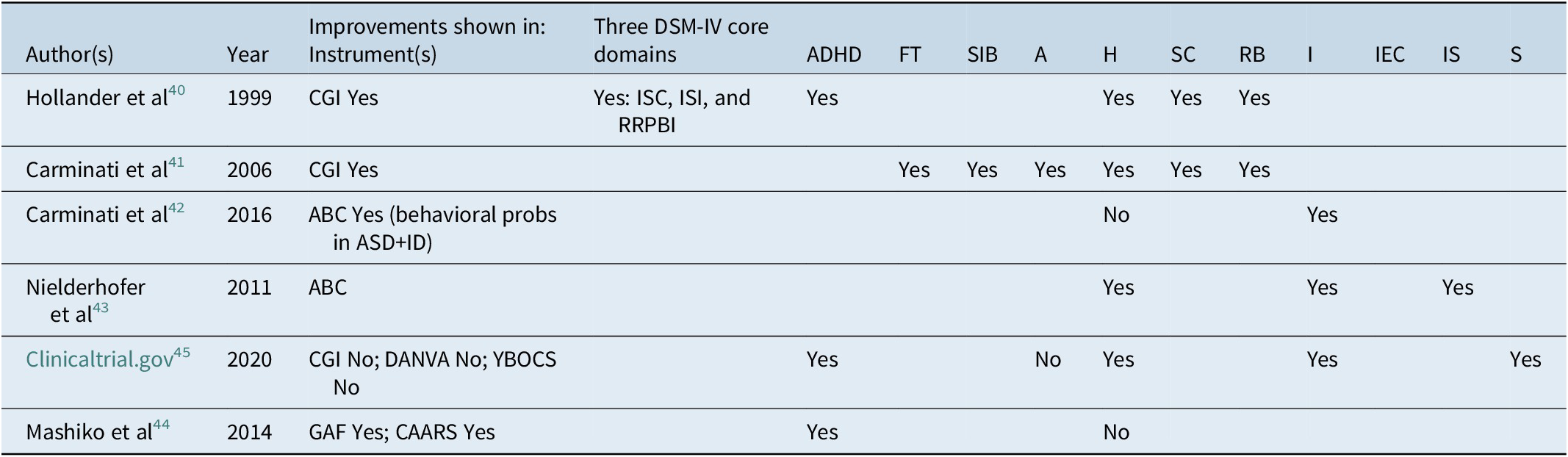
Abbreviations: A, aggression; ABC, Aberrant Behavior Checklist; ADHD, attention deficit hyperactivity disorder; CAARS, Conners’ Adult ADHD Rating Scales-Objective-Screening Version (CAARS-O-SV); CGI, clinical global impression; DANVA, Diagnostic Analysis of Nonverbal Accuracy; FT, frustration tolerance; GAF, Global Assessment of Functioning; H, hyperactivity; I, irritability; IC, impaired social communication; ID, intellectual disability; IEC, inadequate eye contact; IS, inappropriate speech; ISI, impaired social interaction; RB, restrictive behaviors; RRPBI, restrictive and repetitive patterns of behavior and interests; S, stereotypy; SC, social communication; SIB, self-injurious behaviors; YBOCS, Yale Brown Obsessive Compulsive Scale.
In the above studies, Caraminati et alReference Carminati, Gerber and Darbellay 42 conducted a RCT to further evaluate their observations from their prior three-case series and found that venlafaxine at 18.75 mg/day was effective in treating the symptoms of self-injurious behavior (SIB) and ADHD symptoms in partially-respondent adult patients with ASD and intellectual deficits who had been treated with antipsychotics. The authors concluded that the addition of venlafaxine to an antipsychotic at the most demonstrated low dose could be useful in ASD patients with partial response to antipsychotics for both SIB and ADHD symptoms, as well as for at least some other associated symptoms. These findings, although examined so far in limited numbers of patients, supported the earlier observations on effects of venlafaxine in children and adults (age range 3-21 years) by Hollander et al.Reference Hollander, Kaplan, Cartwright and Reichman 40 However, authors of this review reiterate that it is unwise to generalize or overstate the results from a small case series in which patients were already on antipsychotic medications. Noone et alReference Noone, Taylor, Racine and Hollander 55 hypothesized that the reports of improvement in symptoms of autism during fever episodes in children was related to temporary correction in the dysfunction of the locus coeruleus/noradrenergic system. Additionally, they suggested that the newer SNRI milnacipran might reactivate this system and help with symptoms of attention, irritability, repetitive behaviors, and social cognition in adults.Reference Noone, Taylor, Racine and Hollander 55. Mashiko et alReference Mashiko, Ishikawa and Itagaki 44 replicated the above results in a study of 15 Asperger’s patients with comorbid ADHD (in a Japanese sample) who were treated with milnacipran up to 200 mg/day, but they found no statistically significant improvement in hyperactivity or impulsivity. Mashiko et alReference Mashiko, Ishikawa and Itagaki 44 also noted significant improvements in inattention and overall functioning on the GAF. The authors concluded that improvement in social functioning in patients having ADHD symptoms associated with Asperger’s disorder was mediated through improvement of inattention.
These studies have smaller sample sizes, and patients in these studies were noted to be using other psychotropic medication including benzodiazepines or had a history of multiple medication trials in the past. To ascertain the effects and benefits of SNRIs in the treatment of ASD, associated symptoms, and comorbid psychiatric disorder, we need studies with larger sample sizes, ideally in psychotropic-naïve patients, that is, using only SNRI medications to treat these symptoms compared to controls. However, it is difficult to achieve ideal research parameters in studies of patients with ASD due to clinical symptoms and problems in multiple domains that often require different classes of medications. The reports that we reviewed included a mix of case series and pilot studies but three of the reports were RCTs, albeit with limited sample sizes. Given these limitations, a retrospective study can give clinical hints as to pending findings of a future prospective study. In this review, we note that the study by Carminati et alReference Carminati, Gerber and Darbellay 42 is a randomized double-blind placebo-controlled trial, as are the studies by Noone et alReference Noone, Taylor, Racine and Hollander 55 and Mashiko et alReference Mashiko, Ishikawa and Itagaki 44 although with small sample size. Hence, well-designed RCTs with large sample sizes and greater statistical power are needed in the future to validate robust conclusions. Until those studies are achieved, based on current available evidence discussed in this review, we can state the following conclusions.
Conclusions
Presently and at best, the medication venlafaxine is useful in low doses as an adjuvant agent in the treatment of SIB, aggression, and ADHD symptoms in ASD patients. It may also enable lower effective dosing of antipsychotic medications (notably SGAs with added variable anxiolytic, mood stabilizing, and serotonergic potentiating properties) that generally trend toward producing more side effects including metabolic syndrome, which can pose a significant and chronic health hazard. There is no evidence for the use of venlafaxine as a single agent in the treatment of these conditions associated and comorbid with ASD. There are no studies or reports of use of the levo isomer desvenlafaxine for treating conditions associated and comorbid with ASD. Given increasing use in at least the adult population, some clinicians may be increasingly comfortable to extrapolate the results of the study of venlafaxine until adequately powered studies conducted for desvenlafaxine can be carried out.
The case series investigating duloxetine use in ASD does not show any added benefit to treat associated symptoms, and comorbid psychiatric disorder in ASD when compared to other antidepressants. However, the evidence is inadequate to come to any meaningful conclusion about the use of duloxetine in ASD alone.
At least in adults, milnacipran might be, notably given its favorable side-effect profile a useful medication to treat attention, irritability, repetitive behaviors, and social cognition deficits in ASD. One limiting factor on more generalized use and findings regarding this medication, at least in the United States, is lack of approved use of this medication for treatment of MDD. There are neither case reports nor RCTs to report on levomilnacipran use in treating patients with any of the associated symptoms, and comorbid psychiatric disorder discussed in ASD. However, some clinicians again may be increasingly comfortable to extrapolate the results of the study of milnacipran, at least in adults, until adequately powered studies conducted for levomilnacipran can be carried out to add clarity on these points.
Although current pharmacologic evidence is not very robust, notably considering the lack of statistical power within these studies and lack of replication among these studies, it may be clinically beneficial to attempt use of members of the SNRI class of medication to treat some of the clinically challenging behaviors associated with ASD, such as irritability, self-injurious behavior, aggression, hyperactivity, and impulsivity. Considering the potentially serious side effects associated with atypical antipsychotics, it could be advantageous to trial at least low-dose SNRI medication as a strategy to help mitigate these side effects, most notably metabolic syndrome. The authors note, however, that there is no evidence to suggest use of an SNRI in the treatment of core symptoms of ASD alone.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Disclosures
Muralidhara Shankarapura Nanjapp, Emanuel Voyiaziakis, Basant Pradhan, and Srinagesh Mannekote Thippaiah have nothing to disclose.




