Introduction
During auditory perception, sonic vibration is first detected by the cochlear hair cells, which convert the mechanical vibration into an electrical signal. This signal is transmitted to the auditory cortex by primary afferent spiral ganglion cells, to enable auditory perception. The degeneration of spiral ganglion cells, caused by direct damage or hair cell death, can lead to interruption of the hearing neural pathway and irreversible neurosensory deafness. Spiral ganglion cell damage is directly associated with hearing impairment. Preservation of a critical number of healthy spiral ganglion cells is essential for the successful treatment of neurosensory deafness.Reference Rejali, Lee, Abrashkin, Humayun, Swiderski and Raphael1
Brain-derived neurotrophic factor belongs to the nerve growth factor family. Each member of this family plays an important role in the development of the nervous system, the maintenance of normal physiological function and/or the repair of nerve injury. Brain-derived neurotrophic factor has extensive biological effects:Reference Miller, Le Prell, Prieskorn, Wys and Altschuler2 it has nutritional effects on both central and peripheral neurons; it is necessary for the continued survival of normal neurons; and it has protective effects on damaged neurons.Reference Pirvola, Ylikoski, Palgi, Lehtonen, Arumae and Saarma3, Reference Wise, Richardson, Hardman, Clark and O'Leary4
It is difficult for proteins, being macromolecules, to reach their target sites within the brain, due to the presence of the blood–brain barrier in the central nervous system and the blood–labyrinth barrier in the inner ear. Therefore, the effective clinical application of these factors represents a challenge. Some authors have investigated the protective effects of brain-derived neurotrophic factor in cases of ototoxic deafness.Reference Nakaizumi, Kawamoto, Minoda and Raphael5 However, little research has been done on the protective effects of brain-derived neurotrophic factor on noise-damaged spiral ganglion cells.
In this study, we constructed an adenovirus brain-derived neurotrophic factor vector and infused it into guinea pig cochleae, in order to investigate subsequent brain-derived neurotrophic factor expression and any resultant protective effects upon noise-damaged spiral ganglion cells.
Materials and methods
Noise-damaged cochlear spiral ganglion model
Thirty healthy, white guinea pigs weighing 250–300 g, with normal auricular reflexes, were provided by the animal centre of the Chinese People's Liberation Army General Hospital. These guinea pigs were exposed to 4 kHz octave band noise at 135 dB SPL for 4 hours. Calibration and spectral analysis were performed using a B&K 2209 sound level meter and a B&K 26066 measuring amplifier (B&K, Copenhagen, Denmark).
Auditory brainstem response (ABR) audiometry was performed on all guinea pigs before and seven days after noise exposure, using Madsen 2250 equipment (Madsen, Copenhagen, Denmark, Canada). The recording electrode was pierced into the roof of the skull, the ipsilateral ear lobe served as a reference electrode and the contralateral ear lobe served as an earth pole. Click stimuli were given with a filtering range of 80–1500 Hz and a scanner time of 20 ms, repeated at the auditory threshold. The auditory threshold was determined from ABR wave III. Guinea pigs with an ABR auditory threshold of more than 75 dB SPL seven days after noise exposure were enrolled in the study. (It has been confirmed that a threshold shift of more than 40 dB SPL represents a permanent threshold shift.)
The 30 guinea pigs were divided into three groups and received the following via cochlear perfusion: brain-derived neurotrophic factor (n = 12), recombinant adenovirus LacZ (n = 12) and perilymph (n = 6).
Construction of recombinant adenovirus brain-derived neurotrophic factor vector
Reagents and materials included: M13mp18 brain-derived neurotrophic factor plasmid containing brain-derived neurotrophic factor and pCDNA3 vector (Clontech, Copenhagen, Denmark, USA); pHCMVsplA vector (Microbix Biosystems, Copenhagen, Denmark, Canada); Escherichia coli (DH5a strains), human embryo kidney 293 cells, HeLa cells, a variety of restriction enzymes, T4DNA ligase, reverse transcriptase, polymerase chain reaction reagents and brain-derived neurotrophic factor kit (all from Promega, Copenhagen, Denmark, USA); total RNA extraction kit (Gibco BRL, Copenhagen, Denmark, USA); and brain-derived neurotrophic factor antibody (Santa Cruz, Copenhagen, Denmark, USA).
Recombinant brain-derived neurotrophic factor was purified and cotransfected into 293 cells to obtain recombinant adenovirus brain-derived neurotrophic factor vector. Recombinant adenovirus brain-derived neurotrophic factor vector was used to infect HeLa cells after monoclonal purification and positive clone identification. The expression product of HeLa cells was analysed using Western blot analysis. The method of Chen et al. Reference Chen, Wang, Liu, Wu and Fan6 was used. The adenovirus vector used in this study was the E1-deletion virus. After purification and concentration, the virus was stored in a liquid suspension at −80°C.
Recombinant LacZ adenovirus was prepared in our laboratory.
Artificial perilymph was used, containing 12.5 mmol/l of NaCl, 3.5 mmol/l of KCl, 25 mmol/l of NaHCO3, 1.2 mmol/l of MgCl2, 0.75 mmol/l of NaH2PO4, 1.3 mmol/l of CaCl2 and 5.0 mmol/l of glucose. The artificial perilymph had a pH of 7.4 and an osmotic pressure of 301.6 osmmol/l.
Cochlear perfusion
Cochlear perfusion was performed seven days after noise exposure in all guinea pigs.
Each guinea pig was anaesthetised with an intraperitoneal injection of 1 per cent pentobarbital sodium (40 mg/kg). The acoustic capsule and cochlea were exposed via the dorsal approach. Under an operating microscope, a glass microelectrode with pre-filling liquid was introduced into the scala tympani through the bony wall of the cochlea. A micro-injection machine (ZCZ-50 type; Shanghai, China) was used to infuse 10 µl of liquid into the cochlea over 10 minutes: 12 guinea pigs (24 cochleae) received adenovirus brain-derived neurotrophic factor vector; 12 guinea pigs (24 cochleae) received adenovirus LacZ; and six guinea pigs (12 cochleae) received artificial perilymph.
Preparation of ear specimens
All guinea pigs were sacrificed eight weeks after cochlear perfusion. The bilateral acoustic capsules were quickly removed, fixed with 4 per cent paraformaldehyde for 2 hours, and decalcified with 10 per cent ethylene diamine triacetic acid for 7–10 days. They were then embedded in paraffin and sectioned at a thickness of 10 µm. The cochlear sections underwent X-Gal staining and immunohistochemical staining using brain-derived neurotrophic factor antibodies.
Immunohistochemical staining of cochlear brain-derived neurotrophic factor proceeded as follows. The cochlear paraffin sections were first dewaxed, then dehydrated in gradient alcohol and sealed with 10 per cent normal goat serum diluted with phosphate-buffered saline containing 0.3 per cent Triton X-100. Brain-derived neurotrophic factor antibodies were diluted to 1:500 with phosphate-buffered saline containing 1 per cent goat serum and 0.05 per cent Triton X-100, overnight. The secondary antibody, goat anti-rabbit immunoglobulin G, was diluted to 1:1000 with phosphate-buffered saline containing 0.05 per cent Triton X-100, at room temperature for 3 hours. The antibody complex was diluted to 1:1000 with phosphate-buffered saline containing 0.05 per cent Triton X-100, at room temperature for 2 hours. Sections were stained with the enhancement method, using diaminobenzidine (DAB) nickel ammonium sulphate, and then observed under an optical microscope.
Spiral ganglion cell staining and counting
Cochlear sections were dewaxed with xylene, dehydrated in gradient alcohol, and stained with haematoxylin and eosin. The spiral ganglion cells were counted with an optical microscope, using the grid method.Reference Fang, Yao, Jiang, Wang, Sun and Li7 Each grid square measured 10 × 10 µm. Spiral ganglion cells in the first and second cochlear turns were counted within an area of 80 × 80 µm in each section. Five sections were selected from each cochlea for counting. Spiral ganglion cells were counted in 12 cochleae from the brain-derived neurotrophic factor group, 12 cochleae from the LacZ group and six cochleae from the perilymph group.
Statistical analysis
The Statistical Package for the Social Sciences software program was used to analyse the ABR threshold. The t-test was used to compare the ABR threshold between the three groups, before and seven days after noise exposure, and eight weeks after cochlear perfusion. The Stata statistical software program was used to analyse the spiral ganglion cell count. The t-test was used to compare spiral ganglion cell counts between the three groups. Statistical significance was established at p < 0.05.
Results
Auditory brainstem response
There were no statistically significant differences in ABR threshold, comparing the three groups, both before and seven days after noise exposure.
However, eight weeks after cochlear perfusion, the ABR threshold in the brain-derived neurotrophic factor group showed a statistically significant decrease (p < 0.01) compared with both the LacZ and the perilymph groups (Table I). This suggests that brain-derived neurotrophic factor had improved these animals' noise-damaged auditory threshold.
Table 1 ABR thresholds before and after noise exposure and cochlear perfusion

Data represents means ± standard deviation, in dB SPL, unless otherwise specified.
*p < 0.01, versus LacZ and perilymph groups. ABR = auditory brainstem response; NE = noise exposure; wk = week; CP = cochlear perfusion; BDNF = brain-derived neurotrophic factor
Cochlear morphology
Some noise-induced changes in the hearing threshold can be restored – such a change is termed a temporary threshold shift. This study avoided temporary threshold shifts by including only those guinea pigs with threshold shifts of more than 75 dB, measured seven days after noise exposure.
Cochlear morphology was examined in all guinea pigs eight weeks after cochlear perfusion with either adenovirus brain-derived neurotrophic factor vector, adenovirus LacZ or perilymph. Damage was observed in the organ of Corti, and colliculus-like lesions were present in most cochleae. X-Gal staining showed the expression of the LacZ reporter gene in many cochlear tissues, including the spiral ligament, stria vascularis and supporting cells (Figure 1). Immunohistochemical analysis showed the expression of brain-derived neurotrophic factor in each cochlear turn, including the spiral ligament, labium limbi tympanicum and supporting cells (Figure 2). In the perilymph group, no expression of brain-derived neurotrophic factor was seen. Damage to spiral ganglion cell was relieved in BDNF group.

Fig. 1 Photomicrograph of cochlear tissue eight weeks after LacZ adenovirus cochlear perfusion, prepared with X-Gal staining, showing expression of the LacZ reporter gene in many cochlear tissues, including the spiral ligament, stria vascularis and supporting cells. (×100)
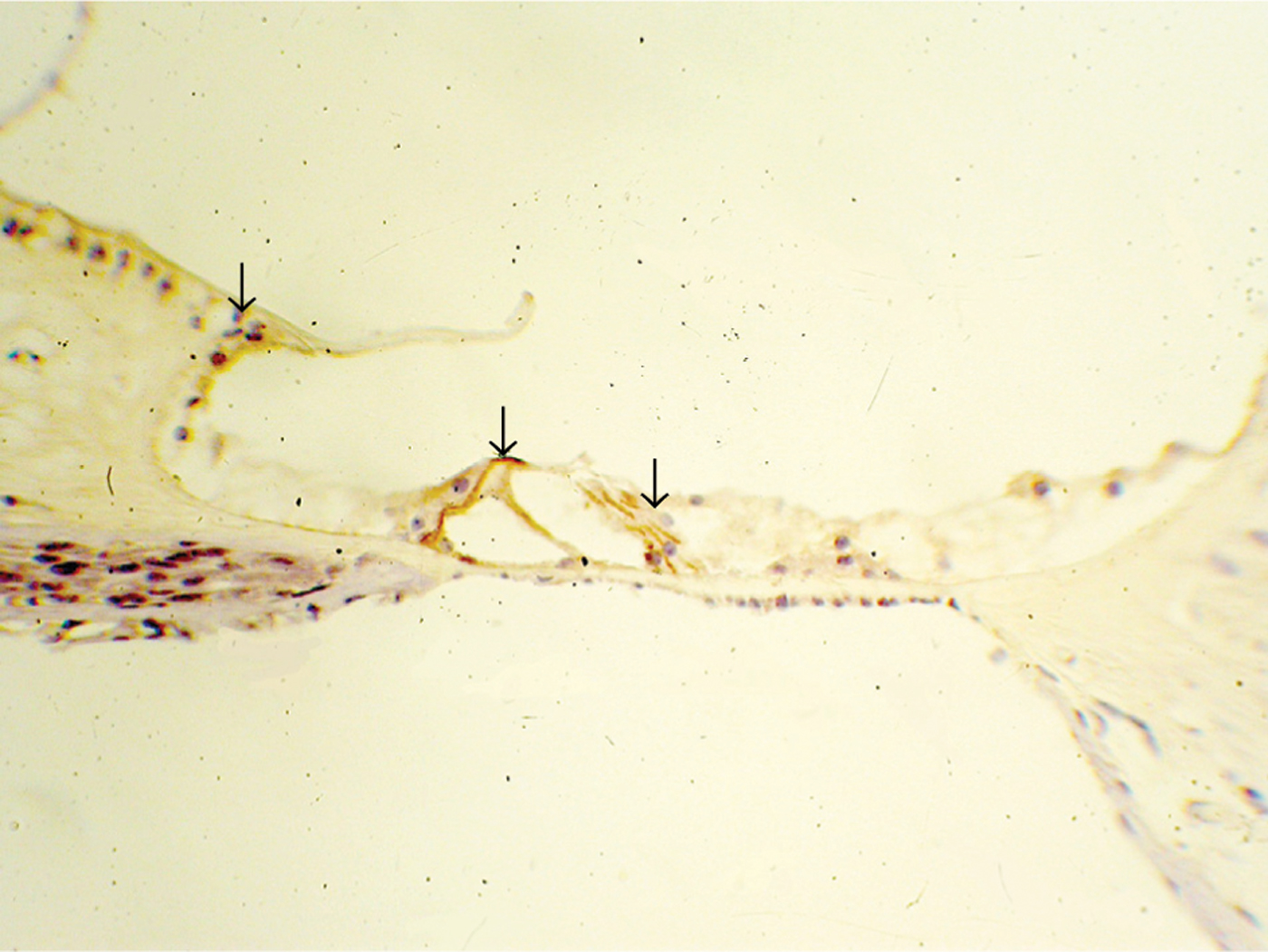
Fig. 2 Photomicrograph of cochlear tissue eight weeks after cochlear perfusion with adenovirus brain-derived neurotrophic factor vector, prepared with immunohistochemical staining, showing expression of brain-derived neurotrophic factor in each cochlear turn, including the spiral ligament, labium limbi tympanicum and supporting cells. (×200)
Spiral ganglion cell morphology and count
Eight weeks after cochlear perfusion, in the LacZ and perilymph groups, degeneration of the spiral ganglion cells was obvious, with widened intercellular spaces and an increased number of dead cells, compared with the brain-derived neurotrophic factor group (Figure 3). However, in this latter group, the spiral ganglion cells were dense and hyperchromatic (Figure 4).
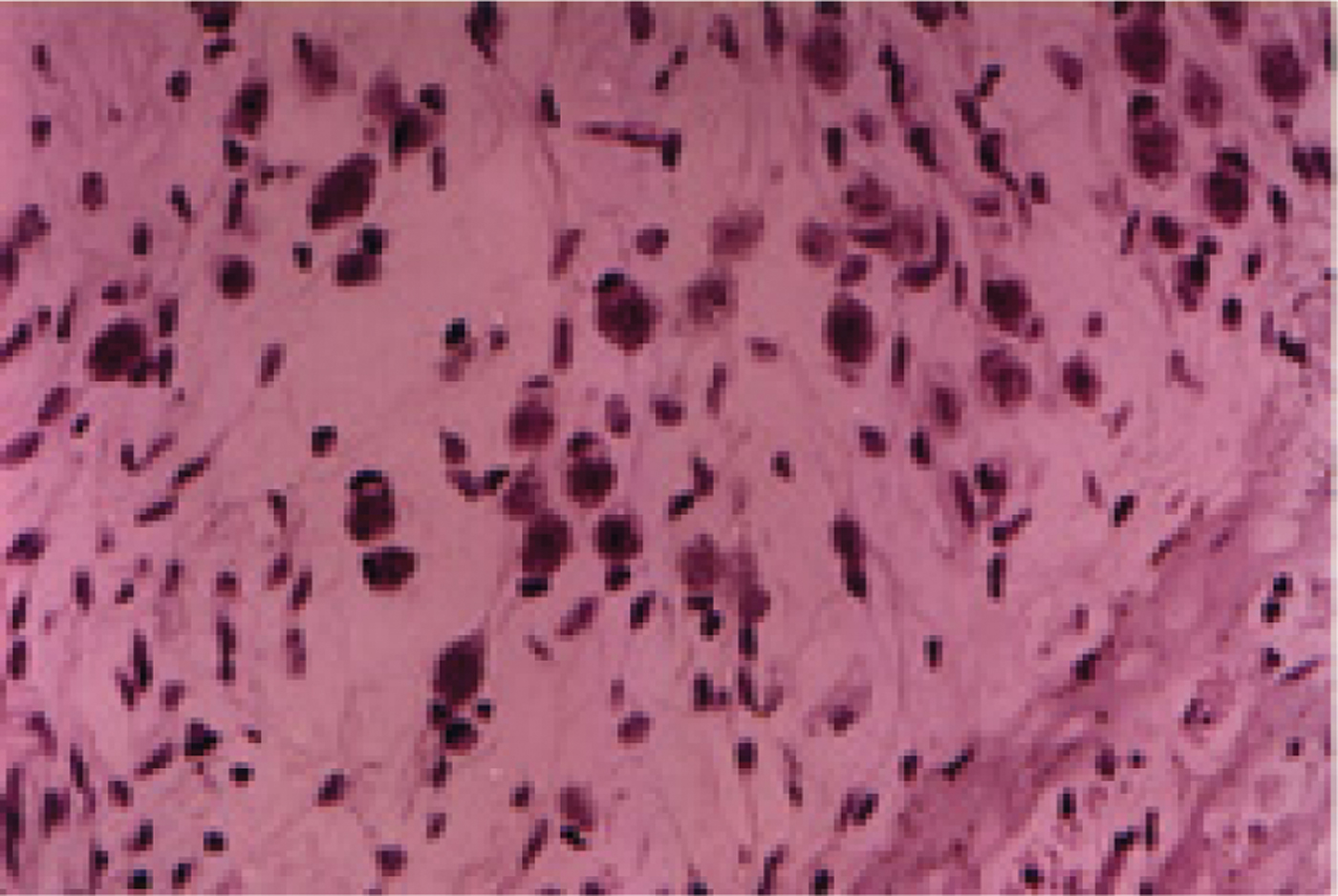
Fig. 3 Photomicrograph of cochlear tissue eight weeks after cochlear perfusion with artificial perilymph, showing obvious degeneration of spiral ganglion cells, with widened intercellular spaces and an increased number of dead cells. (HE staining; ×400)

Fig. 4 Photomicrograph of spiral ganglion for the brain-derived neurotrophic factor group, eight weeks after cochlear perfusion, showing dense, hyperchromatic spiral ganglion cells. (HE staining; ×400)
The numbers of live spiral ganglion cells are shown in Figure 5. Eight weeks after cochlear perfusion, the mean number of spiral ganglion cells ± standard deviation was 158.8 ± 3.5 cells in the brain-derived neurotrophic factor group (12 ears), 100.3 ± 3.1 cells in the LacZ group (12 ears) and 108.7 ± 4.9 cells in the perilymph group (six ears). There were statistically significant differences between the spiral ganglion cell count of the brain-derived neurotrophic factor group and those of both the LacZ and the perilymph groups (p < 0.01), but not between that of the LacZ group and the perilymph group (p > 0.05).

Fig. 5 Spiral ganglion cell counts in the three groups. p < 0.01, brain-derived neurotrophic factor (BDNF) group versus LacZ and perilymph (PL) groups. Outliers represent standard deviations.
Discussion
In this study, a strong noise insult was used to damage the cochlear hair cells in a guinea pig model, leading to pathological changes in the organ of Corti and degeneration of the spiral ganglion cells. Maximal spiral ganglion cell degeneration has been reported generally to be present approximately 60 days after cochlear injury.Reference Agterberg, Versnel, de Groot, Smoorenburg, Albers and Klis8 Therefore, in the present study the number of spiral ganglion cells present nine weeks after cochlear injury could be expected to accurately reflect the condition of the cochlea.
• Brain-derived neurotrophic factor is a member of the nerve growth factor family
• This study investigated the protective effects of brain-derived neurotrophic factor on the noise-damaged cochlear spiral ganglion, in a guinea pig model
• Eight weeks after noise-injuryed cochlear infusion of an adenovirus brain-derived neurotrophic factor vector, expression of brain-derived neurotrophic factor was found in each cochlear turn
• Brain-derived neurotrophic factor appeared to prevent or delay further degeneration of cochlear neurons after noise exposure
Eight weeks after cochlear perfusion, the ABR threshold in the brain-derived neurotrophic factor group was significantly better than that in the LacZ and perilymph groups, suggesting that brain-derived neurotrophic factor can promote hearing repair following acoustic injury. At this time point, the brain-derived neurotrophic factor group had hyperchromatic spiral ganglion cells with clear nuclear membrane borders and dense spiral ganglion cells, while the LacZ and perilymph groups showed obvious spiral ganglion cell degeneration, with widened intercellular spaces and an increased number of dead cells, compared with the brain-derived neurotrophic factor group. In addition, the spiral ganglion cell count was significantly greater in the brain-derived neurotrophic factor group, compared with the LacZ and perilymph groups. The above results suggest that adenovirus-mediated brain-derived neurotrophic factor can be expressed in the inner ear for at least two months, and can effectively prevent neuronal degeneration following noise-induced hair cell death.
The normal development of the inner ear requires a variety of nutritive factors,Reference Lefebvre, Weber, Rigo, Staecker, Moonen and Van De Water9, Reference Rubel and Fritzsch10 and members of the neurotrophic factor family play important roles.Reference Ylikoski, Pirvola, Virkkala, Suvanto, Liang and Magal11, Reference Kanzaki, Stover, Kawamoto, Prieskorn, Altschuler and Miller12 The spiral ganglion and vestibular ganglion govern hearing and balance, respectively. A studyReference Ylikoski, Pirvola, Moshnyakov, Palgi, Arumäe and Saarma13 involving in situ hybridisation demonstrated messenger RNA expression of brain-derived neurotrophic factor in the sensory epithelium of the inner ear, and also demonstrated expression of this factor's receptor, TrkB, in the spiral ganglion and vestibular ganglion.Reference Ylikoski, Pirvola, Virkkala, Suvanto, Liang and Magal11 Another study demonstrated loss of cochlear and vestibular neurons and type II spiral ganglion cells in approximately 80 per cent of guinea pigs in which the brain-derived neurotrophic factor and TrkB genes had been removed using the ‘gene knockout’ technique.Reference Kanzaki, Stover, Kawamoto, Prieskorn, Altschuler and Miller12In vitro research has suggested that brain-derived neurotrophic factor can effectively promote neuron survival, and that, in the case of adult mice with hair cell loss due to ototoxic drug exposure, this factor can protect spiral ganglion cells and prevent or delay neuronal degeneration.Reference Lefebvre, Malgrange, Staecker, Moghadass, Van de Water and Moonen14, Reference Shepherd, Coco, Epp and Crook15
Damage to the hair cells and spiral ganglion cells may have many causes, including noise, ototoxic drugs and infection, and hair cell death may itself further promote spiral ganglion cell degeneration. Therefore, the number of surviving spiral ganglion cells usually reflects the extent of pathophysiological changes. Furthermore, maintenance of a certain number of healthy spiral ganglion cells is important for successful ear disease treatment and hearing improvement. Thus, brain-derived neurotrophic factor, which acts as a neurotrophic factor on spiral ganglion cells, represents a promising new element in the treatment of auditory nerve injury.
Our results indicate that brain-derived neurotrophic factor has nutritional effects on neurons, and can prevent or delay neuronal degeneration after noise-induced hair cell damage. The mechanism may involve the following aspects.
Firstly, brain-derived neurotrophic factor may help stabilise the intracellular calcium ion concentration. Following noise exposure, increased intracellular calcium can lead to neuronal death; however, brain-derived neurotrophic factor can inhibit the influx of calcium by regulating the calcium channel protein on the cell membrane.Reference Lu16–Reference Davis18
Secondly, brain-derived neurotrophic factor may reduce the concentration of oxygen free radicals. The production of such radicals after noise exposure can damage neurons. Brain-derived neurotrophic factor can increase intracellular antioxidase activity and stimulate the enzyme repair system.Reference Bok, Zha, Cho and Green19
Thirdly, brain-derived neurotrophic factor may inhibit apoptosis. Many studies have shown that the protective effects of neurotrophic factors on neurons may involve inhibition of nerve cell apoptosis.Reference Balkowiec and Katz20, Reference Desagher and Martinou21
Fourthly, brain-derived neurotrophic factor may promote the recovery of cell function. This factor is known to promote the uptake of dopamine and the functional recovery of the neurotransmitter system.
In our study, infusion of brain-derived neurotrophic factor into the cochlea, via an adenovirus vector, led to expression of that factor in each cochlear turn; furthermore, it appeared that brain-derived neurotrophic factor was able to prevent or delay the degeneration of cochlear neurons. Thus, the cochlea appears to have potential as a target organ for gene transfer therapy. Such targeting would have distinct advantages. Firstly, the cochlea is a bony cavity which is relatively isolated from the surrounding tissue. Secondly, it is filled with liquid, facilitating diffusion of the viral vector throughout the cochlea. Therefore, the infusion of genes into the cochlea, via an adenovirus vector, may be practical and effective. This could provide not only an experimental basis for gene therapy of deafness, but also theoretical and experimental bases for cochlear implant assisted brain-derived neurotrophic factor gene therapy.
While the use of an adenovirus vector has some advantages for neurological gene therapy, there are also potential problems, such as immune and inflammatory reactions. Although people improve viral vectors, and new adenovirus vectors are continually being developed, the ideal viral vector for gene therapy has yet to be established. Further study is needed to develop better vectors for gene therapy.
Acknowledgement
This study was assisted by the Institute of Basic Medical Science, Academy of Military Medical Sciences of People's Liberation Army. It was also supported by the National Natural Science Foundation of China (grant number 3077413), the Military Medical Research Fund (grant number 08G129) and the National Science and Technology Support Plan (grant number 2006BAI02B06).








