Introduction
Seed formation is a key step that provides many plants a unique opportunity to suspend their life cycle and withstand adverse environmental conditions in a desiccated state. A dominant process characteristic of seed development is the accumulation of reserve carbohydrates, lipids and proteins that are subsequently used following germination as a source of energy, carbon and nitrogen until the seedling becomes autotrophic. In dicot plants, the cotyledons are frequently the main tissue in which reserves accumulate, although in some a persistent endosperm or perisperm may be the major storage tissue. In Arabidopsis, only a single layer of endosperm cells remains in the dry seed, and storage compounds are deposited in the embryo itself (Vicente-Carbajosa and Carbonero, Reference Vicente-Carbajosa and Carbonero2005). In monocots, especially members of the grass/cereal families, the endosperm is the prevailing reserve tissue in which starch and seed storage proteins (SSPs) accumulate. Storage proteins in seeds are the products of a number of specific genes whose expression is tightly regulated during development. Thus, SSP genes provide a powerful system to investigate the regulation of their expression. Several lines of evidence indicate that the synthesis of storage proteins is controlled primarily at the transcriptional level. This review will focus on the current knowledge of the transcriptional regulation driving SSP gene expression in Arabidopsis and cereals, which involves both cis regulatory elements and transcription factors (TFs).
Cis-elements involved in the expression of SSP genes
Extensive analysis of the SSP gene promoters has identified numerous cis regulatory elements involved in controlling seed-specific expression. Among them in dicot species is the widely distributed RY motif (5′-CATGCA-3′), which represents the central core of the previously identified 28-bp-long legumin box (Baumlein et al., Reference Baumlein, Nagy, Villarroel, Inze and Wobus1992), and its deletion abolishes most of the seed-specific promoter activities. The RY motif was originally discovered in the promoters of dicot seed protein genes, but later was found also to be conserved in many of the studied promoters of cereal SSP genes. This observation supports a putative role for the RY box in endosperm-specific gene expression. Mutational analyses in tobacco demonstrated that the RY repeats of legumin, glycinin and β-conglycinin genes are necessary for positive regulation in seed tissues (Baumlein et al., Reference Baumlein, Nagy, Villarroel, Inze and Wobus1992; Chamberland et al., Reference Chamberland, Daigle and Bernier1992; Lelievre et al., Reference Lelievre, Oliveira and Nielsen1992) and negative regulation in vegetative organs (Baumlein et al., Reference Baumlein, Nagy, Villarroel, Inze and Wobus1992).
In the promoters of genes encoding cereal SSPs, the best characterized cis-consensus elements include the prolamin box (P box; 5′-TGT/CAAAG-3′), GCN4 motif (5′-TGAG/CTCA-3′) and AACA motif (5′-AACAAAC-3′) (Takaiwa et al., Reference Takaiwa, Yamanouchi, Yoshihara, Washida, Tanabe, Kato and Yamada1996; Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Moose, Parsons and Schmidt1997; Wu et al., Reference Wu, Washida, Onodera, Harada and Takaiwa2000). Mutations in the GCN4 motif of the GluB-1 gene change its expression from the outer to the inner starchy endosperm, and the extent of expression is severely reduced (90-fold) (Wu et al., Reference Wu, Suzuki, Washida and Takaiwa1998). Multimers of GCN4 fused to a minimal cauliflower mosaic virus (CaMV) 35S core promoter can drive expression in the outer region of the endosperm, whereas a single GCN4 motif cannot (Wu et al., Reference Wu, Suzuki, Washida and Takaiwa1998). In many cases, GCN4 and P box are coupled to each other and separated by only a few nucleotides; they are termed a bifactorial endosperm box. The involvement of these two motifs in regulating gene expression has been demonstrated for numerous cereal prolamin genes (Hammond-Kosack et al., Reference Hammond-Kosack, Holdsworth and Bevan1993; Albani et al., Reference Albani, Hammond-Kosack, Smith, Conlan, Colot, Holdsworth and Bevan1997; Marzabal et al., Reference Marzabal, Busk, Ludevid and Torrent1998). A third motif, AACA, conserved in rice glutelin genes is also involved in controlling the endosperm-specific expression (Washida et al., Reference Washida, Wu, Suzuki, Yamanouchi, Akihama, Harada and Takaiwa1999). Interestingly, AACA is also closely linked with the GCN4 element in most rice glutelin genes (Qu le et al., Reference Qu le, Xing, Liu, Xu and Song2008) and together these have been shown to confer endosperm-specific enhancement to a truncated − 90 CaMV 35S promoter (Yoshihara et al., Reference Yoshihara, Washida and Takaiwa1996).
Major TFs
Major TFs involved in regulation of SSP gene expression in Arabidopsis
Seed-specific traits, such as desiccation tolerance, reserve accumulation and entry into quiescence are acquired during seed maturation ((Wobus and Weber, Reference Wobus and Weber1999; Vicente-Carbajosa and Carbonero, Reference Vicente-Carbajosa and Carbonero2005). In Arabidopsis, this phase is genetically controlled by at least four master regulators, namely FUS3, ABI3, LEC1 and LEC2. FUS3, ABI3 and LEC2 encode TFs containing the conserved B3 DNA binding domain which targets the RY element (Fig. 1) (Giraudat et al., Reference Giraudat, Hauge, Valon, Smalle, Parcy and Goodman1992; Luerssen et al., Reference Luerssen, Kirik, Herrmann and Misera1998; Stone et al., Reference Stone, Kwong, Yee, Pelletier, Lepiniec, Fischer, Goldberg and Harada2001), whereas LEC1 encodes a HAP3 subunit of the CCAAT-binding TF (CBF, also known as NF-Y) (Lotan et al., Reference Lotan, Ohto, Yee, West, Lo, Kwong, Yamagishi, Fischer, Goldberg and Harada1998). In addition, two Opaque2-like (O2-like) factors, AtbZIP10 and AtbZIP25, are also involved in SSP gene expression regulation (Lara et al., Reference Lara, Onate-Sanchez, Abraham, Ferrandiz, Diaz, Carbonero and Vicente-Carbajosa2003). Recently, bZIP53 has been reported to play a pivotal and crucial role in quantitative control of seed maturation gene transcription in cooperation with several TFs (Alonso et al., Reference Alonso, Onate-Sanchez, Weltmeier, Ehlert, Diaz, Dietrich, Vicente-Carbajosa and Droge-Laser2009).
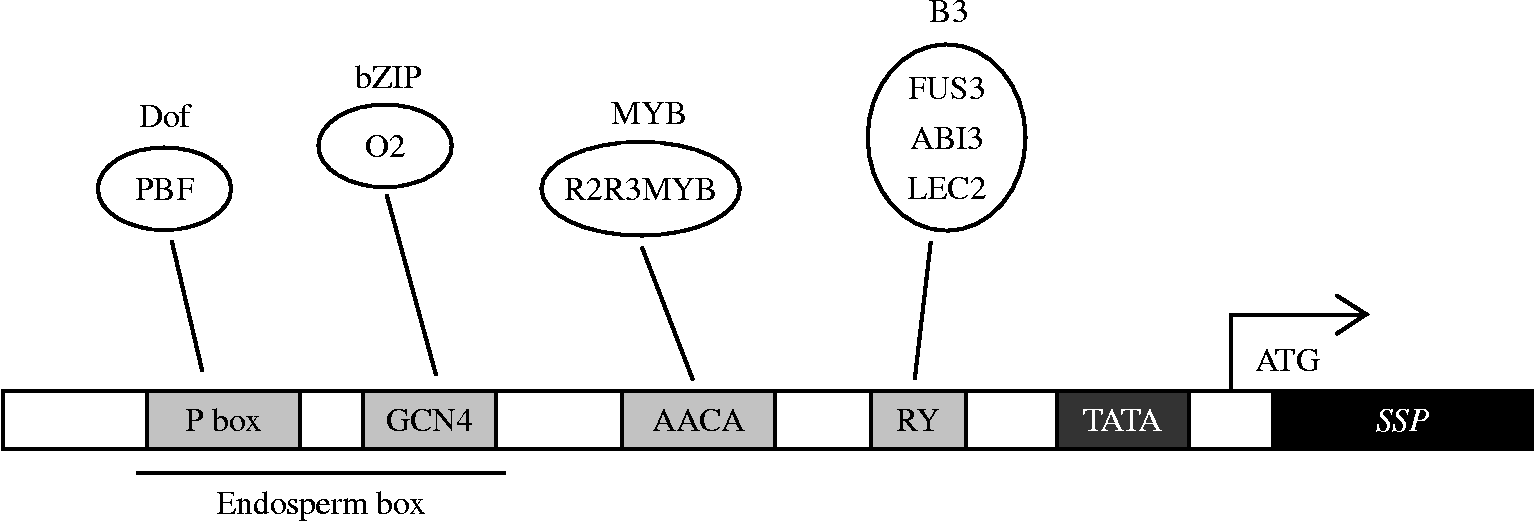
Figure 1 Cis-elements and trans-acting factors involved in SSP gene regulation in cereals and Arabidopsis. Interactions between cis-elements and TFs are indicated by solid lines.
FUS3 is a regulator of seed development that is involved in the establishment of dormancy, desiccation tolerance and cotyledon identity, as well as in the synthesis of SSPs and anthocyanin accumulation (Keith et al., Reference Keith, Kraml, Dengler and McCourt1994; Gazzarrini et al., Reference Gazzarrini, Tsuchiya, Lumba, Okamoto and McCourt2004). ABI3 is a transcription activator required for abscisic acid (ABA) responses in seeds; its importance as a regulator of transcription of seed-specific genes has been demonstrated by the fact that ectopic expression of ABI3 in vegetative tissues confers on them the ability to accumulate seed-specific transcripts in response to ABA (Parcy et al., Reference Parcy, Valon, Raynal, Gaubier-Comella, Delseny and Giraudat1994; Parcy and Giraudat, Reference Parcy and Giraudat1997). LEC1 is required for normal development during early and late phases of embryogenesis and is sufficient to induce embryonic development in vegetative cells (Lotan et al., Reference Lotan, Ohto, Yee, West, Lo, Kwong, Yamagishi, Fischer, Goldberg and Harada1998). LEC2 is a transcriptional regulator that establishes a cellular environment sufficient to initiate embryo development, and is required for the maintenance of suspensor morphology, specification of cotyledon identity, progression through maturation and suppression of premature germination (Stone et al., Reference Stone, Kwong, Yee, Pelletier, Lepiniec, Fischer, Goldberg and Harada2001). All four abi3, lec1, lec2 and fus3 mutants display severely affected seed maturation and share some common phenotypes, such as reduced expression of SSPs. However, they also show some specific phenotypes, such as the absence of chlorophyll degradation (abi3) and of anthocyanin accumulation (fus3, lec1 and lec2), altered chemical composition (lec2), reduced sensitivity to ABA (abi3 and lec1), intolerance to desiccation (abi3, fus3 and lec1) and altered cotyledon identity (fus3, lec1 and lec2) (Parcy et al., Reference Parcy, Valon, Kohara, Misera and Giraudat1997; Lotan et al., Reference Lotan, Ohto, Yee, West, Lo, Kwong, Yamagishi, Fischer, Goldberg and Harada1998; Vicient et al., Reference Vicient, Bies-Etheve and Delseny2000; Raz et al., Reference Raz, Bergervoet and Koornneef2001; Kroj et al., Reference Kroj, Savino, Valon, Giraudat and Parcy2003; To et al., Reference To, Valon, Savino, Guilleminot, Devic, Giraudat and Parcy2006; Angeles-Nunez and Tiessen, Reference Angeles-Nunez and Tiessen2011).
Numerous studies have been performed aiming to uncover the mechanisms through which these genes interact to control the various facets of seed maturation. LEC1 controls the expression of the SSP genes in a hierarchical manner, which involves ABI3 and FUS3 (Kagaya et al., Reference Kagaya, Toyoshima, Okuda, Usui, Yamamoto and Hattori2005). FUS3 and LEC2 seem to act in a partially redundant manner to control SSP gene expression by directly binding to the RY motif present in the promoter regions, and LEC2 locally regulates FUS3 expression in regions of the cotyledons (Kroj et al., Reference Kroj, Savino, Valon, Giraudat and Parcy2003). However, FUS3 also regulates the synthesis of SSPs through TRANSPARENT TESTA GLABRA1 (TTG1) or some other intermediate protein (Tsuchiya et al., Reference Tsuchiya, Nambara, Naito and McCourt2004). Although ABI3 regulation of SSP genes is also dependent on the RY box, it is yet unclear whether it takes place through a direct interaction or by activation of downstream targets such as FUS3 or LEC2.
The complex interactions among the four major regulators are better understood by analysing ABI3 and FUS3 expression in various single, double and triple maturation mutants, which indicated that these regulators are interlocked in a complex hierarchical network of mutual interactions (To et al., Reference To, Valon, Savino, Guilleminot, Devic, Giraudat and Parcy2006). As shown in Fig. 2, LEC1 acts upstream of the main genetic network of seed maturation and positively regulates ABI3 and FUS3. One of the major roles of LEC2 is to upregulate FUS3 and ABI3 expression. Additionally, ABI3 and FUS3 positively regulate themselves and each other, thereby forming feedback loops essential for their sustained and uniform expression in the embryo. Notably, the gene regulatory controls in this network act locally and redundantly (To et al., Reference To, Valon, Savino, Guilleminot, Devic, Giraudat and Parcy2006). In the root tip, FUS3 expression is redundantly controlled by LEC2 and by FUS3 itself; in the embryo axis FUS3 expression is redundantly controlled by LEC2 and ABI3; in the cotyledons, FUS3 expression is under the control of all three regulators (To et al., Reference To, Valon, Savino, Guilleminot, Devic, Giraudat and Parcy2006). In addition to this set of regulatory controls, LEC1 also locally regulates FUS3 expression in cotyledons.
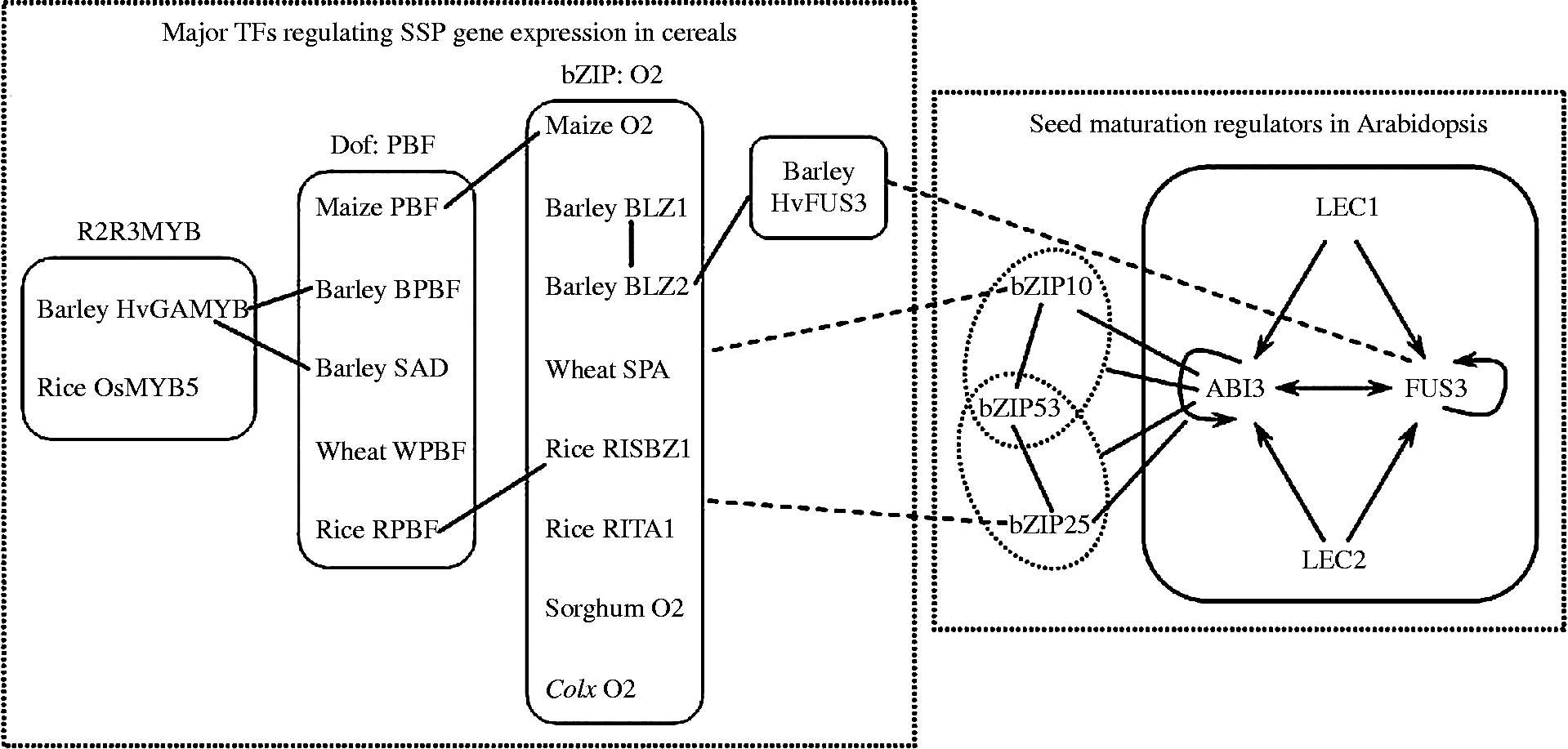
Figure 2 Major TFs involved in the regulation of SSP expression in cereals and Arabidopsis. Major TFs regulating SSP gene expression in cereals are located in the dashed box on the left. Major TFs involved in regulation of SSP expression in Arabidopsis are located in the dashed box on the right. Four master regulators (LEC1, LEC2, FUS3 and ABI3) of seed maturation in Arabidopsis are located in the solid box. An arrow indicates a promotional effect. Interactions between TFs are indicated by solid lines. The interactions between ABI3 and bZIP10/25–bZIP53 heterodimers are also indicated by solid lines. Conservation between bZIP10/25 and O2-like TFs, as well as between FUS3 and HvFUS3, is indicated by the dashed lines.
Major TFs regulating SSP gene expression in cereals
The cis regulatory elements GCN4, P box and AACA, highly conserved in the promoters of genes encoding cereal SSPs, are bound by TFs of the basic leucine zipper (bZIP) (Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Onate, Lara, Diaz and Carbonero1998; Onate et al., Reference Onate, Vicente-Carbajosa, Lara, Diaz and Carbonero1999), DNA binding with one finger (DOF) (Lijavetzky et al., Reference Lijavetzky, Carbonero and Vicente-Carbajosa2003; Yanagisawa, Reference Yanagisawa2004) and R2R3MYB families (Diaz et al., Reference Diaz, Vicente-Carbajosa, Abraham, Martinez, Isabel-La Moneda and Carbonero2002), respectively (Fig. 1).
bZIP proteins contain a basic region and a leucine repeat involved in DNA binding and dimerization, respectively, other domains accounting for activation capabilities or for interaction with ancillary proteins of the transcription machinery (Onate et al., Reference Onate, Vicente-Carbajosa, Lara, Diaz and Carbonero1999). One of the first-described endosperm-specific bZIP TFs in plants involved in regulation of SSP genes is O2 from maize, which can activate the transcription of maize 22 kDa α-zein gene and b-32 ribosome-inactivating protein gene by recognizing the ACGT core motif and a distinct consensus sequence in their promoters, respectively (Lohmer et al., Reference Lohmer, Maddaloni, Motto, Di Fonzo, Hartings, Salamini and Thompson1991; Schmidt et al., Reference Schmidt, Ketudat, Aukerman and Hoschek1992). O2 also binds and activates promoters of SSP genes obtained from other species such as the wheat LMWG-1D1 and rice GluB-1 by binding to GCN4 elements (Holdsworth et al., Reference Holdsworth, Munoz-Blanco, Hammond-Kosack, Colot, Schuch and Bevan1995; Wu et al., Reference Wu, Suzuki, Washida and Takaiwa1998), thus indicating that the O2 has broad binding specificity. The central role of O2-like factors in seed-specific expression has been well documented in other cereal species, such as wheat storage protein activator (SPA) (Albani et al., Reference Albani, Hammond-Kosack, Smith, Conlan, Colot, Holdsworth and Bevan1997), barley BLZ1 and BLZ2 (Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Onate, Lara, Diaz and Carbonero1998; Onate et al., Reference Onate, Vicente-Carbajosa, Lara, Diaz and Carbonero1999), rice RISBZ1 and RITA1 (Izawa et al., Reference Izawa, Foster, Nakajima, Shimamoto and Chua1994; Onodera et al., Reference Onodera, Suzuki, Wu, Washida and Takaiwa2001), sorghum O2 (Pirovano et al., Reference Pirovano, Lanzini, Hartings, Lazzaroni, Rossi, Joshi, Thompson, Salamini and Motto1994) and Coix O2 (Vettore et al., Reference Vettore, Yunes, Cord Neto, da Silva, Arruda and Leite1998). Out of those, wheat SPA, barley BLZ1 and BLZ2, and rice RISBZ1 trans-activate expression of wheat LMWG-1D1, barley B-hordein gene Hor2 and rice GluB-1 by interacting with the GCN4 elements in their promoter regions, respectively (Albani et al., Reference Albani, Hammond-Kosack, Smith, Conlan, Colot, Holdsworth and Bevan1997; Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Onate, Lara, Diaz and Carbonero1998; Onate et al., Reference Onate, Vicente-Carbajosa, Lara, Diaz and Carbonero1999; Onodera et al., Reference Onodera, Suzuki, Wu, Washida and Takaiwa2001).
Dof proteins are a family of plant-specific TFs containing a highly conserved DNA-binding domain, the Dof domain (Yanagisawa, Reference Yanagisawa2002), which includes one single Cys2–Cys2 zinc finger. Numerous Dof proteins are involved in diverse plant processes such as seed germination (Mena et al., Reference Mena, Cejudo, Isabel-Lamoneda and Carbonero2002; Isabel-LaMoneda et al., Reference Isabel-LaMoneda, Diaz, Martinez, Mena and Carbonero2003), photosynthesis (Yanagisawa, Reference Yanagisawa2000), secondary metabolism (Skirycz et al., Reference Skirycz, Reichelt, Burow, Birkemeyer, Rolcik, Kopka, Zanor, Gershenzon, Strnad, Szopa, Mueller-Roeber and Witt2006), phytohormone expression) Baumann et al., Reference Baumann, De Paolis, Costantino and Gualberti1999) and SSP expression (Diaz et al., Reference Diaz, Martinez, Isabel-LaMoneda, Rubio-Somoza and Carbonero2005) in both monocots and dicots. The Arabidopsis and rice genomic projects indicate the presence of 37 and 30 Dof protein-encoding genes, respectively (Yanagisawa, Reference Yanagisawa2002; Lijavetzky et al., Reference Lijavetzky, Carbonero and Vicente-Carbajosa2003). Prolamin-box binding factor (PBF), which belongs to the Dof protein family, is implicated in trans-activation of SSP genes from a wide spectrum of cereal grains. In maize, PBF is able to interact with the P box present in the promoters of 22 kDa α-zein and 27 kDa γ-zein genes (Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Moose, Parsons and Schmidt1997; Marzabal et al., Reference Marzabal, Gas, Fontanet, Vicente-Carbajosa, Torrent and Ludevid2008). Similar types of PBFs have been isolated from barley, wheat and rice, which are designated BPBF (Mena et al., Reference Mena, Vicente-Carbajosa, Schmidt and Carbonero1998), WPBF (Mena et al., Reference Mena, Vicente-Carbajosa, Schmidt and Carbonero1998) and RPBF (Yamamoto et al., Reference Yamamoto, Onodera, Touno and Takaiwa2006), respectively. In barley, BPBF, which activates transcription of a native Hor2 promoter in the barley endosperm through P box-recognition (Mena et al., Reference Mena, Vicente-Carbajosa, Schmidt and Carbonero1998), is a repressor of the gibberellin-induced thiol-protease cathepsin-B-like gene in aleurone layer cells following germination (Mena et al., Reference Mena, Cejudo, Isabel-Lamoneda and Carbonero2002). In wheat, transient expression experiments in co-transfected BY-2 protoplast cells demonstrate that WPBF trans-activates transcription from native α-gliadin promoter through binding to the intact P box (Dong et al., Reference Dong, Ni, Yao, Nie and Sun2007). However, it seems that WPBF transcriptional activity is not specific to wheat storage protein genes since it is not only expressed in the grains but also in other tissues (Dong et al., Reference Dong, Ni, Yao, Nie and Sun2007). Rice RPBF activates the expression of several rice glutelin and prolamin genes by recognizing the P box in their promoters (Yamamoto et al., Reference Yamamoto, Onodera, Touno and Takaiwa2006). In addition, scutellum and aleurone-layer-expressed DOF (SAD) from barley, trans-activates transcription of the cathepsin B-like cysteine protease AI21 promoter in co-bombarded aleurone layers (Isabel-LaMoneda et al., Reference Isabel-LaMoneda, Diaz, Martinez, Mena and Carbonero2003). Also, transient expression experiments in co-bombarded developing barley endosperms demonstrate that SAD trans-activates transcription from B-hordein Hor2 and trypsin-inhibitor BTI-CMe Itr1 promoters through binding to the P box (Diaz et al., Reference Diaz, Martinez, Isabel-LaMoneda, Rubio-Somoza and Carbonero2005).
OsMYB5 is a MYB protein from rice that specifically binds to the AACA motif within the promoter region of the GluB-1 gene (Suzuki et al., Reference Suzuki, Wu, Washida and Takaiwa1998). In barley, GAMYB, a member of the R2R3MYB family, is not only a regulator of gibberellin-responsive genes during and after seed germination (Gubler et al., Reference Gubler, Kalla, Roberts and Jacobsen1995), but also activates gene expression during endosperm development through binding to the AACA motif in native Hor2 and Itr1 promoters (Diaz et al., Reference Diaz, Vicente-Carbajosa, Abraham, Martinez, Isabel-La Moneda and Carbonero2002). In contrast to endosperm-specific O2-like bZIP TFs and PBF-like TFs, HvGAMYB is also expressed in various non-seed tissues (Diaz et al., Reference Diaz, Vicente-Carbajosa, Abraham, Martinez, Isabel-La Moneda and Carbonero2002).
There have been many reports of cereal TFs involved in SSP gene regulation; however, because there are few mutants, most studies have been carried out in vitro (e.g. electrophoretic mobility shift assay for analysis of the interactions between cis-elements and TFs) or in vivo (e.g. transient assays using yeast or plant cells), with a few exceptions. Mutations of O2 lead to pleiotropic phenotypes such as opaque kernels, a substantial reduction in α-zein and increased total lysine and tryptophan contents (Kawakatsu and Takaiwa, Reference Kawakatsu and Takaiwa2010). In contrast, a knock-down (KD) mutant of RISBZI (KD-RISBZ1) or RPBF (KD-RPBF) does not show a large reduction in the SSPs, whereas double KDs of these TFs results in a significant reduction of most SSP gene expression and accumulation (Kawakatsu et al., Reference Kawakatsu, Yamamoto, Touno, Yasuda and Takaiwa2009). In addition, the double KD line contains much less starch and a reduced lipid content compared with the wild type, indicating redundant functions of RISBZ1 and RPBF during rice grain filling (Kawakatsu et al., Reference Kawakatsu, Yamamoto, Touno, Yasuda and Takaiwa2009).
Interactions among TFs
Transcriptional regulation is normally exerted by the concerted action of multiple TFs responding to distinct signals. Consequently, the anticipated extent of gene expression arises from the effects of various TFs acting on a particular promoter and their combinatorial interactions. The importance of such interactions (e.g. ABI3–bZIP10/25, bZIP53–bZIP10/25, bZIP53–bZIP10/25–ABI3, PBF–O2, RPBF–RISBZ1, BPBF–HvGAMYB, SAD–HvGAMYB, BLZ1–BLZ2, HvFUS3–BLZ2) has already been reported in the regulation of seed-specific genes of both Arabidopsis and cereals (Fig. 2). In Arabidopsis, AtbZIP10 and AtbZIP25 are involved in the regulation of SSP gene expression in a concerted manner with ABI3 (Lara et al., Reference Lara, Onate-Sanchez, Abraham, Ferrandiz, Diaz, Carbonero and Vicente-Carbajosa2003). Recently, bZIP53 has been demonstrated to enhance seed maturation gene expression by specific heterodimerization with bZIP10 or bZIP25 (Alonso et al., Reference Alonso, Onate-Sanchez, Weltmeier, Ehlert, Diaz, Dietrich, Vicente-Carbajosa and Droge-Laser2009). Furthermore, these bZIP heterodimers interact with ABI3, which further increases seed maturation gene activation (Alonso et al., Reference Alonso, Onate-Sanchez, Weltmeier, Ehlert, Diaz, Dietrich, Vicente-Carbajosa and Droge-Laser2009). In maize, the interaction between PBF and O2 is involved in the zein protein expression during seed development (Vicente-Carbajosa et al., Reference Vicente-Carbajosa, Moose, Parsons and Schmidt1997). In rice, RPBF activates rice glutelin and prolamin genes in cooperation with RISBZ1 (Yamamoto et al., Reference Yamamoto, Onodera, Touno and Takaiwa2006). Moreover, the combinatorial interactions between them play an essential role during grain filling (Kawakatsu et al., Reference Kawakatsu, Yamamoto, Touno, Yasuda and Takaiwa2009). In barley, BPBF interacts with HvGAMYB through its C-terminal domain to activate barley-endosperm-specific genes during seed development (Diaz et al., Reference Diaz, Vicente-Carbajosa, Abraham, Martinez, Isabel-La Moneda and Carbonero2002). Another Dof protein, SAD, also interacts with HvGAMYB in vivo through its C-terminal domain. This interaction enhances the transcription activation of a gibberellin-induced protease promoter in aleurone layer cells (Isabel-LaMoneda et al., Reference Isabel-LaMoneda, Diaz, Martinez, Mena and Carbonero2003; Diaz et al., Reference Diaz, Martinez, Isabel-LaMoneda, Rubio-Somoza and Carbonero2005). Barley BLZ2 protein can interact with BLZ1 in vivo and activates transcription from the GCN4 motif of B-hordein promoters in the barley endosperm (Onate et al., Reference Onate, Vicente-Carbajosa, Lara, Diaz and Carbonero1999). FUSCA3 from barley, HvFUS3, can interact with BLZ2 in a yeast two-hybrid system, and this interaction is essential for full trans-activation of the seed-specific genes Hor2 and Itr1 (Moreno-Risueno et al., Reference Moreno-Risueno, Gonzalez, Diaz, Parcy, Carbonero and Vicente-Carbajosa2008). Therefore, cooperation of transcriptional regulators by protein–protein interactions provides an efficient mechanism to control gene expression in seeds and explains some of the molecular mechanisms underlying SSP gene expression.
Evolutionary conservation between dicot and monocot species
Most interestingly, the prolamins (the main group of SSPs in many cereal grains) and a major group of dicot seed albumins can be traced phylogenetically to a common ancestor (Kreis and Shewry, Reference Kreis and Shewry1989). Their conservation relates not only to protein structure, but also to regulatory elements and trans-acting factors that are functionally exchangeable between the groups. The RY motif, which is widely distributed in the promoters of dicot species, is also conserved in the promoters of SSP genes of cereals, such as the barley Hor2, maize 22 kDa α-zein and 15 kDa β-zein, wheat α-gliadin and HMW-glutenin, rice GluA-1 and GluB-3, and Coix α-coixin. Characterization of the TFs involved in seed gene expression provides further evidence for evolutionary conservation. In maize, Viviparous1 (VP1), which is the orthologue of Arabidopsis ABI3, is also involved in ABA signalling, establishment of dormancy and activation of maturation-specific genes during seed development (McCarty et al., Reference McCarty, Hattori, Carson, Vasil, Lazar and Vasil1991; Suzuki et al., Reference Suzuki, Ketterling, Li and McCarty2003). Maize VP1, expressed in the embryo and aleurone layer, can complement Arabidopsis abi3 mutants that are impaired in their expression of SSPs (Suzuki et al., Reference Suzuki, Kao, Cocciolone and McCarty2001). In addition, a detailed characterization of AtbZIP10 and AtbZIP25 of Arabidopsis showed that they are both structurally and functionally the most closely related to O2-like cereal TFs, and might represent their Arabidopsis counterparts involved in SSP gene regulation (Lara et al., Reference Lara, Onate-Sanchez, Abraham, Ferrandiz, Diaz, Carbonero and Vicente-Carbajosa2003). Both the barley and Arabidopsis FUS3 genes maintain a conserved functionality for the regulation of SSP genes and anthocyanin biosynthesis in these two distantly related phylogenetic groups. Complementation of the loss-of-function mutant fus3 in Arabidopsis by the barley HvFus3 gene results in restored transcription of the At2S3 gene promoter and normal accumulation of anthocyanins in the seed (Moreno-Risueno et al., Reference Moreno-Risueno, Gonzalez, Diaz, Parcy, Carbonero and Vicente-Carbajosa2008).
Conclusions and outlook
The SSPs are of great importance in determining the quality and end-use properties of the seed. Transcriptional regulation of SSP genes is achieved by a variety of protein–DNA and protein–protein interactions. Understanding the expression pattern and regulation of these genes is important to underpin the attempts to change the content of seed reserves by genetic engineering. While studies on gene regulation governing seed maturation have been performed extensively in Arabidopsis, less progress has been made concerning the mechanisms regulating cereal SSP expression. One of the major reasons is a lack of loss-of-function mutants for cereal storage protein synthesis or the relevant regulatory genes. Thus, identification and characterization of mutants is necessary to better understand the molecular mechanism of cereal SSP gene regulation and to identify new regulators.
Transcriptional regulation of SSP genes does not constitute the full extent of the genetic network underlying seed maturation. Indeed, the spatial and temporal regulation of seed maturation requires the concerted action of several signalling pathways that integrate information from genetic programmes and from both hormonal and metabolic signals. Moreover, SSP levels are regulated by several steps at the transcriptional, translational and post-translational levels (modification, processing, trafficking and deposition). In recent years, our understanding of seed maturation regulation has greatly increased, thanks to the diversity of experimental approaches available. However, some other areas, such as DNA methylation, will have to be considered in future research. DNA methylation is a stable epigenetic modification associated with transposable element silencing and gene imprinting in plant and animals. Recently, two publications have provided evidence for the extensive occurrence of demethylation during Arabidopsis seed development, especially of the endosperm (Gehring et al., Reference Gehring, Bubb and Henikoff2009; Hsieh et al., Reference Hsieh, Ibarra, Silva, Zemach, Eshed-Williams, Fischer and Zilberman2009). In one, it was demonstrated that large-scale methylation changes accompany endosperm development and endosperm-specific gene expression, and transposable element fragments are extensively demethylated in the endosperm (Gehring et al., Reference Gehring, Bubb and Henikoff2009). In the other, it is suggested that virtually the entire endosperm genome is demethylated, coupled with extensive local non-CG hypermethylation of small interfering RNA-targeted sequences (Hsieh et al., Reference Hsieh, Ibarra, Silva, Zemach, Eshed-Williams, Fischer and Zilberman2009). These discoveries provide new insights into the mechanisms regulating SSP expression.
Acknowledgements
This work was supported by the National Natural Science Foundation (Grant No. 30970230), the National Special Program for Research and Industrialization of Transgenic Plants (Grant No. 2009ZX08009-092B), and the Open Project of State Key Laboratory of Crop Biology in Shandong Agricultural University (Grant No. 2011KF10) in China.




