INTRODUCTION: LEISHMANIASIS AND LEISHMANIA SPP
Disease
Leishmaniasis manifests itself in three different clinical forms that are specified primarily by the infecting Leishmania species. The most prevalent form is the cutaneous leishmaniasis (CL) found from West Africa to Central Asia, but also in Central and South America (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). At the inoculation site, lesions develop that may ulcerate (Fig. 1A) and cause substantial scarring after mostly spontaneous healing. Secondary skin lesions may occur in diffuse cutaneous leishmaniasis (DCL, Fig. 1B). Secondary lesions in the mucosa of the nasopharyngeal region are known as mucocutaneous leishmaniasis (MCL, Fig. 1C), a highly disfiguring and due to secondary infections often lethal form that is found in South America. Treatment of CL, if attempted, is often difficult and may be complicated by the immunological predisposition of the patient. Several Leishmania species that cause CL have a zoonotic transmission cycle, compounding control efforts by the health authorities.

Fig. 1. The clinical forms of leishmaniasis. (A) Cutaneous leishmaniasis (CL) caused by L. amazonensis, Costa Rica; (B) Diffuse cutaneous leishmaniasis (DCL) caused by L. guyanensis; (C) Mucocutaneous leishmaniasis (MCL) probably by L. braziliensis; (D) Visceral leishmaniasis (VL) caused by L. donovani, India. The felt pen marks show the enlargement of spleen and liver below the ribcage (dotted line).
Visceral leishmaniasis (VL), also known by its Hindi name Kala-azar, is a chronic, life-threatening infection affecting the entire reticulo-endothelial system, and causing symptoms such as persisting fever, anaemia, weight loss and the hallmark, a massive swelling of liver and spleen (hepatosplenomegaly, Fig. 1D). Unless efficient chemotherapy is administered, VL has a mortality >90%. It is caused by two species, Leishmania donovani (India, Bangladesh, Nepal, East Africa) and Leishmania infantum (southern Europe, northern Africa, Turkey, Northern Brazil). Leishmania infantum also spread from southern Europe to South America where it was known as Leishmania chagasi until its identity was clarified (Mauricio et al. Reference Mauricio, Stothard and Miles2000; Kuhls et al. Reference Kuhls, Alam, Cupolillo, Ferreira, Mauricio, Oddone, Feliciangeli, Wirth, Miles and Schonian2011).
All existing anti-leishmanial drugs are either very costly or fraught with severe side effects limiting the applicability and/or the efficacy of the treatments. Leishmaniasis is counted among the neglected tropical infectious diseases and is considered poverty-related. A recent epidemiological survey places the disease burden at ∼1·5 million new cases of CL and approximately 0·5 million cases of VL per year (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012), not counting a suspected substantial number of unreported cases in the often poverty-stricken endemic regions, e.g. East Africa and northern India/Bangladesh.
The genus Leishmania
Phylogenetically, the leishmaniae are part of the Trypanosomatidae family, order Kinetoplastida, Euglenozoa. They share most of their genetic and cell biological features with the African and American trypanosomes that cause sleeping sickness or Chagas’ disease, respectively. Common to this ancient group of eukaryotes is a complete lack of cis-acting transcription factors and their cognate RNA polymerase II promoters. Instead, the leishmaniae and trypanosomes rely on RNA processing and stability, modulated translation and post-translational modifications to regulate gene expression and protein activity (Clayton, Reference Clayton2002). In fact, this concept was first shown using heat shock genes as a model (Hunter et al. Reference Hunter, Cook and Hayunga1984; Miller, Reference Miller1988; Argaman et al. Reference Argaman, Aly and Shapira1994; Brandau et al. Reference Brandau, Dresel and Clos1995). A view has emerged of the Kinetoplastida translating large stretches of their chromosomes as poly-cistronic pre-RNAs that are subsequently processed into mono-cistronic mRNAs by combined trans-splicing and polyadenylation (Myler et al. Reference Myler, Sisk, McDonagh, Martinez-Calvillo, Schnaufer, Sunkin, Yan, Madhubala, Ivens and Stuart2000). This, by itself, precludes gene-specific regulation of RNA synthesis.
The leishmaniae are eminently suitable for reverse and functional genetics. Due to their high rate of recombination, gene replacement using homologous recombination (Cruz et al. Reference Cruz, Coburn and Beverley1991; Ommen et al. Reference Ommen, Lorenz and Clos2009) is highly effective, allowing the creation of null mutants and subsequent phenotype analysis. Using this strategy the functions of a large number of single-copy genes were successfully analysed, with examples too numerous to list. The functions and roles of several heat shock and co-chaperone genes were analysed in this manner, including Hsp100 (Hübel et al. Reference Hübel, Krobitsch, Horauf and Clos1997; Krobitsch and Clos, Reference Clos and Krobitsch1999; Silverman et al. Reference Silverman, Clos, Horakova, Wang, Wiesgigl, Kelly, Lynn, McMaster, Foster, Levings and Reiner2010b ), Sti1 (Morales et al. Reference Morales, Watanabe, Dacher, Chafey, Osorio y Fortéa, Beverley, Ommen, Clos, Hem, Lenormand, Rousselle, Namane and Spath2010; Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012), HOP2 and HIP (Ommen et al. Reference Ommen, Lorenz and Clos2009), SGT (Ommen et al. Reference Ommen, Chrobak and Clos2010), HslU and HslV (Chrobak et al. Reference Chrobak, Forster, Meisel, Pfefferkorn, Forster and Clos2012).
Several genes of the heat shock gene families, but also of other structural genes, are amplified and often organized in tandem arrays (Lee et al. Reference Lee, Atkinson, Giannini and Van der Ploeg1988; MacFarlane et al. Reference MacFarlane, Blaxter, Bishop, Miles and Kelly1990; Hübel and Clos, Reference Hübel and Clos1996; Zilka et al. Reference Zilka, Garlapati, Dahan, Yaolsky and Shapira2001) extending over up to 70 kb and rendering those genes unlikely candidates for a homologous recombination. Interestingly, different isolates even of the same species may contain different repeat numbers of heat shock genes (Folgueira and Requena, Reference Folgueira and Requena2007) possibly indicating adaptation processes working through heat shock gene copy numbers.
Reverse genetics by use of RNA interference is also not feasible in most of the leishmaniae due to a lack of the required enzymes, e.g. Dicer. However, recent reports suggest this functionality for the South American parasite Leishmania braziliensis, a member of the Viannia subgenus (Lye et al. Reference Lye, Owens, Shi, Murta, Vieira, Turco, Tschudi, Ullu and Beverley2010). The lack of RNA interference, however, paves the way for functional cloning strategies and stable, episomal transfection using circular DNA constructs such as plasmids and cosmids. Since the leishmaniae show a high variability of gene copy numbers, both chromosomal and extrachromosomal, functional cloning and episomal transgenes are valid models for copy number-dependent gene functionality (Clos and Choudhury, Reference Clos and Choudhury2006).
HEAT SHOCK AND LIFE CYCLE CONTROL
In principle, leishmaniae exist in two different life cycle stages, promastigotes (Fig. 2A) and amastigotes (Fig. 2B); however, the promastigote stage is subdivided into rapidly dividing procyclic promastigotes and stationary phase metacyclic promastigotes (Sacks and Perkins, Reference Sacks and Perkins1984; Sacks, Reference Sacks1989; Pimenta et al. Reference Pimenta, Turco, McConville, Lawyer, Perkins and Sacks1992). Promastigotes are found in the arthropod vectors, female sandflies of the genera Phlebotomus (Fig. 2C) or Lutzomyia, attaching to the hindgut epithelium via the surface lipophosphoglycans of their flagella. Here, the procyclic promastigotes amplify by binary fission until a high parasite density is achieved, whereupon the promastigotes elongate their flagella and reduce their cell body volumes to become the highly motile and infectious metacyclic promastigotes. This form distributes through the digestive tract and can be transmitted to a suitable mammalian host during the sandfly's blood meal.

Fig. 2. Leishmania life cycle stages. (A) L. major promastigote (prom) in vitro, stained after Giemsa. k = kinetoplast, ln = leishmania nucleus; (B) Intracellular L. major amastigotes (ama) in murine bone marrow-derived macrophages, stained after Giemsa 48 h post infection. mn = macrophage nucleus; (C) Phlebotomus argentipes on clay-covered wall.
Once the leishmaniae are transmitted into the skin of a mammal, phagocytic immune cells such as neutrophilic granulocytes, dendritic cells and tissue macrophages will engulf the parasites, which will then come to reside within phagosomes. Success of the parasites depends upon their ability to block the fusion of phagosomes and lysosomes, and to establish themselves as aflagellated, non-motile amastigotes inside the macrophage population. Intracellular proliferation and concomitant destruction of macrophages will then trigger inflammatory immune responses that, in turn, cause tissue destruction and further immune cell influx. These eventually manifest themselves as skin lesions or organ enlargement.
Given a sufficient prevalence of infected antigen-presenting cells in the skin and/or the bloodstream, feeding female sandflies may take up infected cells with a blood meal, whereupon the parasites will respond to the lower temperature and sugar-rich, presumably basic, environment by converting into flagellated procyclic promastigotes.
It has been known for some time that the elevated temperature found in a mammalian host as opposed to the ambient temperatures in insects is at least one trigger for the promastigote-to-amastigote stage conversion (Bates et al. Reference Bates, Cobertson, Tetley and Coombs1992; Bates, Reference Bates1993, Reference Bates1994), the single most-important event for parasite survival. Another environmental factor is the acidic pH encountered in phagosomes (Zilberstein and Shapira, Reference Zilberstein and Shapira1994; Barak et al. Reference Barak, Amin-Spector, Gerliak, Goyard, Holland and Zilberstein2005). For at least two species of Leishmania, L. donovani and L. mexicana, the combination of both elevated temperatures and acidic milieu can trigger promastigotes to convert into amastigotes in axenic culture, thereby providing a valuable in vitro life cycle model. This allowed a detailed analysis of stage-specific protein abundance patterns induced during differentiation (Rosenzweig et al. Reference Rosenzweig, Smith, Opperdoes, Stern, Olafson and Zilberstein2008), revealing metabolic changes.
Once axenic amastigotes are resuspended in neutral pH medium at ambient temperature, they will readily revert to the promastigote form and resume rapid proliferation (Krobitsch and Clos, Reference Clos and Krobitsch1999).
EVOLVING VIEW OF HEAT SHOCK PROTEINS IN LEISHMANIA SPP
Stress protection
Initially, heat shock proteins were ascribed a presumed function in stress protection (Hunter et al. Reference Hunter, Cook and Hayunga1984; Lawrence and Robert-Gero, Reference Lawrence and Robert-Gero1985; van der Ploeg et al. Reference van der Ploeg, Giannini and Cantor1985). Life inside the mammalian host was viewed as stress, due to the elevated temperature and the hostile macrophage environment. Part of this view stemmed from the work experience where the easily maintainable promastigote was seen as ‘normal’, whereas axenically grown amastigotes or even intracellular amastigotes were ‘special’. In its natural life cycle however, the period spent in the amastigote stage measures in months and years, whereas the promastigote stage in the sand fly lasts for less than 10 days. Therefore, one can argue that in real life, the amastigote is the standard developmental form to which Leishmania is perfectly adapted (Clos and Krobitsch, Reference Clos and Krobitsch1999). Moreover, being sheltered inside a homoeothermic host for most of its life, an adaptability to extreme temperature stress is neither required nor advantageous in an evolutionary sense. Even in the transmitting sandflies, the promastigotes are not exposed to high temperatures as those insects are night-active and seek shelter in rodent burrows during the day.
Instead, a view has emerged that sees the Leishmania heat shock proteins as part of the signal transduction pathways regulating stage conversion and stage-specific gene expression (Krobitsch et al. Reference Krobitsch, Brandau, Hoyer, Schmetz, Hübel and Clos1998; Wiesgigl and Clos, Reference Wiesgigl and Clos2001; Morales et al. Reference Morales, Watanabe, Dacher, Chafey, Osorio y Fortéa, Beverley, Ommen, Clos, Hem, Lenormand, Rousselle, Namane and Spath2010; Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012), but not as a necessity for survival at mammalian body temperature (Clos and Krobitsch, Reference Clos and Krobitsch1999). This does not mean that heat shock proteins play no role in stress protection. Ongoing analyses of small heat shock proteins in fact point at a role in temperature and stress tolerance (Hombach, unpublished).
Drug resistance
Heat shock proteins may also play a role in innate resistance to antileishmanial drugs, in particular to antimony compounds. Both Hsp70 (Brochu et al. Reference Brochu, Haimeur and Ouellette2004) and Hsp90 (Vergnes et al. Reference Vergnes, Gourbal, Girard, Sundar, Drummelsmith and Ouellette2007) were shown to be over-expressed after in vitro selection under SbIII and in clinically resistant parasite isolates, respectively. The implication would be that parasites that express Hsps to higher levels are better protected against drug-induced programmed cell death (Vergnes et al. Reference Vergnes, Gourbal, Girard, Sundar, Drummelsmith and Ouellette2007). It is also possible that increased expression of Hsps may protect against oxidative stress inside activated macrophages (Wilson et al. Reference Wilson, Andersen and Britigan1994), an activation that is one of the effects of pentavalent antimony, the first-line drug against leishmaniasis. A role for Hsp90 in drug resistance is also supported by recent findings. Leishmania donovani overexpressing Histone H1 show higher sensitivity to SbIII in vitro. This increased sensitivity can be abrogated by the additional over-expression of Hsp90 (Alexandratos et al. Reference Alexandratos, Clos, Samiotaki, Efstathiou, Panayotou, Soteriadou and Smirlis2013).
Interestingly, another eukaryotic pathogen, Candida albicans, depends on Hsp90 and its co-chaperone SGT-1 for its resistance against drugs (Dai et al. Reference Dai, Wang, Li, Xu, Liang, Zhao, Cao, Jia and Jiang2012; Shapiro et al. Reference Shapiro, Zaas, Betancourt-Quiroz, Perfect and Cowen2012). Given the essential role of SGT for general Leishmania viability (Ommen et al. Reference Ommen, Chrobak and Clos2010), a function in drug resistance is difficult to verify. Nevertheless, it appears that a role for Hsp90 in drug resistance may not be restricted to the leishmaniae but is rather a common principle in eukaryotic pathogens.
Antigen
The Leishmania heat shock proteins also play an antigenic role in human infection (Skeiky et al. Reference Skeiky, Benson, Costa, Badaro and Reed1997). Hsp90 was shown to contain T cell epitopes that stimulate peripheral blood mononuclear cells to produce the cytokines IL-2, γ-interferon and tumour necrosis factor-α (Skeiky et al. Reference Skeiky, Benson, Guderian, Whittle, Bacelar, Carvalho and Reed1995). Serum antibodies from South American VL patients also recognize recombinant Leishmania Hsp90, but not the Trypanosoma cruzi homologue (de Andrade et al. Reference de Andrade, Kirchhoff, Donelson and Otsu1992), raising the possibility of using anti-Hsp90 ELISA for serological detection of Leishmania infections. On the other hand, it was shown that Hsp90 elicits IgG4 production that may be part of a weakened T helper cell class 1 response in DCL (Skeiky et al. Reference Skeiky, Benson, Costa, Badaro and Reed1997). It is now clear that several Hsps, including Hsp90, Hsp70, Hsp100 and Hsp60 (CPN60.2), constitute a large part of the protein payload of immune modulatory exosomes of Leishmania (Silverman et al. Reference Silverman, Clos, de'Oliveira, Shirvani, Fang, Wang, Foster and Reiner2010a , Reference Silverman, Clos, Horakova, Wang, Wiesgigl, Kelly, Lynn, McMaster, Foster, Levings and Reiner b ; Hassani et al. Reference Hassani, Antoniak, Jardim and Olivier2011; Lambertz et al. Reference Lambertz, Silverman, Nandan, McMaster, Clos, Foster and Reiner2012), and may thus be presented both by MHC-1 and/or MHC-2.
HSP90 IN LEISHMANIA
Hsp90 as a prototype of a regulatory heat shock protein
The function of Hsp90 as a chaperone for regulatory protein factors was first revealed when its interaction with non-ligand-bound glucocorticoid receptors was discovered (Catelli et al. Reference Catelli, Binart, Jung-Testas, Renoir, Baulieu, Feramisco and Welch1985). Chaperoning signal transduction proteins turned out to be the main ‘occupation’ of Hsp90 in eukaryotic cells, setting it apart from the traditional stress relief roles ascribed to Hsps (Rutherford and Zuker, Reference Rutherford and Zuker1994). To date, more than 200 client proteins have been identified, including oncogene products such as p53, Raf-1 and erbB2, protein kinases, transcription factors including steroid hormone receptors as well as heat shock transcription factors and the cytoskeletal proteins actin and tubulin (Ochel et al. Reference Ochel, Eichhorn and Gademann2001; Picard, Reference Picard2002). Hsp90-dependent client proteins play crucial roles in cellular signal transduction, cell cycle control and transcription regulation (Sanchez et al. Reference Sanchez, Meshinchi, Tienrungroj, Schlesinger, Toft and Pratt1987; Nathan and Lindquist, Reference Nathan and Lindquist1995; Buchner, Reference Buchner1999; Pratt and Toft, Reference Pratt and Toft2003). Apart from assisting regulatory proteins, Hsp90 also acts as an unspecific chaperone for denatured or damaged proteins (Pearl and Prodromou, Reference Pearl and Prodromou2006). The universal importance of Hsp90 is underscored by its high abundance and redundant gene copies (Chen et al. Reference Chen, Zhong and Monteiro2006).
Hsp90 in Leishmania cell cycle and life cycle
The high abundance of Hsp90 in L. donovani (Brandau et al. Reference Brandau, Dresel and Clos1995) and its high gene copy numbers (Hübel and Clos, Reference Hübel and Clos1996; Zilka et al. Reference Zilka, Garlapati, Dahan, Yaolsky and Shapira2001; Ivens et al. Reference Ivens, Peacock, Worthey, Murphy, Aggarwal, Berriman, Sisk, Rajandream, Adlem, Aert, Anupama, Apostolou, Attipoe, Bason, Bauser, Beck, Beverley, Bianchettin, Borzym, Bothe, Bruschi, Collins, Cadag, Ciarloni, Clayton, Coulson, Cronin, Cruz, Davies, De Gaudenzi, Dobson, Duesterhoeft, Fazelina, Fosker, Frasch, Fraser, Fuchs, Gabel, Goble, Goffeau, Harris, Hertz-Fowler, Hilbert, Horn, Huang, Klages, Knights, Kube, Larke, Litvin, Lord, Louie, Marra, Masuy, Matthews, Michaeli, Mottram, Muller-Auer, Munden, Nelson, Norbertczak, Oliver, O'Neil, Pentony, Pohl, Price, Purnelle, Quail, Rabbinowitsch, Reinhardt, Rieger, Rinta, Robben, Robertson, Ruiz, Rutter, Saunders, Schafer, Schein, Schwartz, Seeger, Seyler, Sharp, Shin, Sivam, Squares, Squares, Tosato, Vogt, Volckaert, Wambutt, Warren, Wedler, Woodward, Zhou, Zimmermann, Smith, Blackwell, Stuart, Barrell and Myler2005) in three different Leishmania species were also indicative of an important role in these parasites. A reverse genetic approach to characterize the role and function of Hsp90 in Leishmania was not feasible (see below). Fortunately, specific inhibitors of Hsp90 and thus of the dependent client proteins were introduced to the field in the 1990s. Geldanamycin (GA) (Whitesell et al. Reference Whitesell, Mimnaugh, De Costa, Myers and Neckers1994; Whitesell and Cook, Reference Whitesell and Cook1996; Smith et al. Reference Smith, Whitesell and Katsanis1998), and radicicol (RAD) (Schulte et al. Reference Schulte, Akinaga, Soga, Sullivan, Stensgard, Toft and Neckers1998; Sharma et al. Reference Sharma, Agatsuma and Nakano1998), both bind to the ATP binding pocket of Hsp90 family members and compete with ATP, thus blocking the ATP hydrolysis-dependent functions of the chaperone.
Using GA and RAD, inhibition studies were carried out on L. donovani promastigotes. The effects were manifold and striking (Wiesgigl and Clos, Reference Wiesgigl and Clos2001). First, inhibition of Hsp90 induced the synthesis of several heat shock proteins, including the amastigote-specific Hsp100, thus mimicking temperature elevation even when cultivated at 25 °C. Second, high doses of Hsp90 inhibitors cause a cell cycle arrest in the G2 phase. Third, treatment with GA or RAD induced the synthesis of another amastigote-specific protein family, the A2 proteins (Charest and Matlashewski, Reference Charest and Matlashewski1994; Charest et al. Reference Charest, Zhang and Matlashewski1996). This raised the possibility that reduced Hsp90 activity might be part of the signalling pathways leading to promastigote-to-amastigote conversion. Indeed, treatment of L. donovani promastigotes with GA or RAD at 25 °C and in neutral milieu (pH 7·0) induces a morphological change indistinguishable from heat and pH-triggered axenic amastigote conversion (Wiesgigl and Clos, Reference Wiesgigl and Clos2001; Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012). The morphological conversion is accompanied by a proteome change, again indistinguishable from that observed for temperature and pH-induced amastigote development (Bente et al. Reference Bente, Harder, Wiesgigl, Heukeshoven, Gelhaus, Krause, Clos and Bruchhaus2003). These data suggested a pivotal role for Hsp90 in the maintenance of the rapidly dividing promastigote stage (Wiesgigl and Clos, Reference Wiesgigl and Clos2001) and as a possible antagonist to the amastigote-promoting Hsp100 (Krobitsch and Clos, Reference Clos and Krobitsch1999).
Interestingly, L. donovani responded to non-lethal doses of GA with the spontaneous amplification of Hsp90 genes (Wiesgigl and Clos, Reference Wiesgigl and Clos2001), strongly indicating that the cytosolic Hsp90 is the target of GA and RAD in the context of pharmacologically induced differentiation. This was finally proven by the expression of a RAD-resistant L. donovani Hsp90 that mediated uninhibited growth and promastigote morphology under RAD treatment (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012).
This effect is not restricted to L. donovani. Treatment of Leishmania amazonensis with the GA derivative 17-AAG also results in a morphological change, including rounded cells and a reduction of the flagellum (Petersen et al. Reference Petersen, Guedes, Versoza, Lima, de Freitas, Borges and Veras2012). Interestingly, the closely related L. mexicana, like L. donovani, can be induced to axenic amastigote differentiation by temperature and pH (Bates, Reference Bates1994). In contrast, our attempts to induce Leishmania major amastigote conversion with GA failed (M. Wiesgigl, unpublished), reflecting the more complicated procedures required for in vitro differentiation of this species (Wenzel et al. Reference Wenzel, Bank, Florian, Forster, Zimara, Steinacker, Klinger, Reiling, Ritter and van Zandbergen2012).
Similarly, the related kinetoplastid protozoon T. cruzi, while also showing induced heat shock protein levels and growth inhibition under GA, does not differentiate towards the amastigote stage. Rather, T. cruzi bloodstream forms, trypomastigotes, form so-called spheromastigotes under GA inhibition (Graefe et al. Reference Graefe, Wiesgigl, Gaworski, Macdonald and Clos2002). The arthropod stage, epimastigote, however shows no morphological effects when treated with GA.
In Plasmodium falciparum, GA treatment of ring stages blocked the further development toward trophozoites (Banumathy et al. Reference Banumathy, Singh, Pavithra and Tatu2003). Similarly, the differentiation of Toxoplasma gondii from bradyzoites to tachyzoites and vice versa is impaired by exposure to GA (Echeverria et al. Reference Echeverria, Matrajt, Harb, Zappia, Costas, Roos, Dubremetz and Angel2005). The signals transposed by Hsp90 are therefore not restricted to temperature stress, indicating that the function of Hsp90 in life cycle control is not conserved in the protozoa.
KNOWN ASSOCIATES
Given the vast variety of client proteins that Hsp90 has to chaperone in a regulated manner, a host of so-called co-chaperones are required (Johnson and Brown, Reference Johnson and Brown2009) to form chaperone–chaperone as well as chaperone–client interactions. When interacting with signal transduction proteins, Hsp90 is part of a large complex called the Hsp90 foldosome consisting of a Hsp90 dimer and a dynamic set of chaperones and co-chaperones. The most prominent of those are Hsp70 and Hsp40, Sti1 (stress-inducible protein 1, a.k.a. Hsp organizing protein, HOP), P23, immunophilins, Aha-1 (activator of 90 kDa heat shock protein ATPase) and CDC37 (37 kD cell division control protein). Most of these are also expressed in Leishmania. Several co-chaperones, e.g. Sti1, possess one or more so-called tetratricopeptide repeat domains. Each of these domains usually consists of three 34 amino acid repeats that are known to form interactions with co-chaperone recognition motifs in Hsp90 and Hsp70 (Pearl and Prodromou, Reference Pearl and Prodromou2006). When not part of the Hsp90 foldosome, P23 which are part of the α-crystallin protein family have chaperone activity of their own (Garcia-Ranea et al. Reference Garcia-Ranea, Mirey, Camonis and Valencia2002).
While a number of chaperones and co-chaperones have been investigated in recent years, there are still large gaps in our understanding of the roles of many Hsps.
Hsp70 is a multi-copy gene-encoded (Lee et al. Reference Lee, Atkinson, Giannini and Van der Ploeg1988) protein of very high constitutive abundance (Brandau et al. Reference Brandau, Dresel and Clos1995). Its presumably essential nature, combined with the gene copy number, the presence of several additional Hsp70 family members and a lack of specific inhibitors have so far precluded a functional analysis of this protein in Leishmania. It is known to interact with the co-chaperones Sti1 and SGT (Webb et al. Reference Webb, Campos-Neto, Skeiky and Reed1997; Ommen et al. Reference Ommen, Chrobak and Clos2010; Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012).
Hsp40 gene homologues are numerous in Leishmania (Folgueira and Requena, Reference Folgueira and Requena2007), yet none has been subjected to analysis to date. Given that Hsp40, or dnaJ, specifies client–chaperone recognition, the multiplicity of Hsp40 genes may reflect a wider regulatory role for chaperone complexes in the Kinetoplastida (Kim et al. Reference Kim, Hipp, Bracher, Hayer-Hartl and Hartl2013).
Sti1 is the best-characterized co-chaperone in Leishmania. Its interaction with Hsp90 and Hsp70 was noted early on (Webb et al. Reference Webb, Campos-Neto, Skeiky and Reed1997) and shown to be crucial for all life stages of L. donovani (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012). Moreover, Sti1 was shown to be an amastigote stage-specific phosphoprotein. Reverse genetics combined with single amino acid mutations showed that two phosphorylation sites in Sti1, serine 15 and serine 481, are crucial for L. donovani viability in both promastigotes and amastigotes (Morales et al. Reference Morales, Watanabe, Dacher, Chafey, Osorio y Fortéa, Beverley, Ommen, Clos, Hem, Lenormand, Rousselle, Namane and Spath2010). This identifies Sti1 as a target of stage-specific phosphorylation pathways, i.e. signal transduction.
The LinJ.36.0080 gene was also annotated as a Sti1 gene and dubbed HOP-2 by us. However, it is not essential for L. donovani promastigotes or amastigotes (Ommen et al. Reference Ommen, Lorenz and Clos2009), at least under laboratory conditions. Unlike Sti1, which possesses the canonical three TPR domains, HOP-2 carries only one TPR domain and is not likely to provide Sti1 functionality.
HIP or Hsc70-interacting protein was tentatively identified and found to be non-essential in cultivated stages of L. donovani (Ommen et al. Reference Ommen, Lorenz and Clos2009).
SGT, the small glutamine-rich tetratricopeptide repeat protein, is not glutamine-rich at all in Leishmania, but nevertheless an essential protein that interacts stably with Hsp90, Hsp70 and Sti1 (Ommen et al. Reference Ommen, Chrobak and Clos2010). In addition, HIP may be part of SGT-containing protein complexes. Its function in Leishmania is so far unknown.
P23 was recently identified in our laboratory and found to boost GA resistance of Leishmania Hsp90 much like its yeast homologue Sba 1 (Forafonov et al. Reference Forafonov, Toogun, Grad, Suslova, Freeman and Picard2008; Ommen, Reference Ommen2009). More importantly, the lack of P23 reduces L. donovani proliferation and/or survival inside mouse macrophages after in vitro infection (A. Hombach, unpublished).
MUTATIONAL ANALYSIS OF HSP90 IN L. DONOVANI
The problems with reverse genetics and a solution
As indicated above, Hsp90 was not accessible for a detailed mutational analysis until recently. The reasons for this were manifold. As in all other eukaryotes, Hsp90 is an essential chaperone without which no cell growth is possible. This was confirmed for Leishmania by inhibitor studies (Wiesgigl and Clos, Reference Wiesgigl and Clos2001). Secondly, Hsp90 is encoded by up to 17 identical, tandemly arranged gene copies (Hübel and Clos, Reference Hübel and Clos1996; Zilka et al. Reference Zilka, Garlapati, Dahan, Yaolsky and Shapira2001; Ivens et al. Reference Ivens, Peacock, Worthey, Murphy, Aggarwal, Berriman, Sisk, Rajandream, Adlem, Aert, Anupama, Apostolou, Attipoe, Bason, Bauser, Beck, Beverley, Bianchettin, Borzym, Bothe, Bruschi, Collins, Cadag, Ciarloni, Clayton, Coulson, Cronin, Cruz, Davies, De Gaudenzi, Dobson, Duesterhoeft, Fazelina, Fosker, Frasch, Fraser, Fuchs, Gabel, Goble, Goffeau, Harris, Hertz-Fowler, Hilbert, Horn, Huang, Klages, Knights, Kube, Larke, Litvin, Lord, Louie, Marra, Masuy, Matthews, Michaeli, Mottram, Muller-Auer, Munden, Nelson, Norbertczak, Oliver, O'Neil, Pentony, Pohl, Price, Purnelle, Quail, Rabbinowitsch, Reinhardt, Rieger, Rinta, Robben, Robertson, Ruiz, Rutter, Saunders, Schafer, Schein, Schwartz, Seeger, Seyler, Sharp, Shin, Sivam, Squares, Squares, Tosato, Vogt, Volckaert, Wambutt, Warren, Wedler, Woodward, Zhou, Zimmermann, Smith, Blackwell, Stuart, Barrell and Myler2005), spanning up to 65 kb of genomic DNA. So far, no gene cluster this size could be replaced by homologous recombination in Leishmania. Given the predicted low efficiency of such a gene replacement and the propensity of Leishmania for spontaneous gene amplification (Genest et al. Reference Genest, ter Riet, Dumas, Papadopoulou, van Luenen and Borst2005), a reverse genetic approach to study Hsp90 is not promising.
This quandary was resolved by the discovery in 2009 that RAD resistance of Hsp90 in a RAD self-producing fungus, Humicola fuscoatra, is achieved by a single leucine to isoleucine exchange in the ATP binding pocket. Moreover, it was shown that the equivalent exchange in the Saccharomyces cerevisiae Hsp90 also rendered that yeast RAD resistant (Prodromou et al. Reference Prodromou, Nuttall, Millson, Roe, Sim, Tan, Workman, Pearl and Piper2009).
A similar result was achieved for L. donovani whose Hsp90 was subjected to targeted mutagenesis to create a Leu33 to Ile33 amino acid exchange mutant, Hsp90rr. The expression of this mutated Hsp90 from an episomal transgene did not affect L. donovani growth in vitro. However, once under RAD inhibition, parasites expressing the Hsp90rr variant showed normal growth and morphology, while wild type L. donovani and parasites over-expressing the natural Hsp90 were arrested in growth and developed an amastigote-like shape (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012) (Fig. 3). The latter result shows that the effect of Hsp90rr is sequence-specific and not a gene dose effect.

Fig. 3. Validation of RAD-resistant Hsp90rr. Morphological effect of RAD on promastigotes of L. donovani overexpressing Hsp90 (L. donovani [Hsp90]) or Hsp90rr (L. donovani [Hsp90rr]). Promastigotes were incubated without (– RAD) or with (+ RAD) the drug for 48 h, fixed and prepared for scanning electron microscopy (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012).
This combined strategy of inhibition and complementation can now be used to conduct a detailed mutagenetic analysis of Hsp90. Using the Hsp90rr variant as a base, additional mutations can be introduced. After transfection into L. donovani promastigotes, cell growth is ensured by the endogenously encoded wild type Hsp90. The phenotypes of the mutant transgenes will only be revealed under RAD inhibition of the endogenous Hsp90.
Hsp90 and Sti1: not just growth
The first target for the new inhibition/complementation strategy was the Hsp90-Sti1 interaction. Cytosolic Hsp90 possesses a C-terminal Sti1 recognition motif, in particular a 5 amino acid sequence, MEEVD, at the extreme C terminus. In Leishmania spp. and also in T. cruzi, the C-terminal sequence is slightly diverged, reading MEQVD instead. Nevertheless, this C-terminal pentapeptide was targeted for mutation. One mutant lacked the pentapeptide entirely while the other had the glutamine (Q) replaced with the canonical glutamic acid (E) found in most other Hsp90 at that position. Expressing the Hsp90rr lacking the pentapeptide under RAD inhibition resulted in a growth arrest and a RAD-induced shift to an amastigote-like shape. The Q to E exchange showed slightly enhanced growth under RAD (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012). These results showed a critical importance of the Sti1 recognition motif for promastigote growth and maintenance, confirming the essential functions of both Hsp90 and Sti1.
We also looked into the role of the Hsp90-Sti1 interaction during the amastigote stage. In vitro infection experiments using ex vivo macrophages and RAD pre-treated parasites expressing those Hsp90 variants showed that functional Hsp90 and the Hsp90-Sti1 interaction are both critical for the survival and proliferation of intracellular amastigotes (Hombach et al. Reference Hombach, Ommen, Chrobak and Clos2012). This forces a change of the views of Hsp90 as promastigote-promoting chaperone towards a critical function throughout the life cycle. It also explains the success of Hsp90 inhibitors in experimental treatment against Leishmania infection (Petersen et al. Reference Petersen, Guedes, Versoza, Lima, de Freitas, Borges and Veras2012).
Stage-specific phosphorylation of Hsp90 and its impact
An analysis of the L. donovani phosphoproteome (Morales et al. Reference Morales, Watanabe, Dacher, Chafey, Osorio y Fortéa, Beverley, Ommen, Clos, Hem, Lenormand, Rousselle, Namane and Spath2010) revealed that several heat shock proteins and co-chaperones are among the main targets of amastigote stage-specific phosphorylation. Hsp90 and its co-chaperone, Sti1, are both phosphoproteins, and, as mentioned above, at least two phosphorylation sites in Sti1 are critical for both life cycle stages.
During the past years, our laboratory has utilized the inhibition/complementation system described above to investigate the impact of the known and suspected phosphorylation sites in the L. donovani Hsp90. By replacing serines or threonines at eight known or suspected phosphorylation sites with the structurally neutral alanine amino acid or the phosphomimetic aspartic acid, we found that all replacements caused moderate growth reductions of the promastigote stages. However, two phosphorylation site alanine exchanges had a significant negative impact on intracellular proliferation. Moreover, replacing the phosphorylated amino acids with aspartic acid, a negatively charged amino acid that mimics a phosphorylated side chain, restored intracellular proliferation. This indicates a special function for the phosphorylated Hsp90 and for the upstream kinase(s) during the mammalian stage of the parasite's life cycle (Hombach, Reference Hombach2013).
Hsp90 depletion as signal for stage conversion?
From the data known so far, we hypothesize that depletion of Hsp90 causes the morphological changes of in vitro promastigote-to-amastigote conversion in L. donovani and possibly in other species. When promastigotes encounter elevated temperatures, some proteins will suffer stress-induced damage, thus attracting molecular chaperones such as Hsp90 and Hsp70. At the same time, the stress-induced synthesis of Hsp100 (Hübel et al. Reference Hübel and Clos1995) increases the channelling of Hsp90 and other chaperones into the exosome-based export pathway (Silverman et al. Reference Silverman, Clos, de'Oliveira, Shirvani, Fang, Wang, Foster and Reiner2010a ; Hassani et al. Reference Hassani, Antoniak, Jardim and Olivier2011), further decreasing the chaperone pools. This depletion of chaperones that can be mimicked by Hsp90 inhibition (Wiesgigl and Clos, Reference Wiesgigl and Clos2001) then leads to stage conversion, as shown schematically in Fig. 4.
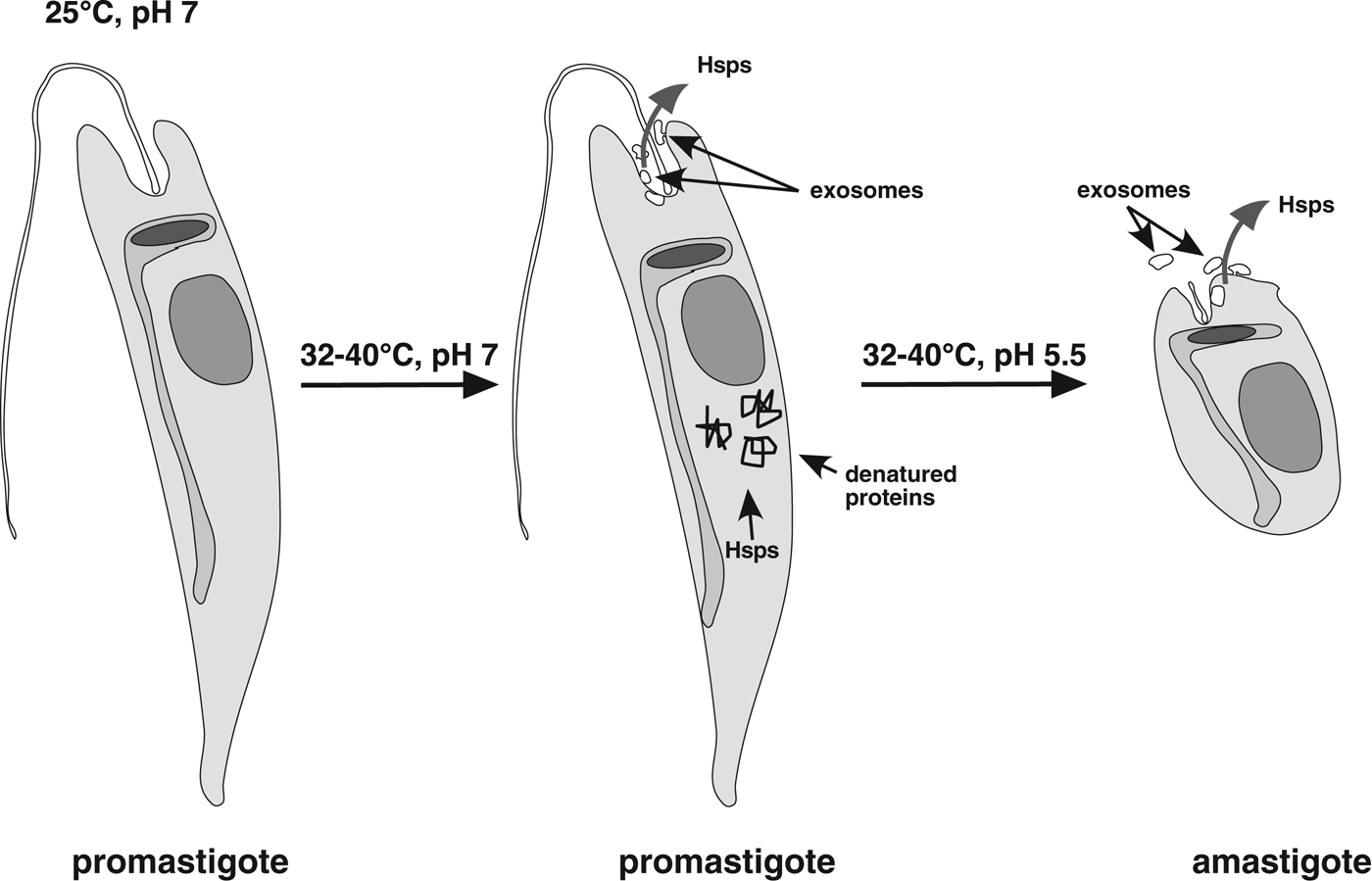
Fig. 4. Schematic model of Hsp depletion underlying stage conversion. At elevated temperatures, heat shock proteins are sequestered by denatured proteins. In addition, elevated exosome-based export of Hsp90 and other chaperones further depletes Hsp levels leading to morphological differentiation towards the amastigote.
HSP90 AS A DRUG TARGET
Hsp90 has been promoted as a target for anti-tumour drugs. Neither GA itself nor RAD are promising drugs for human use due to their liver toxicity and instability in vivo, respectively. However, a GA derivative, 17-AAG, has undergone clinical testing up to phase 2 (Whitesell and Lindquist, Reference Whitesell and Lindquist2005; Workman et al. Reference Workman, Burrows, Neckers and Rosen2007). Unfortunately, the few published reports from phase 2 trials (Gartner et al. Reference Gartner, Silverman, Simon, Flaherty, Abrams, Ivy and Lorusso2012; Oki et al. Reference Oki, Copeland, Romaguera, Fayad, Fanale, Faria Sde, Medeiros, Ivy and Younes2012) did not show promising results. Also, as of 2010, all development of 17-AAG, tested under the name Tanespimycin, was halted by the owners (The Myeloma Beacon, Reference Simkovich2010). Therefore, the drug is not likely to be available as an antiparasitic drug unless the owners offer its usage as an orphan drug. This is regrettable since initial experiments showed 17-AAG to be efficient against Leishmania at doses that are not harmful to the mammalian host cells (Petersen et al. Reference Petersen, Guedes, Versoza, Lima, de Freitas, Borges and Veras2012).
Derivatives of RAD and GA, including 19-methyl- and 19-phenylgeldanamycin, reviewed in Kitson and Moody (Reference Kitson and Moody2013), are being pursued and might prove to be viable anti-tumourigenic compounds that may even trickle down into antiparasitic usage.
Other Hsp90 inhibitors that do not bind to the N-terminal ATP binding pocket may also have potential, and therefore should be tested. Celastrol was shown to block the interaction of Hsp90 with Cdc37, but not ATP binding or interaction with HOP/Sti1 (Zhang et al. Reference Zhang, Hamza, Cao, Wang, Yu, Zhan and Sun2008). No homologue of CDC37 could be found in Leishmania (Johnson and Brown, Reference Johnson and Brown2009), but the binding of Celastrol may affect the binding of other co-chaperones, e.g. P23.
Various other inhibitors were identified, some of them already in use as drugs, such as paclitaxel (taxol) which has a proven effect on Leishmania Hsp90 (Wiesgigl and Clos, Reference Wiesgigl and Clos2001; Kitson and Moody, Reference Kitson and Moody2013). As they reach certified lead status, they should be tested as antiparasitics given the crucial role of Hsp90 in Leishmania intracellular proliferation.
Future directions
The most pressing question concerns the signal transduction pathways behind the post-translational modification events that are critical for the amastigote stage-specific functionality of Hsp90. Once identified, these pathways may serve as therapeutic targets. It is also quite conceivable that protein kinases in turn require a stage-specific Hsp90 activity for their maturation and activation. A carefully controlled phospho-proteome analysis of parasites expressing Hsp90 with varying phosphorylation site mutations may shed light on such effects.
Other post-translational modifications of Hsp90 must also be considered. It has been reported that Hsp90s from eukaryotic model organisms are subject to acetylation (Scroggins et al. Reference Scroggins, Robzyk, Wang, Marcu, Tsutsumi, Beebe, Cotter, Felts, Toft, Karnitz, Rosen and Neckers2007) and S-nitrosylation (Retzlaff et al. Reference Retzlaff, Stahl, Eberl, Lagleder, Beck, Kessler and Buchner2009). An analysis of the leishmanial Hsp90 amino acid sequences reveals that the respective residues subject to modification are conserved. However, care must be taken not to assume that all conserved residues have similar functions in Leishmania as in yeast or mammals, as we have seen in at least two instances that the effects of amino acid exchanges were quite different from the analogy-based expectations.
One may also envisage implementation of the inhibition/complementation strategy to the Hsp90s of other microorganisms that lack an RNAi system, i.e. T. cruzi or Plasmodium spp., to unravel the structure-function relationships of this chaperone in other host-parasite interactions.
Another direction worthy of pursuit is the utilization of Hsp90 and other chaperones as drug targets and as diagnostic markers, given the extreme abundance, immunogenicity and essential function of Hsp90 in Leishmania.
ACKNOWLEDGEMENTS
We thank Gerald Späth and Martin Wiese for stimulating discussions and the free communication of unpublished data, Andrea MacDonald for performing the scanning electron microscopy, Eugenia Bifeld for the Giemsa-stained Leishmania major images and Laura Lee for a careful reading of the manuscript.
FINANCIAL SUPPORT
This work was performed using intramural funding.







