Introduction
Since the establishment of the Suez Canal in 1869, hundreds of Indo-Pacific marine species migrated from the Red Sea to the Mediterranean (Galil et al., Reference Galil, Froglia and Noël2002), where they invaded new areas and changed the composition of the local communities, or in some cases modified the regional biodiversity (Por, Reference Por2010). Many authors have reported that these newly introduced species can affect the structure and functioning of Mediterranean ecosystems, in particular through competition with native species, which may alter the physical habitat and influence trophic resources (Galil, Reference Galil, Galil, Clark and Carlton2011; Katsanevakis et al., Reference Katsanevakis, Wallentinus, Zenetos, Leppäkoski, Çinar, Oztürk, Grabowski, Golani and Cardoso2014). According to Galil et al. (Reference Galil, Marchini, Occhipinti-Ambrogi, Minchin, Narščius, Ojaveer and Olenin2014), most alien crustacean species which penetrated into eastern and western Mediterranean came mainly via the Suez Canal.
The blue swimming crab, Portunus segnis (Forskål, 1775), is widely distributed in the Indo-Pacific, particularly in tropical and subtropical waters (Romano & Zeng, Reference Romano and Zeng2008). It is distributed from the eastern Mediterranean to east Africa in the Indian Ocean, and to Pakistan, the Red Sea and Persian Gulf (Ropme, 1999). It has also entered the eastern Mediterranean Sea, as a Lessepsian migrant through the Suez Canal (Ekman, Reference Ekman1967; Özcan et al., Reference Özcan, Katagan and Kocatas2005; Yokes et al., Reference Yokes, Karhan, Okus, Yüksek, Aslan-Yilmaz, Noyan-Yilmaz, Demirel, Demir and Galil2007) and become established as far north as the northern Tyrrhenian Sea (Crocetta, Reference Crocetta2006).
According to Lai et al. (Reference Lai, Ng and Davie2010), P. segnis appears confined to the western Indian Ocean from Pakistan to South Africa, and is a Lessepsian migrant into the Mediterranean from the Red Sea. Portunus segnis also lives in a wide range of inshore and continental shelf areas, including sandy, muddy or algal and seagrass habitats, from the intertidal zone to at least 50 m depth. It is usually found in large numbers in shallow bays with sandy substrates. Portunus segnis supports important fisheries in many countries such as Iran and South East Asia (Williams, Reference Williams1974; Batoy et al., Reference Batoy, Sarmago and Pilapil1987; Atar & Sector, Reference Atar and Sector2003, Wu et al., Reference Wu, Zhou, Cheng, Zeng, Wang and Feng2010).
Portunus segnis was recorded in Tunisian waters, for the first time, in October 2014, when a few individuals were collected in shallow sandy areas covered mostly by seagrass and algal beds (Rifi et al., Reference Rifi, Ounifi Ben Amor, Ben Souissi and Zaouali2014; Rabaoui et al., Reference Rabaoui, Arculeo, Mansour and Tlig-Zouari2015). In late August 2015, the species became very abundant in the region, leading to a ‘bloom’ in the coastal areas of the Gulf of Gabes (Crocetta et al., Reference Crocetta, Agius, Balisteri, Bariche, Bayhan, Cakir, Ciriaco, Corsini-Foka, Deidun, El Zrelli, Erguden, Evans, Ghelia, Giavasi, Kleitou, Kondylatos, Lipej, Mifsud, Ozvarol, Pagano, Portelli, Poursanidis, Rabaoui, Schembri, Taskin, Tiralongo and Zenetos2015); and its presence became more and more relevant on the south-eastern Tunisian coasts.
The present study was undertaken to investigate, for the first time in the Mediterranean, the food and feeding habits of P. segnis collected from the south-eastern coasts of Tunisia and for informing future potential farming projects for this species in the future.
Materials and methods
The study was carried out in the south-eastern waters of Tunisia represented by the Gulf of Gabes, where the species is fished by trawlers, seiners and by coastal fishing boats using trammel nets, gill nets and traps. Crabs of 50–168 mm carapace width were collected monthly between October 2015 and September 2016 at the main landing points of the species. Studies on food and feeding were adapted from Sukumaran (Reference Sukumaran1995). After recording morphometric data, crabs were dissected; the fullness of the stomach was visually examined and assessed as 0, 25, 50, 75 or 100%. The foreguts of the crabs were then removed and their exteriors washed in water, holding both ends closed with forceps. Each foregut was then opened and its contents emptied into Petri dishes containing fresh water.
No single method of stomach contents analysis completely describes the diet of a predator (Hyslop, Reference Hyslop1980); hence, the vacuity index (VI) was calculated (as follows: number of empty stomachs divided by total number of stomachs multiplied by 100), as well as Gastro-somatic index (GaSI) (as follows: weight of stomach (g) divided by crab total body weight (g) multiplied by 100) to describe the trophic behaviour of the species. Individual food organisms were sorted and identified to the lowest possible taxonomic level. Most food items were found to be identifiable as the examined crabs were fished the same day and were directly frozen to stop the digestion process. For crushed prey, only the hard structures that could be identified were relied upon for determining food composition and further analysis. The number of prey found in each stomach was recorded to determine the feeding pattern of P. segnis. Each prey item was wet weighed to the nearest 0.001 g.
The frequency of occurrence method was used for analysis of food items (Williams, Reference Williams1981, Reference Williams1982). This method is widely used in dietary studies of fish and crabs, and gives a measure of the regularity with which food has been taken up in the sample or population. It is specifically recommended when different food items contribute to the diet (Dahdouh-Guebas et al., Reference Dahdouh-Guebas, Giuggioli, Oluoch, Vannini and Cannicci1999). This method entailed recording the number of stomachs containing individuals of each food category, expressed as percentage of all the stomachs examined according to the formula: percentage frequency of occurrence (%F): number of stomachs in which a food item was found, expressed as a percentage of the total number of full stomachs. The food item with the highest value was taken as the most important one.
In order to perform a qualitative and quantitative description of the diet, the following indices were also used:
• Percentage of numerical abundance (%Cn): number of each prey item expressed as a percentage of the total number of food items in all stomachs.
• Percentage of gravimetric composition (%Cw): total weight of each prey item, expressed as a percentage of the total weight of stomach contents.
• Index of relative importance (IRI) (Pinkas et al., Reference Pinkas, Oliphant and Iverson1971) as modified by Hacunda (Reference Hacunda1981), to estimate the contribution of prey items in the fish diet:
where %F is the percentage frequency of occurrence, %Cn is the percentage of numerical abundance and %Cw is the percentage of gravimetric composition.
The index was expressed as a percentage as follows:
It facilitates comparisons to other studies, provides a single measure of the diet, and is less biased than weight, frequency or number alone (Cortès, Reference Cortès1997).
Variations in food habits were investigated in relation to sex, crab size and seasons. Crabs were grouped into four size classes: <80 mm (juveniles); 80–109 mm (medium size crabs or subadults); 110–139 mm and ≥140 mm carapace width (adults). Variation of food habits with respect to season, sex and size were analysed using two-way analysis of variance followed by a Chi-square test, where significant differences were present at P < 0.05 (Zar, Reference Zar1999). The differences of food habits with regard to ovigerous and non ovigerous females were tested using χ 2 test.
Results
Trophic behaviour
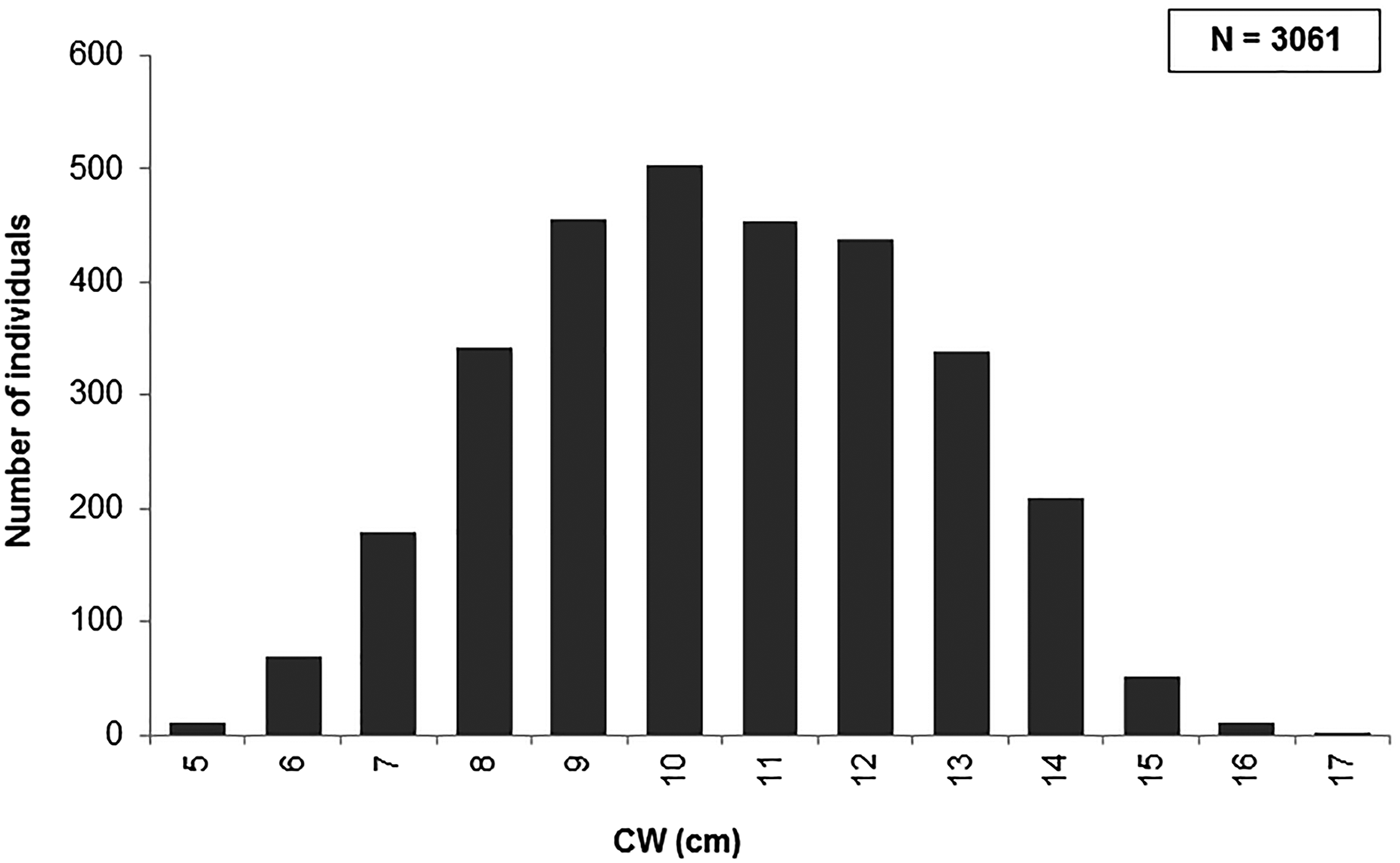
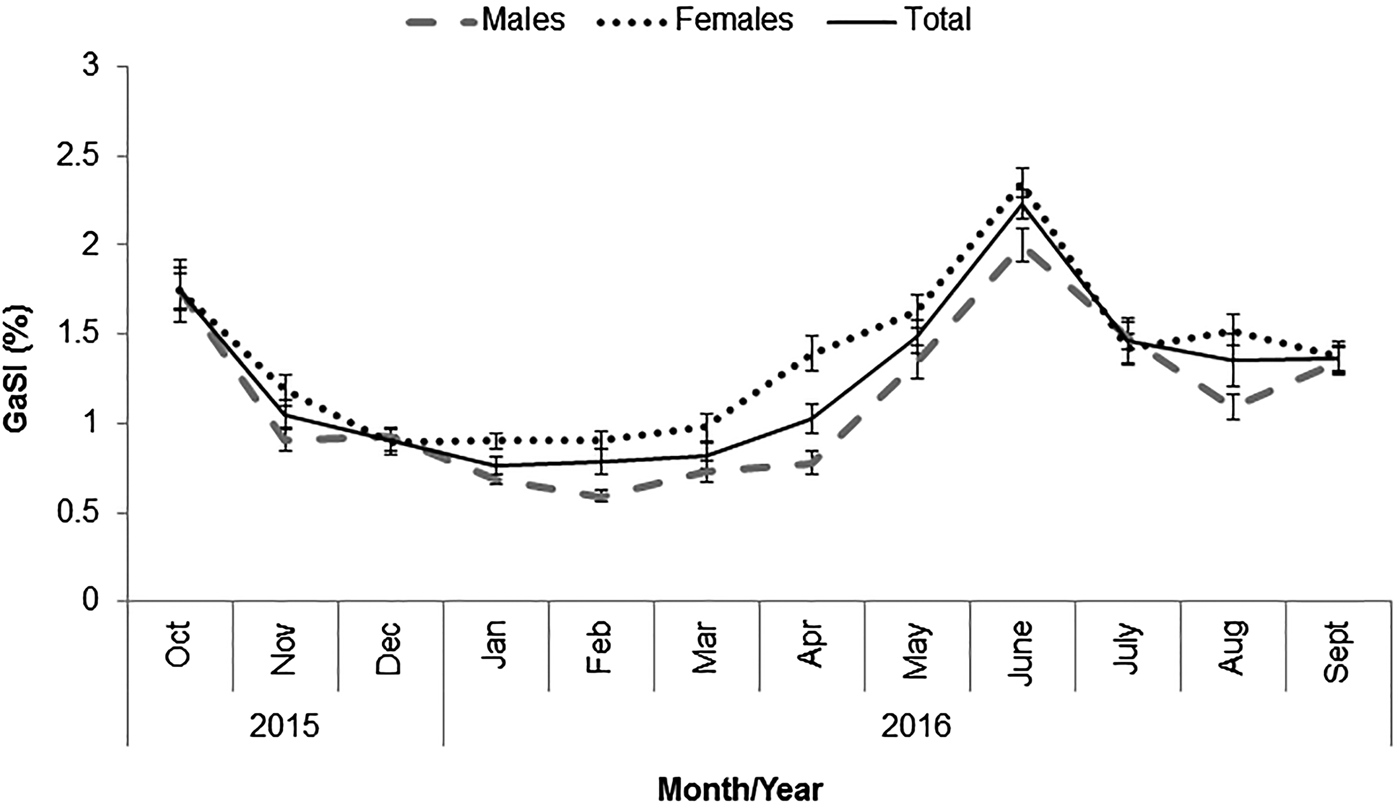
In total, 3061 individuals (1538 males and 1523 females) of P. segnis were analysed (Figure 1), with 1065 male crabs (69.3%) presenting trace-full stomachs and 473 (30.7%) with empty stomachs, while 1163 females (76.4%) had trace-full and 360 (23.6%) with empty stomachs. The Gastro-somatic Index (GaSI) had minimum value in January and increased until maximum value in June (Figure 2). The vacuity index (for both sexes) showed monthly increase fluctuations from October 2015 until January 2016 and decline fluctuations from February 2016 to September 2017. The VI (for both sexes) had a minimum and maximum in October and June, and January–February, respectively. Moreover, the highest and lowest values for males were observed in February (57.6%) and October (2.5%). While, in female crabs, the highest and lowest VI were observed in February (44.5%) and June (3.5%), respectively (Figure 3).

Fig. 1. Length frequency distribution of examined Portunus segnis. CW, carapace width.

Fig. 2. Monthly variation of the Gastro-somatic Index (GaSI) in Portunus segnis.

Fig. 3. Monthly variation of the vacuity index (VI) in Portunus segnis.
There were highly significant differences between means of VI for both sexes in different months (for males: χ 2calculated = 146.4>> χ 2theoretical = 19.7; for females: χ 2calculated = 146.1>> χ 2theoretical = 19.7, P < 0.0001), and also a significant difference (P < 0.05) between means of VI for females and males. The VI varies significantly among sizes (χ 2calculated = 30.5>> χ 2theoretical = 7.8), being at its lowest value (23.2%) for 8–10.9 cm CW and at its highest value (32.8%) for 11–13.9 cm CW.
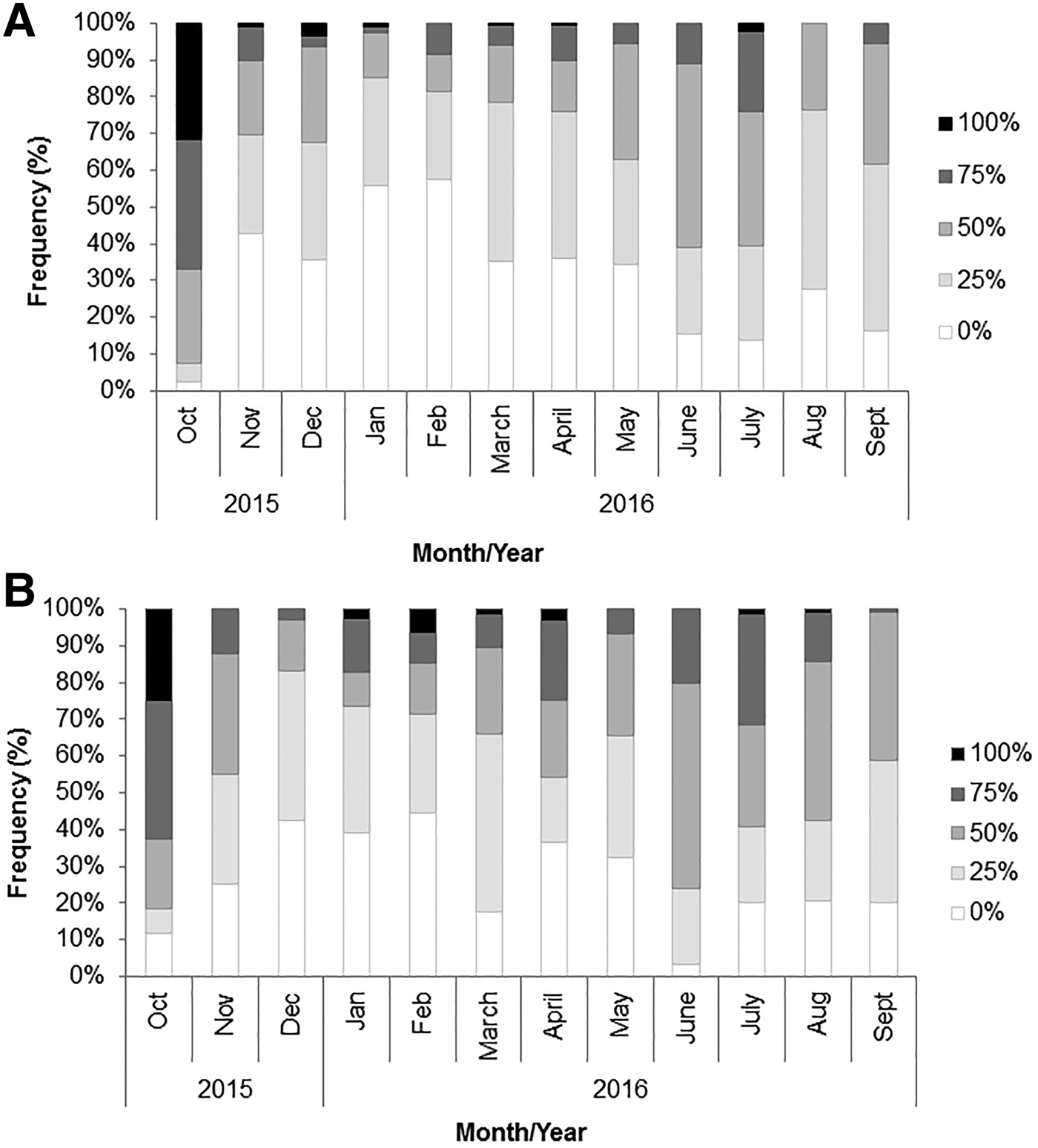
Out of all male stomachs examined, 32.8% were full or empty (30.8%), with the intermediate categories ranging from 9.2–33.1%; for females 26.1% were represented by full or empty (23.4%) stomachs, and the others in a range of 13.5–31.8%, with inferior limit for full category represented by stomachs with 75% of fullness. Details by month are given in Figure 4. The maximum number of full stomachs was observed in the largest individuals whilst the minimum was recorded in juveniles (Figure 5). However, stomach fullness did not show significant differences between the different size groups (χ 2calculated = 2.3 < χ 2theoretical = 21).

Fig. 4. Stomach fullness during various months in males (A) and females (B) of Portunus segnis.

Fig. 5. Stomach fullness in different size groups (CW, carapace width in mm) of Portunus segnis.
Among examined females, 124 were ovigerous, ranging in size between 5–15.9 cm CW, and 1999 were non-ovigerous, ranging in size between 8.9–15.6 cm CW. A comparison of the feeding behaviour of ovigerous (8.14%) and non-ovigerous females (91.86%) showed that there was no significant difference neither in the VI (χ 2calculated = 0.08 < χ 2theoretical = 3.84) nor in the GaSI (ANOVA, F = 1.172; P > 0.05) between the two groups.
Diet composition
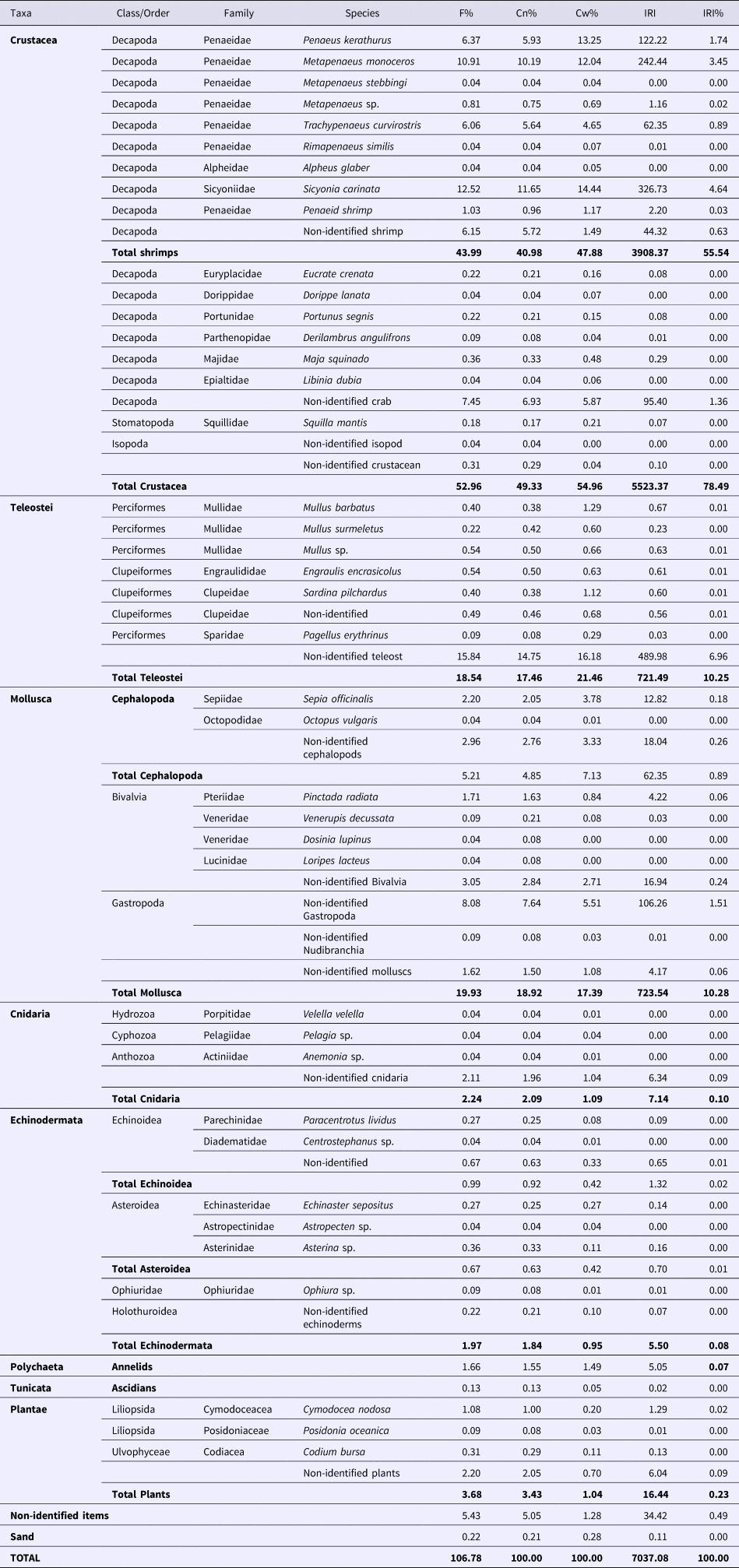
The stomach contents of P. segnis appeared to consist mainly of large quantities of crustaceans, teleosts and molluscs, and small quantities of echinoderms, cnidarians, annelids, tunicates, plant remains and debris (Table 1). These six latter items were grouped and considered as ‘miscellaneous’. In the percentage of IRI, crustaceans formed the most dominant food group, and were found in 78.5% of the stomachs ‘with food’. This fraction consisted primarily of decapods such as Sicyonia carinata (Brünnich, 1768) and Metapenaeus monoceros (Fabricius, 1798), stomatopods and isopods. Molluscs and teleosts formed the second and the third most important food items with 10.3%IRI and 10.2%IRI, respectively. Shrimps were the main ingested crustaceans (55.5%IRI), whilst for molluscs, shellfish were the most important prey (4%IRI). The other food items were of minor importance.
Table 1. Diet composition of Portunus segnis in the Gulf of Gabes.

F%: the percentage frequency of occurrence; Cn%: the percentage of numerical abundance; Cw%: the percentage of gravimetric composition; and IRI: the index of relative importance.
The ‘miscellaneous’ group mainly comprised echinoderms (Echinoidea, Ophiuridae, for example) and plant material as well as cnidarians, annelids, tunicates and debris was present in 15.3% of the stomachs with food and represented 1.15%IRI.
Diet composition in relation to season
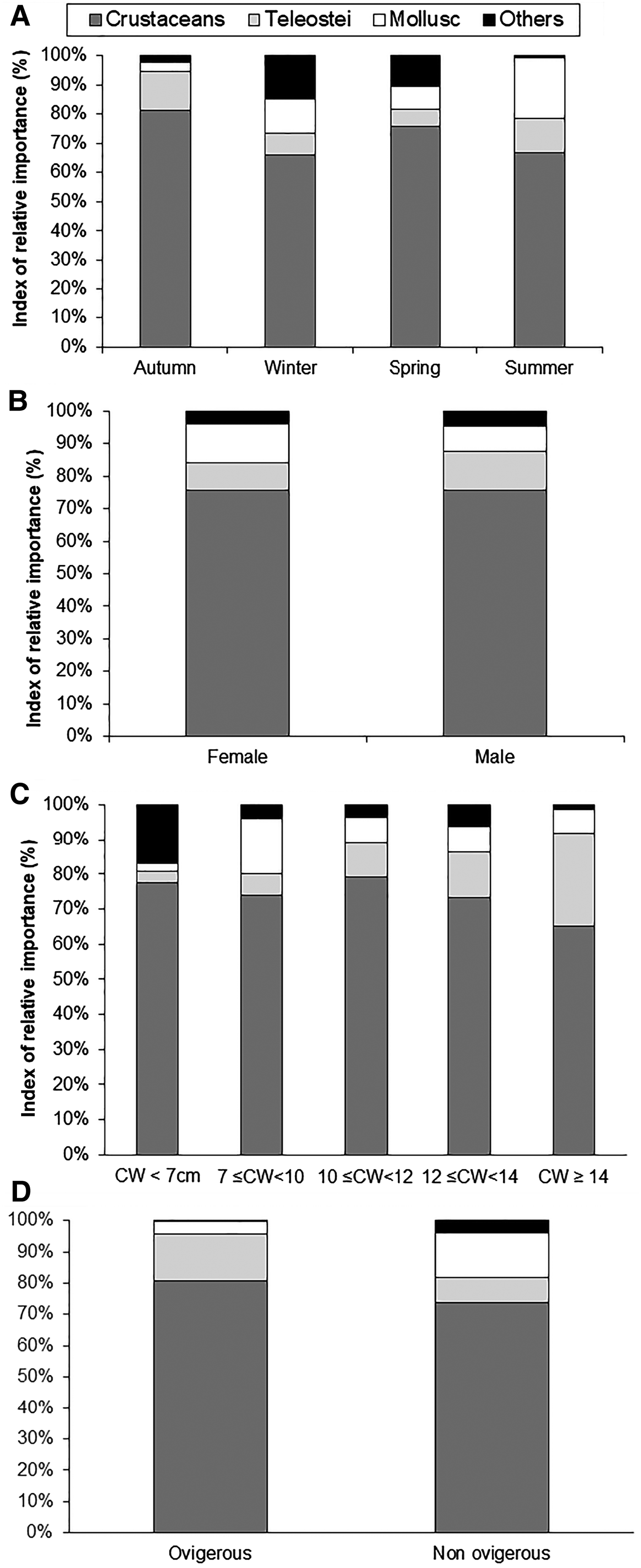
Some seasonal variation in the foregut contents was detected for P. segnis. Crustaceans were consumed throughout the whole period but in term of %IRI, were the most dominant in autumn, and were found in 81.4% of the stomachs with food (Figure 6A). Molluscs ranged between 3.4–20.8% in various seasons, and were more abundant as food during summer. Teleosts ranged between 5.6–13.0% in spring and autumn, respectively. There were highly significant differences in the preference for food items in the different seasons of the crab (χ 2calculated = 162.2>> χ 2theoretical = 16.9, P < 0.05). In fact, there was a significant variation of molluscs, teleosts and miscellaneous items between seasons.

Fig. 6. Diet composition of Portunus segnis among season (A), sex (B), and size classes (C) based on percentage index of relative importance (IRI) values of major prey groups in the Gulf of Gabes.
The mean number of prey items (Nm/ST) and mean weight of prey items (Wm/ST) per stomach was more important during the summer–autumn period than during the winter–spring period. The mean number of prey items (ANOVA, F = 1.1; P > 0.05) was not significantly different among seasons while the mean weight of prey items per stomach (ANOVA, F = 4.071; P < 0.05) was significantly different between seasons (Figure 7A).

Fig. 7. Variation of the mean weight of prey per stomach (Wm/ST) and mean number of prey items per stomach (Nm/ST) of Portunus segnis among seasons (A) and size classes (B).
Diet composition in relation to sex
Considering the dietary composition for both sexes, there were slight dietary differences between sexes (χ 2 = 8.5, P < 0.05) (Figure 6B). Crustaceans were the main prey in both males and females, reaching 75.8%IRI and 75.5%IRI respectively. Molluscs were the second most dominant group in females, constituting 12.0%IRI (7.7% IRI in males). However, teleosts were the second main prey for males and constituted 11.9%IRI (8.4%IRI in females).
Diet composition in relation to size
Ontogenetic differences in the diet of P. segnis were apparent (χ 2 = 50.67; P < 0.05), and the frequency of molluscs (χ 2 = 18.3; P < 0.05) and teleosts (χ 2 = 15.5; P < 0.05) varied significantly with predator size, while the frequency of crustaceans did not show any significant variation (χ 2 = 0.96; P > 0.05), when the individuals grow up.
In crabs <8 cm CW, crustaceans, teleosts, molluscs and miscellaneous accounted for 77.8, 6.1, 9.4 and 6.7%IRI respectively. Whilst in the following size-group (8–10.9 cm), ‘miscellaneous’ were of lesser importance (3.8%IRI) and the contributions of teleosts (7.0%IRI) and molluscs (14.2%IRI) increased. However, the diet of larger crabs CW≥11 cm consisted almost exclusively of crustaceans (65.3–76.7%IRI), while teleost fish (12.3–26.7%IRI) formed the second group and molluscs (6.1–6.7%IRI) represented the third group (Figure 6C).
The mean number of prey items (Nm/ST) and mean weight of prey items per stomach were found to increase with increased size of the predator. Portunus segnis seems to ingest more and larger prey when growing up. However, the Nm/ST (ANOVA, F = 1.731; P > 0.05) and Wm/ST (ANOVA, F = 2.790; P > 0.05) were not significantly different between sizes (Figure 7B).
Diet composition in relation to female reproductive state
A comparison of food habits of ovigerous and non-ovigerous females is presented in Figure 6D. Significant differences were found in the food preferences between the two groups of females (χ 2 = 15.03; P < 0.05). The latter group was found to ingest more molluscs and miscellaneous food items, and less crustaceans and teleosts than the former group.
Discussion and conclusions
In the Mediterranean, several migrants have now become common in local fish landings and markets (Oral, Reference Oral2010). In the 1920s the blue swimming crab Portunus segnis was fished for sale in the markets at Alexandria and at Haifa (Fox, Reference Fox1927) and by 1962 (to 1983) it dominated decapod fisheries near Alexandria (Abdel-Razek, Reference Abdel-Razek1987). Crab production is one of the most valuable fishery resources on the Mediterranean coast of the Sinai Peninsula (Abdel Razek et al., Reference Abdel Razek, Taha and Ameran2006). In the 2000s, it was sold in huge quantities at the fish markets of Sicily (Crocetta, Reference Crocetta2006). The species is also commercially important on the Mediterranean coast of Turkey, particularly at local fish markets in Mersin and Iskenderun Bays (Ozcan, Reference Ozcan2012). Portunus segnis invaded southern Tunisian waters by the end of 2015 and became an important component of the Gulf of Gabes fisheries and has a growing economic value. In fact, the species is commercially important in some local markets in Tunisia, but is also becoming a value-added product for export to Asian and European markets. Until November 2018, a quantity of almost 3100 tons of P. segnis were exported mainly to Thailand, Malaysia and Vietnam (GIPP, 2018).
Knowledge of an animal's dietary habits is essential for nutritional requirements, interactions with other organisms and for its breeding (Santos & Borges, Reference Santos and Borges2001). Crabs inhabit many different environments (Chande et al., Reference Chande, Nikundiwe and Kyomo1999; Dahdouh-Guebas et al., Reference Dahdouh-Guebas, Giuggioli, Oluoch, Vannini and Cannicci1999; Kyomo, Reference Kyomo1999; Bryceson & Massinga, Reference Bryceson and Massinga2002) and this is reflected in the diversity of food eaten. Portunus segnis feeds on macroscopic food. Researchers therefore use the contents of the foregut to identify the food consumed (e.g. Williams, Reference Williams1981; Cannicci et al., Reference Cannicci, Dahdouh-Guebas, Anyona and Vannini1996). There is, as yet, no established methodology for the quantitative description of gut contents in crustaceans that feed on macroscopic food items. Several studies of foregut contents have used the percentage of occurrence of food types as the only measure of relative intake of different food items (Donaldson, Reference Donaldson1975; Hill, Reference Hill1976; Williams, Reference Williams1982; Dahdouh-Guebas et al., Reference Dahdouh-Guebas, Giuggioli, Oluoch, Vannini and Cannicci1999). Other studies have used the points method, in which each food category is awarded points proportional to its estimated contribution to stomach volume, taking into account the size and abundance of the food item (Hynes, Reference Hynes1950; Hyslop, Reference Hyslop1980).
In this research, the VI for the whole study was 72.8%, ranging between 2.5–57.6% and 3.5–44.5% for males and females, respectively. These values were similar to those reported by Safaie (Reference Safaie2016) for P. segnis in the northern coastal waters of Iran, in which VI varied between 6.9–56% for males and 16.5–48% for females. Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) noted a global VI of 22.4% for P. segnis from Persian Gulf.
In the Gulf of Gabes, P. segnis appeared to be an omnivorous species with a preference for animal material. This finding conforms to those of several authors working on the same species or other portunid crabs. Josileen (Reference Josileen2011) and Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) as well as Zainal (Reference Zainal2013) and Safaie (Reference Safaie2016) described this species as omnivorous, and that its diet preference for animal matter was evident. The results showed that P. segnis in the Gulf of Gabes feeds primarily on crustaceans, followed by molluscs and teleost fish. The species of crustacean that occurred in the stomach content of this crab were, mainly, the Mediterranean rock shrimp Sicyonia carinata, the speckled shrimp Metapenaeus monoceros, the caramote prawn Penaeus kerathurus and the archer shrimp Trachysalambria curvirostris. Algae and seagrass were of minor importance in the diet of P. segnis. This finding suggests that plant materials may be ingested accidentally as the main ingested prey items are, generally, gleaned from among algae and seagrass (Williams, Reference Williams1981). Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) and Tadi et al. (Reference Tadi, Pazooki and Safaie2012) also observed that crustaceans constitute the most favoured item in the diet of P. segnis, followed by molluscs and fish, in the Persian Gulf. According to Zainal (Reference Zainal2013), fish and crustaceans were the dominant components of the diet of P. segnis along the coastal waters of the kingdom of Bahrain. The author highlighted, also, that the diet of this crustacean appeared to be very variable and indicated an ability to adopt different modes, and that the species would ingest any food item depending on the availability. In the northern coastal waters of Iran, crustaceans constitute the most favoured item in the diet of P. segnis, followed by fish and molluscs (Safaie, Reference Safaie2016).
Furthermore, this type of diet has been widely reported for many large portunid crabs, particularly P. pelagicus, on which Patel et al. (Reference Patel, Chhaya and Bhaskaran1979), in India, reported pieces of crabs, gastropods and bivalve shells, and sometimes fish to be the main food types, while Williams (Reference Williams1982) in Moreton Bay, Queensland, reported benthic invertebrates such as bivalves, polychaetes and crustaceans as its diet. The importance of food types in the diet differs depending on the nature of the habitat and their availability. The composition of the diet recorded for P. pelagicus by Potter & De Lestang (Reference Potter and De Lestang2000) demonstrates, that in south-western Australia, this species can be regarded as essentially a benthic carnivore and feeds mainly on slow-moving or relatively sessile benthic macroinvertebrates (e.g. amphipods, polychaetes and bivalves and gastropod molluscs) and also occasionally on benthic teleosts (e.g. gobies). Josileen (Reference Josileen2011) noted that crustaceans were the most dominant food group, while molluscs and teleost fish formed the second and the third most important food item for P. pelagicus on the Mandapam coast in India.
In the present study, slight dietary differences between sexes in terms of food item preference were observed. Crustaceans were the main prey in both males and females; while molluscs and teleosts were the second most dominant groups in females and males, respectively. Zainal (Reference Zainal2013) noted that there were no significant differences between sexes in term of food preference except for the presence of fish tissues which were more present in the stomachs of males than in females. This author reported that greater stomach fullness was also observed in males. Furthermore, ovigerous and non-ovigerous females have different feeding habits. Comparison of ovigerous and non-ovigerous females revealed that both groups fed on the same food items, but the percentage of relative importance of crustaceans and teleosts were higher for ovigerous females. However, Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) concluded also that the frequency of occurrence of the food items for ovigerous and non-ovigerous females is different.
In the southern Tunisian waters, in all size groups, crustaceans were the most ingested prey group. In sub-adults (8–10.9 cm CW), ‘miscellaneous’ were of lesser importance and the contributions of teleosts and molluscs increased. However, the diet of adults (11–13.9 cm CW) and the larger adult crabs (CW≥14 cm) consisted almost exclusively of crustaceans, while fish formed the second group and molluscs represented the third group. Differences in the preference for food items between different crab sizes were also reported for P. segnis by several authors (Pazooki et al., Reference Pazooki, Hosseini and Zadeh2012; Safaie, Reference Safaie2016). Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) observed that crustaceans were the major food item for all size groups, and were followed by molluscs in juvenile and adult crabs, molluscs and fish in the subadult group and fish and molluscs in larger size adult crabs. Furthermore, Safaie (Reference Safaie2016) reported that crustaceans formed the main prey item group in juvenile, subadult and adult crabs; while fish dominated in the stomach contents of the larger adults. The difference in the preference for food items among crab size is presumably related to the influence of an increase in the size of the chela and muscle mass on the type of prey that is most susceptible to predation (Freier et al., Reference Freier, Paz Sampedro and Gonzáles-Gurriáran1996) and to greater ability of larger crabs to ingest larger prey such as polychaetes (Nereididae).
There were highly significant differences in the preference for food items of P. segnis in the different seasons in the Gulf of Gabes. In fact, ANOVA performed for each dependent variable (group taxa) indicated that molluscs were the source of difference among sexes whereas teleosts, molluscs and miscellaneous were responsible for the statistically significant differences among size classes. Molluscs were involved in all interactions. These findings are in contrast with those reported by Pazooki et al. (Reference Pazooki, Hosseini and Zadeh2012) and Safaie (Reference Safaie2016). Furthermore, Zainal (Reference Zainal2013) highlighted that there was some evidence of temporal variation in the stomach fullness of P. segnis along the coastal waters of the Kingdom of Bahrain.
In conclusion, P. segnis is a large crab, which has successfully invaded the south-eastern coasts of Tunisia. Despite its importance for fisheries, there was no information on the diet and relative importance of food types of this Indo-Pacific invader in Tunisian waters. The aim of the present study, therefore, was to investigate the food habits of P. segnis along the coast of the Gulf of Gabes with respect to sex, size, reproductive state, as well as season. The large size and abundance of the blue swimming crab, P. segnis, make it an important predator of crustaceans, molluscs and fish. Portunus segnis is an opportunistic predator, the diet of which depends on local availability of food items. The distribution and biological aspects (e.g. growth, reproduction) of crabs are largely dependent on the availability of preferred prey organisms. Diet analysis data provide the basis for the species ecology and behaviour and its potential for mariculture. Indeed, the international market demand for P. segnis is mainly targeted on the fresh, live product. This has prompted many investors to think about crab farming for export purposes. So, the information provided by this study will be of great interest for developing future farming projects for the species in this area. Feeding is the dominant activity in the entire life cycle of this species, thus detailed knowledge of food and feeding habits is essential for successful culture.
Author ORCIDs
Olfa Ben Abdallah-Ben Hadj Hamida, 0000-0001-9361-6709.
Acknowledgements
We wish to express our gratitude to several colleagues, in particular Z. Hentati and F. Ltifi, who helped with sample treatment and processing, and data archiving.