Introduction
Skin cancer is the most common cancer afflicting humans. More than one million additional cases of skin cancer are expected to be newly diagnosed in the US in 2007.Reference Jemal, Siegel, Ward, Murray, Xu and Thun1 The skin of the head and neck accounts for less than 10 per cent of the body's surface area; however, as a result of greater sun exposure in occupational and recreational activities, this region accounts for 70 to 80 per cent of skin cancers. Skin carcinogenesis is a multistep process in which an accumulation of genetic events within a single cell line leads to a progressively dysplastic cellular appearance, deregulated cell growth and finally carcinoma.Reference Tsao, Kim and Hong2
Thus, chemoprevention represents a promising strategy for inhibition of carcinoma occurrence before invasive tumour develops. However, the standard chemotherapeutic approaches currently used for the treatment of early skin lesions are far from satisfactory, especially given their significant toxicity. Such toxicity also prevents the use of adequate doses and sufficient treatment periods to achieve the desired results.
The topical application of chemopreventive agents has been proposed, in order to reduce the risk of systemic toxicity while still preserving treatment efficacy.Reference Wang, Polavaram and Shapshay3 The development of both effective drugs and practical delivery approaches is key to the establishment of effective topical chemoprevention.
Cyclo-oxygenase and lipoxygenase are two important enzyme classes involved in carcinogenesis. Cyclo-oxygenase-2 selective inhibitors exhibit chemopreventive activity against ultraviolet (UV) light induced skin carcinogenesis in mice. However, cyclo-oxygenase-2 inhibitors alone have limited effectiveness in inhibiting skin cancer.Reference Wilgus, Koki, Zweifel, Rubal and Oberyszyn4, Reference Wilgus, Breza, Tober and Oberyszyn5 Therefore, enhancing the tumour-fighting capabilities of cyclo-oxygenase-2 inhibitors, by targeting additional pathways involved in tumour growth, may prove to be beneficial in inducing tumour regression. A number of studies have shown that aberrant arachidonic acid metabolism, especially cyclo-oxygenase-2 and 5-lipoxygenase pathways, are activated during various carcinogenetic processes, including skin cancer development.Reference Steele, Holmes, Hawk, Kopelovich, Lubet and Crowell6, Reference Ye, Wu, Shin, Bruce, Wong and Cho7 Dual inhibitors that block both the cyclo-oxygenase and lipoxygenase metabolic pathways of arachidonic acid have an advantage over agents inhibiting cyclo-oxygenase alone.Reference Li, Sood, Wang, Fang, Wang and Sunet8 At present, the chemopreventive effects of 5-lipoxygenase pathway inhibitors have been demonstrated in animal models of skin cancer.Reference Yan, Wang, Zuo and Qu9 However, to the best of our knowledge, there is little current information on the chemoprevention of skin cancer using dual inhibitors of 5-lipoxygenase and cyclo-oxygenase-2, especially when applied topically.
Celecoxib (a Cyclo-oxygenase-2 inhibitor) and zileuton (a specific inhibitor of 5-lipoxygenase) inhibit tumour growth by targeting different arachidonic acid metabolic pathways. In the current study, our aim was to determine whether combination of these two inhibitors would result in an additive effect on cancer chemoprevention. Previously, we had observed their efficacy in early treatment to prevent and inhibit the growth of skin squamous cell carcinoma (SCC) cells in a nude mouse model. Recent studies have provided evidence that a specific microemulsion with high permeation rate and significant anti-inflammatory activity may serve as a vehicle for topical delivery of pharmaceutical agents to skin lesions.Reference Subramanian, Ghosal and Moulik10 In this study, topical delivery of zileuton and celecoxib was achieved using such a microemulsion system.
Materials and methods
This study was performed in accordance with the Public Health Service policy on human care and use of laboratory animals at US. The animal use protocol was approved by the institutional animal care and use committee at the Boston University School of Medicine. Animal care was in accordance with institutional guidelines.
Cell culture and animals
All experiments used a cell line of human skin SCC (ATCC, Rockville, Maryland, USA). Cells were grown in a monolayer growth medium, at 37°C, in a humidified atmosphere of 5 per cent CO2 and 95 per cent air. The cells were harvested on day six, followed by trypsinisation and centrifugation, and re-suspended in normal saline to achieve a concentration of 106 cells/ml. Homozygous (nu/nu) mice aged 40–50 days were purchased from Taconic Farms (Germantown, New York, USA). Under general anaesthesia, the flank skin of each mouse was inoculated intradermally with the cell suspension. Inoculation was conducted under an operating microscope (at a magnification of×10) using a 30-gauge hypodermic needle. Each mouse received four inoculations in the flank skin (with 50 000 cells per single injection in 0.05 ml saline) to create four seeding sites in each animal. All mice were weighed before inoculation and after the last day of the topical chemoprevention course.
Microemulsion preparation
Microemulsions consisted of 22 per cent isopropyl myristate, caprylic/capric mono-/di-glycerides (as Capmul MCM) (2:1), 30 per cent polysorbate 80 and water (all weight for weight). Isopropyl myristate and polysorbate 80 were purchased from Sigma (Sigma-Aldrich, Wisconsin, USA). The Capmul MCM was a gift sample from Abitec (Abitec, Northampton, UK) and comprised an approximately 5:1 mixture of C8/C10 mono-diglycerides with 2 per cent free glycerol and the following percentages of fatty acids: caprylic (C8), 83 per cent; capric (C10), 15.5 per cent; caproic (C6), 1.0 per cent; and palmitic (C16), <1.0 per cent. Doubly distilled water was used in all experiments.
Treatment and tumour measurement
In this study, 24 mice (with a total of 96 inoculated skin sites) were randomly divided into three groups of eight mice (32 sites) each. Group one served as a control and received no treatment. Group two (receiving 6 per cent celecoxib) and group three (receiving 6 per cent celecoxib plus 6 per cent zileuton) were treated topically with the respective chemopreventive agent. The topical microemulsion was painted onto the skin to cover a 5.0 × 5.0 mm skin area overlying the targeted inoculation sites, and this was repeated twice per day. The treatment was performed for five days, weekly for two weeks. The number of inoculated skin sites that developed a visible tumour was recorded daily. Tumour size was measured using a sliding caliper and was calculated using the formula V = abc (∏/6) (where a, b and c were three orthogonal diameter measurements). The animal inoculations and tumour measurements were conducted under general anaesthesia induced by CO2 inhalation for 5–7 seconds. The results were also documented photographically.
Statistical analysis
Tumour volumes across the three groups were analysed by repeated measure analysis of variance (ANOVA) for global differences among the three groups as a whole; data then underwent the least significant difference (LSD) t-testing for comparison of two-tailed p values. The mice's body weights were measured once a week. Chemopreventive agent administration and tumour measurement were terminated at day 14. The animals were then sacrificed by CO2 inhalation.
Results
The time required for the first signs of tumour to appear in 50 per cent of the skin inoculation sites, referred to hereafter as ‘time to tumour visibility’, was 3 days for the control group, 5 days for the celecoxib-only group and 6 days for the combined treatment group (Figure 1). The time to tumour visibility was approximately twice as long in the two treatment groups compared with the control group. The time to tumour visibility in the combined group was one day longer than that in the celecoxib-only group.

Fig. 1 Tumour yields of the three groups after carcinoma cell inoculation. T50 marks the time point at which 50 per cent of the first signs of tumour had appeared. Cele = celecoxib; zil = zileuton
Notable differences in tumour growth appearance were found among the three groups (Figure 2).
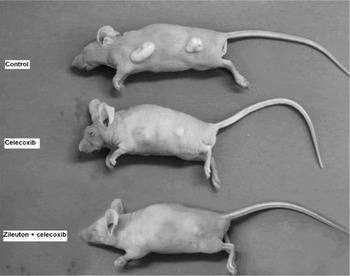
Fig. 2 Appearance of mice from the three groups at day 14.
Tumour volume was calculated in each of the groups as a function of time after inoculation (Figure 3), and significant differences were found between the three groups (F = 11.81, p < 0.01). After tumour cell inoculation, the combined treatment group showed significantly greater inhibition of tumour growth compared with the control group (p < 0.01). The celecoxib-only treatment group showed a significantly reduced visible tumour incidence and tumour volume growth, compared with the control group (p < 0.05). Figure 3 shows that there was little difference in tumour volume growth between the two treatment groups in the first 6 days of treatment; however, from days 7 to 14, the combined treatment group had the best results. Thus, the combination of 5-lipoxygenase and cyclo-oxygenase-2 inhibitors resulted in an additive inhibitory effect on the incidence and tumour growth of skin SCC.

Fig. 3 Tumour growth over time in the three groups. Repeated measure analysis of variance among three groups (F = 11.81, LSD t-test): *p < 0.05, **p < 0.01 for cele + zil group vs control group; αp < 0.05, ααp < 0.01 for cele group vs control group; βp < 0.05, ββp < 0.01 for cele + zil group vs cele group. Cele = celecoxib; zil = zileuton, Ca = carcinoma
All 24 mice were carefully observed in order to detect any potential toxicity associated with celecoxib, zileuton or the microemulsion transport vehicle. No side effects were observed in any of the three groups. No significant change in body weight was observed in any of the treatment groups, over the course of the study (p > 0.5).
Analysis and discussion
Molecular studies, based on the well known relationship between polyunsaturated fatty acid metabolism and carcinogenesis, have revealed novel molecular targets for cancer chemoprevention. Polyunsaturated fatty acids, including arachidonic acid, can enhance tumourigenesis.Reference Shureiqi and Lippman11 Cyclo-oxygenase and lipoxygenase are two important enzyme classes which metabolise polyunsaturated fatty acids, and arachidonic acid acts as a substrate from which both lipoxygenase and cyclo-oxygenase form various metabolites.Reference Ara and Teicher12 Therefore, both lipoxygenase and cyclo-oxygenase have been suggested to play important roles in carcinogenesis.
An abundance of evidence now exists demonstrating a role for cyclo-oxygenase-2 in chronically ultraviolet B(UVB)-irradiated skin, as well as in UVB-induced skin SCC.Reference Wilgus, Koki, Zweifel, Kusewitt, Rubal and Oberyszyn13 Prostaglandin E2 has been shown to play a critical role in mediating the contribution of the cyclo-oxygenase-2 pathway to cancer development, and prostaglandin E2 can stimulate increased proliferation, altered adherence, increased migration and enhanced invasiveness of cancer cells.Reference Buchanan, Wang, Bargiacchi and DuBois14 Some results suggest that such prostaglandin E2 effects are most important in the very early stages of the tumour promotion process.Reference Pentland, Schoggins, Scott, Khan and Han15
However, the 5-lipoxygenase pathway seems to play an even more important role in skin carcinogenesis. Mounting evidence suggests that lipoxygenase-catalysed products have a profound influence on the development and progression of human cancers. Inhibition of the 5-lipoxygenase pathway appears to be antiproliferative and proapoptotic for cancer cells.Reference Steele, Holmes, Hawk, Kopelovich, Lubet and Crowell6 Five-lipoxygenase pathway inhibitors have shown chemopreventive effects in animal models of skin cancer.Reference Yan, Wang, Zuo and Qu9 Among the products and biological mediators of 5-lipoxygenase which appear to promote cancer cell growth are 5-hydroxyeicosatetraenoic acid and leukotriene B4. Of these two compounds, leukotriene B4 (the terminal product of the 5-lipoxygenase metabolic pathway) would appear to be more powerful than 5-hydroxyeicosatetraenoic acid. Leukotriene B4 inhibits apoptosis and has been shown to be procarcinogenic.Reference Hebert, Takano, Holthofer and Brady16
Zileuton (N-(1-benzo(b)-thien-2yl) ethyl-N-hydroxyurea), marketed as Leutrol (or Zyflo®, Critical Therapeutics, Inc, Lexington of Mass, US), is a specific 5-lipoxygenase inhibitor. It represents the first active leukotriene inhibitor suitable for oral administration, and has shown clinical efficacy in humans. It is able to selectively inhibit the lipoxygenase-mediated signalling pathways. It appears to directly reduce fatty acid metabolite production, with concomitant damping of the associated inflammatory, proliferative and metastatic activities associated with carcinogenesis.Reference Read, Astbury, Evans, Goodwin and Rowlands17
Studies have found that cyclo-oxygenase-2 inhibitor results in decreased prostaglandin E2 and increased leukotriene B4 concentrations. In contrast, 5-lipoxygenase inhibitor results in a reduced leukotriene B4 concentration but an unchanged prostaglandin E2 concentration.Reference Ye, Wu, Shin, Bruce, Wong and Cho7 Inhibition of cyclo-oxygenase-2 may lead to a shunt of arachidonic acid metabolism towards the leukotriene pathway during tumourigenesis. Suppression of 5-lipoxygenase does not induce such a shunt and thus could be expected to produce a better response. Therefore, 5-lipoxygenase inhibitor may be more effective than cyclo-oxygenase-2 inhibitor, and blockade of both cyclo-oxygenase-2 and 5-lipoxygenase may result in a superior anticancer profile. Interestingly, recent studies in oral cancer also suggest that zileuton is effective in inhibiting biosynthesis of multiple arachidonic acid metabolites, including leukotriene B4 and prostaglandin E2, while celecoxib only suppresses prostaglandin E2 biosynthesis at a high dose.Reference Sun, Sood, Li, Ramji, Yang and Newman18 This finding may explain why cyclo-oxygenase-2 inhibitors alone are not as effective as expected for skin cancer prevention. The use of dual inhibitors which block both cyclo-oxygenase and lipoxygenase pathways of arachidonic acid metabolism could be expected to have clinical advantages over the use of cyclo-oxygenase inhibitors alone. This study further confirms this theory. During the first four days of treatment, application of celecoxib alone significantly reduced the incidence of visible tumours and the tumour volume growth rate, compared with the control group. Subsequently, however, celecoxib alone seemed to lose its treatment efficacy over time. Over the whole treatment period, there was no statistically significant difference in the number and size of tumours which developed, comparing the celecoxib-only and control groups.
Our results for a combination of zileuton and celecoxib clearly show an additive effect regarding inhibition of the incidence and development of human skin SCC in an inoculated nude mouse model, comparing the initiation and post-initiation stages. The time to tumour visibility was significantly greater in the combination treatment group compared with the control group. On day 14, there were still five inoculated sites without any visible tumour development in the combination treatment group, whereas all 32 inoculated sites had visible tumour growth at day 11 in the control group (Figure 1). Furthermore, inhibition of tumour growth was more significant in the combination treatment group than the control group.
Thus, combined treatment with zileuton and celecoxib appeared to be more effective than that with celecoxib alone.
• Skin cancer is the most common cancer and often occurs in the head and neck
• This is the first study to identify both 5-lipoxygenase and cyclo-oxygenase-2 as targets for skin cancer chemoprevention, by topical application of inhibitors of both compounds via microemulsion
• Results indicated that combined application of both compounds, applied topically via microemulsion, had very promising results in inhibiting skin squamous cell carcinoma growth in a nude mouse model
• Further studies are required to explore the possible use of this therapy in the human population
Cardiovascular and other significant side effects have been reported in patients treated systemically with celecoxib. The topical use of this drug for skin cancer chemoprevention would appear to be a safer alternative; one would hope to reduce the risk of systemic side effects without sacrificing chemoprevention efficacy. Our previous research found that topical application of celecoxib in polymer film was able to inhibit tumourigenesis in a mouse skin model.Reference Wang, Polavaram and Shapshay3 Recent studies reported that topical delivery of celecoxib via a microemulsion resulted in a higher permeation rate and significant anti-inflammatory activity. Thus, the use of a microemulsion vehicle would appear to have great potential for topical treatment of skin lesions.Reference Subramanian, Ghosal and Moulik10, Reference Yener, Gonullu, Uner, Degim and Araman19 A microemulsion formulation has also been found to have the ability to stabilise labile drugs, to control their release, and to increase their solubility and bioavailability. This drug-incorporated microemulsion has prospects for topical application.Reference Natesan, Saroj, Asis and Satya20 Thus, in the current study, we chose a microemulsion as our topical delivery system, and found that this delivery system enabled effective permeation of pharmaceutically active agents into the mouse epithelium. We would suggest that topical treatment, via such a microemulsion, has a significant inhibitory effect on skin cancer, at least when treatment is commenced at an early stage.
Ideally, this study should have been conducted in an animal model using topical exposure to carcinogens (e.g. UV light) in order to simulate real skin cancer development. However, this would be a slow, long-term process requiring a much longer study duration. The model used in this study served as a simple, expedient means of exploring the feasibility of a new treatment strategy. Such a skin model, using cell inoculation, has been used and accepted in the cancer research community. Additionally, this was a pilot study, designed only to explore treatment feasibility and to determine whether combined celecoxib and zileuton treatment could be more efficient than celecoxib alone. Further studies employing more control groups (e.g. a group receiving microemulsion alone) and using immunohistochemical and molecular investigative methods would be highly desirable in order to obtain a deeper understanding of such treatment mechanisms. Although we did not directly assess drug toxicity in this study, we believe that the risk of systemic toxicity must be reduced due to the lower doses of active agent systemically absorbed. Usually, systemic toxicity with chemopreventive agents is dose-dependent; therefore, a low dose should reduce the toxicity of the treatment. Theoretically, even assuming total absorption of the topically applied celecoxib and zileuton, the resulting systemic concentrations should be well below those following routine oral administration. As described previously, the topical application of microemulsion has been used for many years in the treatment of inflammation and other skin problems.Reference Subramanian, Ghosal and Moulik10, Reference Natesan, Saroj, Asis and Satya20 In literatures, there is no evidence to suggest that the microemulsion itself would have any anticancer or other significant treatment effects. Therefore, in this preliminary study, we did not use an additional control group treated only with microemulsion.
Acknowledgement
This work was supported by grants from the US National Institutes of Health (R03CA112670).





