Introduction
In the field of planetary sciences new observations and results of recent and on-going missions continuously fuel the debate on the habitability of Mars, especially regarding measurements of the planet's surface radiation environment (Hassler et al. Reference Hassler2014), its detailed mineralogy (Williams et al. Reference Williams2013; Grotzinger et al. Reference Grotzinger2014), new announcements of MSL findings and their implications for the surface conditions at Gale crater (Ming et al. Reference Ming2014; Vaniman et al. Reference Vaniman2014) as well as the occurrence of water in the equatorial regions of Mars (Feldman et al. Reference Feldman, Prettyman, Maurice, Plaut, Bish, Vaniman and Tokar2004; Bibring et al. Reference Bibring2005; Poulet et al. Reference Poulet, Bibring, Mustard, Gendrine, Mangold, Langevin, Arvidson, Gondet and Gomez2005; McEwen et al. Reference McEwen, Dundas, Mattson, Toigo, Ojha, Wray, Chojnacki, Byrne, Murchie and Thomas2012).
Based on the observation that all terrestrial life unquestionably depends on water, the search for at least temporarily available sources of water is one of the main topics in astrobiological research related to Mars. A fact is also mirrored by NASA's ‘follow the water’ strategy for Mars exploration (Hubbard et al. Reference Hubbard, Naderi and Garvin2002). Mars bears an estimated near surface water reservoir of about 5 000 000 km3 mostly bound in the polar ice caps and ice-rich subsurface layers (Christensen Reference Christensen2006). Below 60° of latitude it is locally found in concentrations of up to 18%, polewards of 70° of latitude it mostly exceeds 25% and approaches 100% at the poles itself (Feldman et al. Reference Feldman, Prettyman, Maurice, Plaut, Bish, Vaniman and Tokar2004). Nonetheless, summarizing the increasing knowledge of water abundance and its physical conditions, Mars’ surface is classified as a hyper-arid cold desert (Marchant & Head Reference Marchant and Head2007).
Due to Mars’ low surface temperatures water is predominantly frozen (Christensen Reference Christensen2006). At the Viking landing sites temperatures range from −17° to −107°C at 1.5 m above the surface; in winter they drop to minima of about −143°C at the polar caps but frequently surpass 0°C during summer daytime in subtropical to equatorial regions with soil maxima of up to 27°C (Hess et al. Reference Hess, Henry, Leovy, Ryan and Tillman1977; Tillman Reference Tillman1988; Murphy et al. Reference Murphy, Leovy and Tillman1990). Although the amount of water vapour in Mars’ atmosphere is small by terrestrial standards, the relative humidity (RH) can be quite high (Davies Reference Davies1979) depending on daytime, season, latitude and orographic factors (Briggs et al. Reference Briggs, Klaasen, Thorpe, Wellman and Baum1977). Viking revealed the occasional presence of morning fogs and Viking Orbiter 2 showed large amounts of water vapour over the north polar remnant cap during summer (Farmer et al. Reference Farmer, Davies, Holland, LaPorte and Doms1977) while the infrared thermal mapper of Viking Orbiter 2 implied that the atmosphere is near or at saturation at some seasons (Kieffer et al. Reference Kieffer, Martin, Peterfreund, Jakosky, Miner and Palluconi1977).
On the other hand, liquid surface water is considered to be restricted to narrow, temporary and rare conditions. Besides it is not yet understood (seasonal) outflow events from subsurface groundwater reservoirs into gullies (Malin et al. Reference Malin, Edgett, Posiolova, McColley, Dobrea and Noe2006). Möhlmann (Reference Möhlmann2010) depicted three possible conditions for liquid water: undercooled liquid interfacial (ULI) water, undercooled liquid water in cryo-brines and liquid bulk water in the subsurface of ice areas. ULI water that occur on soil grains is discussed as reversibly bound and partially unfrozen films of adsorption water with a thickness of several micrometres only but with an water activity (a w) above the assumed minimum for terrestrial life of 0.6 (Jakosky et al. Reference Jakosky, Nealson, Bakermans, Ley and Mellon2003; Möhlmann Reference Möhlmann2004, Reference Möhlmann2008). Besides sulphatic salts, cryo-brines might be formed by hygroscopic minerals as hydrogen peroxide (Houtkooper & Schulze-Makuch Reference Houtkooper and Schulze-Makuch2009) and perchlorates (Houtkooper & Schulze-Makuch Reference Houtkooper and Schulze-Makuch2010) of which the latter was recently detected on Mars by the Phoenix lander (Hecht et al. Reference Hecht2009; Rennó et al. Reference Rennó2009; Smith et al. Reference Smith2009).
Such utmost scarcity of liquid water, even on a rather Earth-like celestial body like Mars, raises the interest of astrobiologists in extremophile and extremotolerant organisms that resist highly restricted and infrequent water availability in their natural habitat. These organisms are found in arctic, antarctic, alpine and desert environments where intense desiccation stress is accompanied by high insolation, high levels of UVR and extreme temperatures. Such habitats are considered to be as close as possible to Martian conditions and therefore investigated as Mars Analogue Sites. For example, the Andean Atacama Desert (McKay et al. Reference McKay, Friedmann, Gomez-Silva, Caceres-Villanueva, Andersen and Landheim2003) and the dry valley cold deserts in continental Antarctica (Marchant & Head Reference Marchant and Head2007; Harańczyk et al. Reference Harańczyk, Pytel, Pater and Olech2008; Sun et al. Reference Sun, Nienow, McKay, Doran, Lyons and McKnight2010) are among the most Mars-like terrestrial habitats on Earth.
Lichens are organisms that are able to tolerate severe environmental conditions (Kappen Reference Kappen, Ahmadjian and Hale1973, Reference Kappen and Galun1988, Reference Kappen1993) and successfully colonize such extreme polar, alpine and arid habitats (Lange Reference Lange1992). Therefore, they represent useful model systems in astrobiological research (Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007; de Vera et al. Reference de Vera, Möhlmann, Butina, Lorek, Wernecke and Ott2010). Lichens are symbiotic associations constituted of a heterotrophic fungus (mycobiont) and a photoautotrophic partner (photobiont) that form a unique thallus structure with emergent morphological and physiological adaptations towards the harsh conditions at the natural habitat (Kappen Reference Kappen and Galun1988; Lange et al. Reference Lange, Green and Reichenberger1999, Reference Lange, Green and Heber2001). Among these adaptations, three adaptive traits are chiefly relevant in constituting extremotolerance and protecting both symbionts: a number of morphological and anatomical features (Meeßen et al. Reference Meeßen, Sánchez, Brandt, Balzer, de la Torre, Sancho, de Vera and Ott2013), a diverse set of secondary lichen compounds (SLCs, Meeßen et al. Reference Meeßen, Sánchez, Sadowsky, de la Torre, Ott and de Vera2014) and, most important in the present context, anhydrobiosis. It allows lichens to tolerate intense desiccation by passing into an ametabolic state (Kranner et al. Reference Kranner, Cram, Zorn, Wornik, Yoshimura, Stabentheiner and Pfeifhofer2005). Moreover, anhydrobiosis protects lichens against stressors that come along with drought, including severe temperatures and high levels of light or ultraviolet radiation (UVR, Nybakken et al. Reference Nybakken, Solhaug, Bilger and Gauslaa2004). In nature, the availability of water is often correlated to more moderate climatic conditions and rehydration by liquid water or high air humidity (>80%, Sigfridsson & Oquist Reference Sigfridsson and Oquist1980; Lange et al. Reference Lange, Kilian and Ziegler1986) which leads to metabolic re-activation. Regarding astrobiology, the protective effect of anhydrobiosis is stressed by recent studies on lichen UVC-resistance in anhydrobiosis, and when physiologically active (Sánchez et al. Reference Sánchez, Meeßen, Ruiz, Sancho, Ott, Vílchez, Horneck, Sadowsky and de la Torre2014).
In the context of Martian habitability, the adaptation of extremophile and extremotolerant organisms towards severe and lasting desiccation is of peculiar interest. The detailed analysis and understanding of water uptake and retention kinetics of organisms is beneficial to understand the constraints of Martian habitability. Thus, three experimental set-ups applied three parameters that are clearly linked to Martian environmental conditions: (i) irradiation with UVC, which is an environmental stressor at Martian ground level but not on Earth, (ii) a set of three temperatures that represent moderate Martian conditions of up to −17°C at the Viking landing sites and up to 27°C in low latitudes (Hess et al. Reference Hess, Henry, Leovy, Ryan and Tillman1977; Tillman Reference Tillman1988; Murphy et al. Reference Murphy, Leovy and Tillman1990), (iii) a RH that can occasionally become saturated and produce morning fogs, although the amount of atmospheric water vapour is low compared to Earth (Briggs et al. Reference Briggs, Klaasen, Thorpe, Wellman and Baum1977; Farmer et al. Reference Farmer, Davies, Holland, LaPorte and Doms1977; Kieffer et al. Reference Kieffer, Martin, Peterfreund, Jakosky, Miner and Palluconi1977; Davies Reference Davies1979). Furthermore, it may also contribute to the understanding of the resistance of terrestrial organisms towards simulated space and Martian condition (de Vera et al. Reference de Vera, Horneck, Rettberg and Ott2003, Reference de Vera, Horneck, Rettberg and Ott2004a, Reference de Vera, Horneck, Rettberg and Ottb, Reference de Vera, Rettberg and Ott2008, Reference de Vera, Möhlmann, Butina, Lorek, Wernecke and Ott2010, Reference de Vera, Schulze-Makuch, Khan, Lorek, Koncz, Möhlmann and Spohn2013; Sánchez et al. Reference Sánchez, Mateo-Martí, Raggio, Meeßen, Martínez-Frías, Sancho, Ott and de la Torre2012, Reference Sánchez, Meeßen, Ruiz, Sancho, Ott, Vílchez, Horneck, Sadowsky and de la Torre2014) in previous (de la Torre et al. Reference de la Torre, Sancho, Pintado, Rettberg, Rabbow, Panitz, Deutschmann, Reina and Horneck2007, Reference de la Torre2010; Sancho et al. Reference Sancho, de la Torre, Horneck, Ascaso, de los Ríos, Pintado, Wierzchos and Schuster2007; Raggio et al. Reference Raggio, Pintado, Ascaso, de la Torre, de los Ríos, Wierzchos, Horneck and Sancho2011; Onofri et al. Reference Onofri2012; Scalzi et al. Reference Scalzi, Selbmann, Zucconi, Rabbow, Horneck, Albertano and Onofri2012; Brandt et al. Reference Brandt, de Vera, Onofri and Ott2014) and current space exposure experiments (i.e. BIOMEX, Biology and Mars Experiment at the EXPOSE-R2 facility on board the ISS, launched in July 2014, start of ~12 month-exposure in October 2014). An important issue to be tackled in this research is the interaction of moisture of the planet's atmosphere with hypothetical organisms of the planet's surface because of the essential role of water for life. Based in previous studies (Jänchen et al. Reference Jänchen, Meeßen, Ott, Sànches and de la Torre2013, Reference Jänchen, Bauermeister, Feyh, de Vera, Rettberg, Flemming and Szewzyk2014a, Reference Jänchen, Herzog, Meeßen, Ott, Feist and de Verab) we examined the water vapour interaction and water-bearing properties of the three astrobiological model lichens Buellia frigida, Xanthoria elegans and Circinaria gyrosa. The experiments were partially conducted after irradiation with UVC to simulate an environmental parameter typically for Mars but not found on Earth. Our aim is to contribute to an improved knowledge of extremotolerant organisms under astrobiological aspects within the frame of BIOMEX with an emphasis on the effects of UVC irradiation on water retention properties. The results may also support data evaluation of future Mars simulation studies or in-situ missions such as ExoMars.
Experimental
Biological samples
All three lichens, B. frigida Darb. (1910), C. gyrosa Sohrabi (Reference Sohrabi2012) and X. elegans (Link) Th. Fr. (1860), represent symbiotic and eukaryotic associations formed by an ascomycete fungus (Lecanoromycetes) and a green alga (Trebouxiophyceae). While B. frigida is an extremophile Antarctic endemite (collected 2010 at Gerlach Inlet, Gondwanaland, Antarctica, 74°38′S, 164°13′E) and C. gyrosa originates from arid highland areas of central Spain (collected 2010 in Zaorejas, Spain, 1260 m a.s.l.), X. elegans is found in exposed alpine and circumpolar habitats (collected 2008 at Col du Sanetsch, Valais, Switzerland, 2140 m a.s.l.). All three species are well adapted to drought, high levels of insolation and UVR experienced at their natural habitats but also to extremes of cold or heat, respectively (Sancho et al. Reference Sancho, Schroeter and del Prado2000; Øvstedal & Smith Reference Øvstedal and Lewis Smith2001). C. gyrosa was found to survive real and simulated space exposure (de la Torre et al. Reference de la Torre, Sancho, Pintado, Rettberg, Rabbow, Panitz, Deutschmann, Reina and Horneck2007, Reference de la Torre2010; Raggio et al. Reference Raggio, Pintado, Ascaso, de la Torre, de los Ríos, Wierzchos, Horneck and Sancho2011; Onofri et al. Reference Onofri2012) without forming relevant amounts of UVR-shielding SLCs but pronounced amounts of intermedullary calciumoxalate monohydrate crystals (Böttger et al. Reference Böttger, Meeßen, de la Torre, Frias, Rull, Sánchez, Hüber and de Vera2013). In contrast, B. frigida and X. elegans form substantial amounts of melanin and parietin as dominant SLCs in their respective cortices. X. elegans was also shown to survive real and simulated space exposure (de Vera et al. Reference de Vera, Horneck, Rettberg and Ott2003, Reference de Vera, Horneck, Rettberg and Ott2004a, Reference de Vera, Horneck, Rettberg and Ottb) as well as to photosynthesize under simulated Martian conditions (de Vera et al. Reference de Vera, Rettberg and Ott2008, Reference de Vera, Möhlmann, Butina, Lorek, Wernecke and Ott2010; de la Torre et al. Reference de Vera, Möhlmann, Butina, Lorek, Wernecke and Ott2010). B. frigida and C. gyrosa are part of the current BIOMEX space exposure mission outside the ISS. Detailed information on these lichens’ morphological–anatomical adaptations towards extreme environmental conditions and their SLC inventory can be found elsewhere (Meeßen et al. Reference Meeßen, Sánchez, Brandt, Balzer, de la Torre, Sancho, de Vera and Ott2013, Reference Meeßen, Sánchez, Sadowsky, de la Torre, Ott and de Vera2014). Besides the thalli of all three species, we investigated the isolated mycobiont of B. frigida and X. elegans, the photobiont of B. frigida as well as pure whewellite (calciumoxalate monohydrate) and chitin which is one of the main dry weight components of lichen fungi.
UVC sample treatment
A set of samples consisting of isolated mycobionts of B. frigida and X. elegans as well as of the isolated photobiont of B. frigida was exposed to UVC at λ = 254 nm under dry (D) and wet (W) conditions. The irradiation was performed in an air circulation cabinet (Mühlenkamp GmbH) equipped with a HNS 30W G13 G30T8/OF UVC lamp (Puritec®, Osram, >93% emission at 254 nm). After 20 min pre-run, the UVC254nm irradiance was adjusted to 480 μWcm−2 (measured with an UVP UVX dosimeter, sensor 25 at 254 nm). The exposure times were 0 h, 10 h (accumulated dose of 17 280 J cm−2) and 50 h (accumulated dose of 86 400 J cm−2). The corresponding sample codes are D0, D10, D50 for dry treatment and W0, W10, W50 for exposure of wet samples, respectively. After exposure the samples were dried over silica gel assuring comparably low initial water content and safe storage/transport. Prior to thermogravimetry (TG) the samples were preconditioned as described below. The isotherm measurements were started with the ‘silica gel dry’ samples as delivered.
Physico-chemical methods
The dehydration properties and the thermal decomposition (TG, differential thermogravimetry, DTG and differential thermoanalysis, DTA) were measured on a Netzsch STA 409 apparatus with a heating rate of 10 K min−1 up to 1273 Kusing Al2O3 crucibles. The purge gas flow (N2, Air Liquide, 5.0) was 70 ml min−1. Prior to the TG experiments the lichens and their symbionts were preconditioned at controlled atmosphere for 6 days over saturated ammonium chloride solution in an evacuated desiccator at p/p s = 0.79,equal to RH of RH = 79%.
Supplementary to conventional (TG, DTG, DTA) thermoanalytical (TA) measurements, simultaneously coupled mass spectrometry (TA-MS) investigations were performed. A Netzsch thermoanalyzer STA 409 C Skimmer ® system, equipped with a Balzers QMG 421, was used to record the TA curves together with the ionic current (IC) curves in the multiple ion detection (MID) mode (Emmerich & Post Reference Emmerich and Post1997; Kaisersberger & Post Reference Kaisersberger and Post1997). A DTA-TG sample carrier system with platinum crucibles (beaker, 0.8 ml) and Pt/PtRh10 thermocouples was used. Samples of 13–20 mg each were measured against an empty reference crucible. A constant purge gas flow of 70 ml min−1 N2 (Messer-Griesheim, 5.0) and a constant heating rate of 10 K min−1 were applied. The raw data were evaluated with the manufacturer's software Proteus® (v. 4.3) and QuadStar 422 (v. 6.02) without further data treatment, e.g. smoothing. The initial (T i), extrapolated onset (T on ex), and peak temperatures (T p) were determined following standard recommendations (Hill Reference Hill1991).
The base line shift of the IC signals for practically all mass numbers at higher temperatures is the characteristic of the Skimmer ® system (Hanss et al. Reference Hanss, Kalytta, Reller, Kapsch and Hollering2003) where the gas sampling system and the sample holder have the same temperatures to avoid condensation phenomena. Consequently, the MS signal becomes temperature-dependent as the lower gas viscosity affects an increasing gas flow through the sampling orifices of the coupled TA-MS cell, thus leading to an increasing IC intensity. A real substance release from the sample can be deduced only from a distinctly expressed additional IC maximum on the constantly increasing baseline which was not the case here. The mass number assignment is abbreviated in the form of m18 representing m/z = 18.The hydration state of the sample for the present TA-MS study was ‘room-dry’ (RH < 0.79) different to the TG experiments described above.
The sorption/desorption (hydration/dehydration) isotherms were measured gravimetrically from 255 to 293 K with a McBain–Bakr quartz spring balance (McBain & Bakr Reference McBain and Bakr1926) equipped with MKS Baratron pressure sensors covering a range of 10−5–103 mbar. This allowed measurements of the isotherms in the range P = 0.01–P = 20 mbar water vapour pressure. The dilation/contraction of the spring was followed with a cathetometer. The sensitivity of the quartz spring was 4 mm mg−1 and the resolution of the cathetometer 0.01 mm. So the resolution for the water uptake (using 100 mg sample) was 0.0004 g g−1. Prior to each sorption experiment, about 100 mg sample was degassed over night at 293 K and P < 10−5 mbar. This state is regarded as ‘vacuum dry’.
Results and discussion
Thermogravimetry
The TA curves give a comparably easy access to mass loss data and thermal information as a function of temperature including the release of sorbed water. Figure 1 provides records of the (reversibly) physisorbed water (mass loss up to ca. 450 K) as well as the thermal decomposition up to 900 K for all three lichens investigated. Due to the different mass losses up to 450 K it can be concluded that C. gyrosa from the dry warm environment took less water (at RH = 79%) than B. frigida (and less pronounced for X. elegans) which are adapted to the dry cold Alpine or Antarctic habitats. Moreover, the decomposition and carbonization process upon further heating exhibits two steps more in the TG of C. gyrosa when compared to X. elegans and B. frigida. The reason for that fact seems to be the extra component calciumoxalate monohydrate (whewellite) of C. gyrosa (Böttger et al. Reference Böttger, Meeßen, de la Torre, Frias, Rull, Sánchez, Hüber and de Vera2013). Whewellite is a monohydrate and decomposes in three steps according to Schultze (Reference Schultze1971). Figure 2 depicts the three phases beginning with the release of the crystal water from CaC2O4·H2O at ~490 K, the formation of CaCO3 with CO release at ~760 K, and the formation of CaO under release of CO2 at ~1050 K. According to the DTA results (not shown) these three steps are endothermal. Principally, these three phases were identified in the TG/DTG profiles of C. gyrosa as well (dark blue curve in Fig. 2). The temperatures of these steps differed slightly which is probably due to morphological differences between the synthetic and natural whewellite crystals in the lichen, e g. its size and distribution. Calciumoxalate monohydrate did not sorb any humidity at ambient temperature. So it showed no TG step below 450 K for the release of physisorbed water as did the lichen (Fig. 2). Equivalent to the DTG curve of B. frigida, the DTA profile (not shown) exhibits endothermic peaks except for the huge TG/DTG signal at about 600 K. This considerable mass loss is not expressed in the DTA trace.

Fig. 1. TG and DTG profiles after hydration at RH = 79% in a desiccator: Circinaria gyrosa thalli (dark blue); Xanthoria elegans thalli (green) and Buellia frigida thalli (red); TG in solid lines, DTG in dashed/dotted lines.

Fig. 2. TG and DTG profiles upon hydration at RH = 79% in a desiccator: pure calciumoxalate monohydrate (red) and Circinaria gyrosa thalli (dark blue); TG in solid lines, DTG in dashed/dotted lines. All DTG-minima of calciumoxalate monohydrate and the lichen correspond with endothermic peaks in the DTA (not shown).
Figure 3 shows the TG and DTG curves of B. frigida, the mycobiont of B. frigida and pure synthetic chitin, one of the main dry weight components of mycobionts. The pure chitin showed a pattern in between the lichen and the two-step-curve of the mycobiont. Despite these differences, the main components of the released volatiles of all three samples were undoubtedly H2O and CO2. The second step of the mycobiont, however, includes some hydrocarbons as seen below.

Fig. 3. TG and DTG profiles after hydration at RH = 79% in a desiccator: comparison of Buellia frigida thalli (green), B. frigida mycobiont (red) and pure chitin (black); TG in solid lines, DTG in dashed/dotted lines.
After demonstrating the hydration and dehydration behaviour of lichens as an entire organism and of its isolated mycobiont, Figs 4 and 5 show the comparison of the TG/DTG profiles of B. frigida's mycobiont irradiated with different doses of UVC under dry and wet conditions. The curves of D0, D10 and D50 (Fig. 4) were characterized by three TG steps or DTG minima, respectively. The first minimum at 360 K indicated the release of physisorbed water. The second stage around 560 K was due to stronger bonded water or hydroxyls and the beginning decay. The last step at 660 K was dominated by the thermal decomposition of the mycobiont, in particular of its main component, i.e. chitin (sum formula of (C8H12NO5) n ).

Fig. 4. TG and DTG profiles of the mycobiont of Buellia frigida after irradiation with UVC over 0, 10 and 50 h under dry conditions: D0 (green), D10 (red) and D50 (blue). TG after hydration at RH = 79% in a desiccator. TG in solid lines, DTG in dashed/dotted lines.

Fig. 5. TG and DTG profiles of the mycobiont of Buellia frigida after irradiation with UVC over 0, 10 and 50 h under wet conditions: W0 (green), W10 (red) and W50 (blue). TG after hydration at RH = 79% in a desiccator. TG in solid lines, DTG in dashed/dotted lines.
The wet-irradiated samples (Fig. 5) show a very similar behaviour except a new step/minimum at 420 K. This step increases with the doses of UVC indicating an impact of the UVC treatment on the release of stronger physisorbed water. To test this behaviour we repeated the irradiation experiments with the mycobiont of X. elegans but found no comparable effect (Fig. 6). Therefore we hypothesize that this effect is due to melanin, which is formed in large amounts and deposited in/on its cell wall by the mycobiont of B. frigida but not by the one of X. elegans. Melanin pigments are found to dissipate over 99.9% of absorbed UV radiation into heat (Meredith & Riesz Reference Meredith and Riesz2004), representing a potent PAR- and UVR-protective compounds. Strongly interacting with UVR, it might also be involved in the activation of additional UVC-induced sorption site for water such as the -NH, -COOH or -OH substituents. In support, it was already demonstrated that UVC (254 nm) changes the electronic structure of fungal melanin and enhances its electron transfer properties in the NADH redox reaction by a factor of 3.9 (Dadachova et al. Reference Dadachova, Bryan, Huang, Moadel, Schweitzer, Aisen, Nosanchuk and Casdevall2007). Such interactions may also affect the water sorption properties of melanin as shown by the present study. Nonetheless, the mycobiont of X. elegans tended to sorb some more weakly-physisorbed water upon irradiation of the wet sample. This causes higher mass losses at T < 450 K and, may explain the higher total mass loss up to 900 K. According to the results shown in Figs 4 and 5, this is not observed for B. frigida. As expected, the DTA exhibits an endothermal peak at T = 370 K and an exothermal one at T = 590 K.

Fig. 6. TG and DTG profiles of the mycobiont of X. elegans after irradiation under dry and wet conditions for 50 h: non-irradiated control (green), D50 (red), W50 (blue). The DTA exhibited an endothermic peak at T = 370 K and an exothermic one at T = 590 K. TG after hydration at RH = 79% in a desiccator. TG in solid lines, DTG in dashed/dotted lines.
TA-MS investigations
The thermal behaviour of the mycobiont of B. frigida under Ar = argon is represented by the data in Figs 7 and 8 as obtained by online-coupled TA-MS measurements. Two main findings should be emphasized: first, the structured water release at ca. 415 K preceded by an additional shoulder at ca. 360 K, and, second, the chemical information deduced from the fragments occurring during (and around!) the two main steps of the mycobiont decomposition (DTG maxima at 565 and 662 K). The DTA traces are not shown in the plots as the weak exothermicity (under N2) of the two steps is still less pronounced under Ar. On the other hand, several details of the chemical processes during the thermal decomposition can be clearly deduced from an inspection of the ion current (IC) curves under Ar.

Fig. 7. TG-MS curves for the mycobiont of Buellia frigida, W50 (lab-dry) in Ar (Pt beakers) with the IC curves for the mass numbers m/z 12(C+), 18(H2O+), 27(HCN, C2H3 +) and 44(CO2 +, CH3CHO+). The amplifications refer to the most intense IC signal (m/z 18), the intensity of which is given on the scale on the right hand. The IC curves are shifted for a better legibility. Note the qualitatively identical curve shape for 12 and 44, but not for 18 (shoulder at 360 K) and 27.
The TA data in Fig. 7 support our assumption that the wet irradiation causes the formation of additional adsorption sites for water and, furthermore, creates a greater mobility in the system, thus explaining the simultaneous release of water and C-containing species already in the low-temperature range at ca. 415 K. This is clearly evidenced by the IC intensities for m/z 12 and 44 appearing in parallel to the m/z 18.
The most important feature for a correct attribution of fragments to the same (or to another) molecule formed during a multi-step process is the identical (or non-identical) curve shape of different mass numbers to be assigned. So the mentioned parallel curvature of m/z 12 and 44 proves that they originate from the same molecule (most probably CO2), whereas m/z 27 only partly follows the qualitative shape of m/z 12. This means that the mass number m/z 27 does not represent the same molecule during the integral intensity evolution. It most probably indicates C2H3 + (unspecific for hydrocarbons) when compared with the m/z 12 curve, whereas about 660–700 K it indicates HCN (formed from chitin, see below).
If m/z 12 and 44 exhibit the same curvature, the most probable explanation is the attribution to CO2, but other molecules can contribute to the m/z 44 intensity as well. A considerable part of the organic matter indicated by the CO2 release can be chitin which is known to be an essential part of the cell walls of many fungi (Daum Reference Daum2005). Chitin, containing the acetamido group bound to a polysaccharide matrix, can explain not only the formation of CO2 via deacetylation (the well-known chitosan process, Daum Reference Daum2005), but also of HCN and of less-specific fragments such as C2H3 +. Furthermore, the CH3CO part of the acetamido group (m/z 43) can contribute to m/z 44 after hydrogen attraction which is known not only for chemical reactions, but for reorganizations in the MS ionization chamber as well.
The ‘early’ organic matter together with the additional water release yields a mass loss of 6.7%; it can be assumed, however, that the major part is due to water. Both the IC signal for m/z 18 and the DTG curve exhibit an additional shoulder at ca. 360 K, thus indicating two different bonding situations of water: the shoulder, obviously, represents weaker bound water, i.e. the physisorbed one, whereas the main peak has to be attributed to water which was fixed as a consequence of the irradiation. Both water release steps can be detected in the data presented in Fig. 5 as well, but their intensity ratio differs. This is due to the humidity state of the sample measured with TA-MS: the sample was ‘lab-dry’ (no hydration at RH = 79%),therefore the first step/minimum of the physisorbed water at 360 K is lacking in Fig. 7. The two following water release steps with IC maxima at 560 and 670 K have to be explained by another chemical process: this is so-called reaction water which is, together with CO2, the product of multiple decomposition and inner-molecular redox reactions upon decomposition.
In Fig. 8 further fragment intensities are shown. Especially m/z 34(H2S+) and 64(SO2 +) clearly prove the presence of sulphur-containing substances. Please note that the intensity evolution of m/z 34 and 64 is quite different from those for m/z 12, 15, 18 and, obviously, represent independent decomposition steps.
Isotherm measurements
The reversibly physisorbed water, sorbed at RH = 79% and released in the TG at T < 450 K, was investigated in more detail by hydration/dehydration isotherm measurements. Figure 9 gives an overview for all three lichens at T = 293 K. As expected by the TG results documented in Fig. 1, the water uptake of C. gyrosa was retarded compared to the other two lichens starting at around RH = 50%. The samples for the TG experiment were saturated at RH = 79% so that the comparison has to refer to the respective value in Fig. 9. The isotherm measurements confirm the TG results in respect to changes in the water retention ability of lichens adapted to different environmental conditions. Interestingly, the isotherms become steeper at RH > 80% where the thallus water content was found to be sufficient to start photosynthesis in various lichen species (Lange Reference Lange1969; Sigfridsson & Oquist Reference Sigfridsson and Oquist1980; Lange et al. Reference Lange, Kilian and Ziegler1986). With rising RH the lichens sorb more and more water at polar groups and other sites of their polymeric materials culminating in pure condensation at surfaces when approaching RH = 100%.The latter may also happen in mesopores or small μm-pores of the thallus as reported by Valladares et al. (1993) for another family of lichens.

Fig. 9. Comparison of the water sorption isotherms at 293 K (+20°C) of the lichens Xanthoria elegans (green), Buellia frigida (red) and Circinaria gyrosa (blue, from top to bottom) as a function of RH.
Regardless of the hydration mechanism, the isotherms provided a quantitative information about the amount of physisorbed water if the RH (or a w) of the lichens environment is known. Measurements at various temperatures gave detailed thermodynamic information of the sorption system. The Figs 10–12 summarize the results of the water physisorption behaviour at different temperatures found for C. gyrosa (Fig. 10) and B. frigida. (Figs 11 and 12). The measurements were performed close to Martian environmental conditions in terms of temperature and pressure. The tested temperatures of 293, 273 and 255 K meet those frequently found during the Martian day in equatorial and subtropical regions while the applied atmospheric pressure range (<20 mbar, but comprised of water vapour only) is equivalent to that of the Martian atmosphere, which is ranging from 6 mbar at zero elevation level to ca. 11.6 mbar at the bottom of Hellas Planitia. To exemplify the vapour pressure values, the isotherms of B. frigida are plotted in Fig. 11 as a function of the absolute pressure as they were experimentally determined. If the isotherms are recalculated and plotted as a function of the RH, all isotherms for different temperatures of one organism should be found in one bundle of curves. The value of RH is the relative pressure (or a w) multiplied by 100. C. gyrosa in Fig. 10 perfectly fulfilled this condition following the thermodynamic rules.

Fig. 10. Identical hydration/dehydration isotherms of the lichen Circinaria gyrosa at 256 K (−17°C, red), 273 K (0°C, orange) and 293 K (+20°C, blue) as a function of RH.
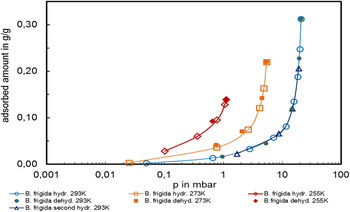
Fig. 11. Hydration/dehydration isotherms of the lichen Buellia frigida at 255 K (−18°C, red), 273 K (0°C, orange) and 293 K (+20°C, blue) as a function of vapour pressure. Note the logarithmic x-axis.

Fig. 12. Hydration/dehydration isotherms of the lichen Buellia frigida at 255 K (−18°C, red), 273 K (0°C, orange) and 293 K (+20°C, blue) as a function of RH.
Interestingly, B. frigida (Fig. 12) deviates from this rule and showed clear temperature-dependence between 298 K (+20°C) and the lower temperatures of 273 K (0°C) and 256 K (−17°C). A repeated determination of the isotherm at 298 K (indicated as second hydration in Figs 11 and 12) gives an identical curvature. It is important to note that these measurements were performed after the determination of the isotherms at the lower temperatures and at the same sample. A comparison of the two single symbionts of B. frigida in Fig. 13 with the entire lichen thallus does not give an obvious explanation of this ‘non-conformist’ behaviour; it could not be consistently attributed to one of the two symbionts. At T = 273 K or lower the isotherm of the entire lichen is very close to that of the mycobiont and at T = 298 K the isotherm of the photobiont is close to the symbiotic lichen organism.

Fig. 13. Hydration/dehydration isotherms of the lichen Buellia frigida at 273 K (0°C, blue); its isolated photobiont (green) and its isolated mycobiont (red) at 273 K (0°C) and 293 K (+20°C), respectively, as a function of RH.
The results consistently stressed the full reversibility of the isothermes (water uptake and release exhibit the same curvature) and revealed relatively fast kinetics of the hydration/dehydration process. In all investigated lichens the time required for equilibration ranged between minutes and ca.1 h, whereas, as an example from own non-published work, Nostoc commune or an unspecified moss species required more than a day. These observations are in line with the poikilohydric nature of lichens. On the one hand, lichens are able to take up water quickly and thus enhance the utilization of limited water supply. On the other hand, many lichen species do not retain water but also desiccate as rapidly. Lichens are more susceptible to damaging effects of high levels of UVR and heat when wet (Kappen & Valladares Reference Kappen, Valladares, Pugnaire and Valladares1999). Therefore, rapid desiccation in case of unfavourable environmental conditions resembles a protective adaptation and fast shifts in the hydration status allow poikilohydric organisms to profit by favourable conditions but to omit unfavourable ones.
Besides liquid water, high air humidity (≥80 to 97%, Sigfridsson & Oquist Reference Sigfridsson and Oquist1980; Lange et al. Reference Lange, Kilian and Ziegler1986) result in re-hydration and activation of the lichen metabolism and the optima for photosynthesis were found to be rather low in drought-adapted lichens (as 65% in Umbilicaria pustulata–90% in Peltigera canina, Ried Reference Ried1960; Smith Reference Smith1962). Lichen photosynthesis can reach its maximum at relatively low water content and depends on habitat-specific adaptations (Henssen & Jahns Reference Henssen and Jahns1974). For example, the lichen Ramalina maciformis reaches its CO2-compensation point (CO2-uptake by photosynthesis compensates CO2-evolution by respiration) at ca. 80% RH which resembles ca. 20% of thallus water content (TWC, Lange Reference Lange1969). At 30% TWC the lichen obtains 50% of its maximum photosynthetic rate which is reached at 60% TWC. At the natural habitat, such metabolic re-activation is possible by rising air humidity and dewfall during night, leading to positive net photosynthesis and up to 90% of maximum photosynthesis rate after 6–8 h of exposure in saturated air (Lange Reference Lange1969). The present results (Fig. 9) show that such physiologically relevant TWC for dry-adapted lichens of ≥20% is achieved during the course of the experiments by the lichens X. elegans and B. frigida, but not by C. gyrosa at 293 K (+20°C). B. frigida showed a maximum TWC of 30% and X. elegans of 35% while C. gyrosa did not surpass 18% TWC. Moreover, temperature-dependent measurements of hydration/dehydration isotherms show that B. frigida achieves >20% TWC at 273 K (0°C) but not at 255 K (−18°C); C. gyrosa did not reach a TWC >18% at any of the measured temperatures (Figs 10–12).The results of the isolated symbionts of B. frigida demonstrate slightly higher hydration rates for the photobiont than the mycobiont at >50% RH (Fig. 13). Hale (Reference Hale1976) reported that the respiration of a lichen (to which the mycobiont is the main contributor) decreases faster with desiccation than the photosynthesis (performed only by the photobiont). The present results suggest that the hydration state of the photobiont is higher than that of the mycobiont, reaching the crucial hydration level of ca. 20% TWC at lower air humidity (Fig. 13).
Finally, the impact of UVC irradiation on the hydration/dehydration isotherms of a symbiont, as a first step, will be discussed. Figure 14 shows the sorption isotherms of water on the untreated mycobiont of B. frigida and the irradiated W50 sample, respectively. The isotherms are plotted again as a function of the RH. There is an obvious difference between both samples at RH >70%. The wet irradiated sample sorbs more water compared with the untreated mycobiont which is in line with the TG results showing some extra physisorbed water (step at 420 K).

Fig. 14. Hydration/dehydration isotherms of the mycobiont of Buellia frigida at 273 K (0°C) and 293 K (+20°C) without UVC irradiation (red) and after 50 h of irradiation in the wet state (blue) as a function of RH.
As already discussed for the TG/DTG analysis, the UVC-treatment apparently improved the ability of physisorption of water by creating extra sorption sites in the mycobiont. This may be due to the presence of melanin in the mycobiont of B. frigida. Melanin is an aromatic polymer with polar ligands such as OH, aldehyde and carboxyl groups being well-known as specific sorption sites for water. Another explanation may be the ‘damage’ of parts of the acetamido groups of the chitin in B. frigida upon UVC exposure. Thus, irradiation of these components of the wet mycobiont change the polarity of their specific groups and stronger sorption sites for the water molecule could be formed.
Conclusion
Besides general information on the complete decomposition at elevated temperatures, the thermoanalytic investigations, including mass spectroscopy, of the lichens X. elegans, B. frigida and C. gyrosa, yielded valuable information about their water physisorption, dehydroxylation and decarboxylation behaviour. Calciumoxalate monohydrate significantly influenced the decomposition pattern of C. gyrosa and was recently reported as a particular intermedullary component (Böttger et al. Reference Böttger, Meeßen, de la Torre, Frias, Rull, Sánchez, Hüber and de Vera2013).
In regard to water retention properties, the gravimetric hydration/dehydration studies close to Martian environmental conditions revealed differences between C. gyrosa (adapted to dry warm conditions) on the one hand, and X. elegans and B. frigida (adapted to cold arctic or alpine conditions) on the other hand. C. gyrosa is less hydrophilic than the other two lichens, leading to a maximum TWC <20%, while B. frigida, X. elegans as well as the isolated symbionts of B. frigida were found to achieve TWC values of 30–35% when the air humidity exceeds 80% RH. A TWC of >20% was found to be crucial to re-activate photosynthesis in drought-adapted lichens (Lange Reference Lange1969). Thus, it might be hypothesized that – at least to some extent – B. frigida and C. gyrosa could accumulate enough water to become metabolically active during the hydration and dehydration experiments under the conditions described above. Furthermore, the present results indicate that exposure to UVC – a stressor not found on Earth but on Mars or in space – may influence the water retention ability of organisms. Under wet exposure conditions, the mycobiont of B. frigida formed additional water sorption sites. Since a similar reaction is not found in the mycobiont of X. elegans, that effect might be attributed to melanin which is only produced by the mycobiont of B. frigida. Another effect might be the increasing ‘damage’ of the acetamido groups of the chitin upon UVC exposure. Nonetheless, chitin is the dominant cell wall component in both mycobionts while the peculiar effect is only found in the mycobiont of B. frigida. However, these two options might be interpreted as first indicators of UVC-induced damage.
In general, the examination of the water sorption properties of organisms may contribute to an improved understanding of important astrobiological topics, as the resistance of extremotolerants and extremophiles towards harsh and/or extraterrestrial environmental factors, their capacity to thrive under conditions of limited water availability, and the damages induced by exposure to such conditions. These experiments may support a deeper evaluation of in-situ climatic measurements from Mars in the context of astrobiology. With respect to the occasionally high water vapour content in the Martian atmosphere (Kieffer et al. Reference Kieffer, Martin, Peterfreund, Jakosky, Miner and Palluconi1977), the water uptake of lichens from air humidity alone and above the level of physiological activity might be of further interest. Because B. frigida and C. gyrosa are astrobiological model lichens and part of the current BIOMEX space exposure mission, the present results obtained from both of these currently investigated organisms will also enlarge our knowledge about possible effects on the physical and physiological properties of lichens caused by the conditions of space and Mars-like exposure on the International Space Station.
Acknowledgements
This research was supported by the Helmholtz Association through the research alliance ‘Planetary Evolution and Life’ and is part of the current BIOMEX mission (ESA call, 2009, Ref.-No. ILSRA-2009-0834).

















