Introduction
The in vitro production of embryos has become a traditional tool to increase the reproductive efficiency, to augment the genetic gain and to reduce generation intervals in livestock animals (Keefer et al., Reference Keefer, Baldassarre, Keyston, Wang, Bhatia, Bilodeau, Zhou, Leduc, Downey and Lazaris2001; Peippo et al., Reference Peippo, Kurkilahti and Bredbacka2001; Conceição et al., Reference Conceição, Moura, Ferreira-Silva, Cantanhêde, Chaves, Lima and Oliveira2016). However, retardation and low development rate of embryos have been induced due to their exposure to free radicals and reactive oxygen species (ROS) that arise during in vitro culture conditions (Agarwal et al., Reference Agarwal, Saleh and Bedaiwy2003; Pasqualotto et al., Reference Pasqualotto, Agarwal, Sharma, Izzo, Pinotti, Joshi and Rose2004; Mehaisen & Saeed, Reference Mehaisen and Saeed2015). Excessive ROS causes detrimental injuries to embryos, including membrane’s lipid peroxidation, metabolism disruption, intracellular milieu impairment, cell death and apoptosis (Maity et al., Reference Maity, Bindu, Dey, Goyal, Alam, Pal, Reiter and Bandyopadhyay2009; Succu et al., Reference Succu, Pasciu, Manca, Chelucci, Torres-Rovira, Leoni, Zinellu, Carru, Naitana and Berlinguer2014; Poprac et al., Reference Poprac, Jomova, Simunkova, Kollar, Rhodes and Valko2017; Sharma et al., Reference Sharma, Roychoudhury, Alsaad and Bamajbuor2017; Zidane, Reference Zidane2017). In addition, the expression of several genes responsible for embryo compaction and development, such as GJA1 and POU5F1 were found to be suppressed as consequences of exposure to oxidative stress during in vitro culture (Gomez et al., Reference Gomez, Caamano, Bermejo-Alvarez, Diez, Munoz, Martin, Carrocera and Gutierrez-Adan2009; Kawasumi et al., Reference Kawasumi, Unno, Matsuoka, Nishiwaki, Anzai, Amano, Mitani, Kato, Saeki and Hosoi2009).
The natural metabolites of retinol (RT) such as all-trans retinoic acid (t-RA) and 9-cis RA are collectively known as retinoids and are considered as non-enzymatic antioxidants (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001). Recent protocols have used these compounds in the culture medium of embryos taking into account their deep effect upon cellular survival, proliferation, differentiation and embryonic morphogenesis (Noy, Reference Noy2010; Rajesh et al., Reference Rajesh, Shankar and Deecaraman2010; Rhinn & Dollé, Reference Rhinn and Dollé2012; Conceição et al., Reference Conceição, Moura, Ferreira-Silva, Ramos-Deus, Silva, Cantanhêde, Chaves, Lima and Oliveira2015; Conceição et al., Reference Conceição, Moura, Ferreira-Silva, Cantanhêde, Chaves, Lima and Oliveira2016). It was found that the addition of retinoids to the culture medium enhances the subsequent preimplantation embryonic development in bovine embryos (Lima et al., Reference Lima, Oliveira, Goncalves, Montagner, Reichenbach, Weppert, Neto, Pina and Santos2004; Ahmed et al., Reference Ahmed, Dutta and Nashiruddullah2016), and in goat embryos (Duque et al., Reference Duque, Diez, Royo, Lorenzo, Carneiro, Hidalgo, Facal and Gomez2002a,Reference Duque, Gómez, Hidalgo, Facal, Fernández and Diezb; Lima et al., Reference Lima, Oliveira, Santos, Reichenbach, Weppert, Paula-Lopes, Neto and Gonçalves2006; Chiamenti et al., Reference Chiamenti, Aguiar Filho, Freitas Neto, Chaves, Paula‐Lopes, Lima, Goncalves and Oliveira2010, Reference Chiamenti, Filho, Moura, Paula-Lopes, Neves, Neto, Gonçalves, Lima and Oliveira2012; Conceição et al., Reference Conceição, Moura, Ferreira-Silva, Ramos-Deus, Silva, Cantanhêde, Chaves, Lima and Oliveira2015). The positive effect of retinoids on embryo development could be induced directly by modulating growth factor gene expression or indirectly by improving the quality of mRNAs (Livingston et al., Reference Livingston, Eberhardt, Edwards and Godkin2004). Furthermore, it was found that retinoids can affect the cell antioxidant defence mechanisms by reducing the content of ROS and lipid peroxidation (Pu et al., Reference Pu, Wang, Bian, Zhang, Yang, Li, Zhang, Liu, Fang and Cao2014), and by increasing the levels of antioxidant enzymes such as superoxide dismutase and catalase (Livingston, Reference Livingston2003; Rajesh et al., Reference Rajesh, Shankar and Deecaraman2010).
To the best of our knowledge, there are not enough reports discussing the effect of retinoids on the in vitro development of embryos recovered from livestock animals, particularly in rabbit species. Therefore, the present study aims at evaluating the effect of retinol (RT) on the in vitro development of preimplantation rabbit embryos, focusing on the morphological aspects of embryo development. The antioxidant biomarkers in developed embryos were also assayed via evaluating the levels of malondialdehyde (MDA) and total antioxidant capacity (TAC) as oxidative substrates, and the activities of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) as antioxidant enzymes. In addition, the effect of RT on the expression of two developmental genes namely gap junction protein alpha 1 (GJA1) and POU class 5 homeobox 1 (POU5F1), and an antioxidant gene of superoxide dismutase 1 (SOD1) were analyzed in the developed embryos.
Materials and methods
Chemicals and reagents
All the chemicals and reagents in this experiment were purchased from Sigma-Aldrich (S.A., Egypt) unless otherwise stated.
Source of embryos
In total, 60 nulliparous 5–6-month-old Red Baladi breed (Khalil, Reference Khalil2002) rabbit does (average 2.5 kg wt) were used in the current study. During the study period, all experimental animals were housed in a semi-closed housing system (Agricultural Experiment Station, Faculty of Agriculture, Cairo University) and kept in individual cages, supplied with feeding hoppers made of galvanized steel sheets and nipples for automatic drinker. They were raised under the same standard environmental conditions, fed with the same commercial diet and had free access to water.
The nulliparous females were synchronized for the receptivity by an intramuscular (im) injection with 20 IU eCG (Folligon, Intervet, The Netherlands) 60 h before insemination. The females were artificially inseminated with a semen pool from adult males of the same breed and immediately received an im injection of 2 µg buserelin acetate (Receptal, Intervet Co., Egypt). The method of semen recovery, evaluation and subsequent insemination was described earlier (Lavara et al., Reference Lavara, Lavara, Vicente and Mocé2000). The embryos were collected after sacrificing the does, 72 h post-insemination, by uterine flushing at room temperature (20–25°C). The flushing medium consisted of 1 L Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 0.132 g calcium chloride, 0.2% bovine serum albumin (BSA) and 0.1% antibiotics (10,000 units penicillin-G and 10 mg streptomycin per ml; penicillin–streptomycin solution 100×, BioShop, Canada Inc.).
Treatments and embryo culture
Retinol solution was added to the embryo culture medium (TCM199 + 20% fetal bovine serum + 1% antibiotics) in four different levels (0, 10, 100 or 1000 nM). Only the normal recovered embryos (compact morulae with intact mucin coat and zona pellucida) from each donor doe were randomly distributed to the four retinol treatment groups in a 4-well embryo culture dish (Nunc A/S, Thermo Fisher Scientific, Roskilde Site, Denmark). In total, 574 embryos were cultured; 141 as controls, 147 in 10 nM RT, 144 in 100 nM RT, and 142 in 1000 nM RT. The embryos were cultured for 48 h at 38.5°C, 5% CO₂ and saturated humidity. The numbers of total blastocysts, expanded and hatched blastocysts were recorded at the end of culture period for each treatment group.
Antioxidant biomarkers assay
Three samples per treatment group (each sample consisted of 1 ml culture medium with at least 10 developed blastocysts) were centrifuged at 1030 g for 10 min at 4ºC. The precipitates were washed three times with phosphate-buffered saline (PBS) and collected by centrifugation. The embryo pellets were re-suspended in 1 ml of deionized water, and then were snap-frozen and stored at −80°C until further biochemical analysis. The samples were thawed, shook up, and centrifuged at 1030 g for 15 min at 4ºC. The supernatant was then collected to evaluate the levels of MDA and TAC as oxidative substrates, and the activities of CAT, SOD and GPx as antioxidant enzymes, using colorimetric assay kits as mentioned below. The data were obtained for all analyses by using an automatic scanning spectrophotometer (CE1010, Cecil Instruments Limited, Cambridge, United Kingdom) and the calculations were adjusted per mg protein for each assay.
First, the total protein content was determined in the supernatant using the protein-Biuret colorimetric method (Gornall et al., Reference Gornall, Bardawill and David1949). According to the kit’s protocol (TP-2020, BioDiagnostic, Inc., Egypt), 25 µl of the sample or the standard solution were mixed with 1 ml Biuret reagent and incubated at 37°C for 10 min. The absorbance of the sample (Asample) and standard (Astandard) against blank reagent was read at 550 nm after colour stability for 1 h and linearity up to 10 g/dL. The protein concentration was calculated using the equation: Asample/Astandard × 5.
The level of MDA was determined using colorimetric assay kits (MDA-2529, BioDiagnostic, Inc., Egypt) according to the methods described by Kei (Reference Kei1978). In summary, 200 µl of the sample or standard was mixed with 1.0 ml of chromogen, heated in boiling water bath for 30 min and then cooled. The absorbance of sample (Asample) against blank and standard (Astandard) against distilled water was measured at 534 nm with colour stability for 6 h and linearity up to 100 nM/ml. The MDA in the sample = Asample/Astandard × 10.
The TAC was also determined using colorimetric kits (TAC-2513, BioDiagnostic, Inc., Egypt) according to previously described methods (Koracevic et al., Reference Koracevic, Koracevic, Djordjevic, Andrejevic and Cosic2001). Briefly, 20 µl of each sample was added to 500 µl of substrate (H₂O₂) then incubated at 37°C for 10 min. After that, 500 µl of working reagent (1.0 ml chromogen + 1.0 ml enzyme buffer) was added to the reaction mixture and incubated for 5 min at 37°C. The absorbance of blank (AB) and sample (AS) against distilled water was read immediately at 505 nm and linearity up to 2.0 mM/l. The TAC concentration was calculated as (AB – AS × 3.3).
CAT activity was assayed using colorimetric assay kits (CAT-2517, BioDiagnostic, Inc., Egypt) according to previous methods (Aebi, Reference Aebi1984). In brief, 50 µl of each sample or standard solution was mixed with 0.5 ml phosphate buffer (pH 7.0) and 100 µl of diluted H₂O₂ then incubated for 1 min at 25°C. After that, 200 µl of chromogen-inhibitor and 0.5 ml of peroxidase 4-aminoantipyrine enzyme were added to the mixture and incubated for 10 min at 37°C. The same steps were repeated for the sample and standard blanks (without addition of H₂O₂). The absorbance of the sample (Asample) against sample blank and the standard (Astandard) against standard blank was read at 510 nm after colour stable for 1 h. The CAT activity was calculated as (Astandard − Asample/Astandard × 1000).
The SOD activity was measured using colorimetric assay kits (SOD-2521, BioDiagnostic, Inc., Egypt) according to the methods previously described (Nishikimi et al., Reference Nishikimi, Roa and Yogi1972). Briefly, 100 µl of the sample or control (distilled water) was mixed well with 1.0 ml of working reagent [10 ml phosphate buffer pH 8.5+1.0 ml nitroblue tetrazolium (NBT) + 1.0 ml NADH], and then added to 100 µl of phenazine methosulphate (PMS) to initiate the reaction. The increase in absorbance at 560 nm over 5 min following the addition of PMS was measured for the control (ΔAcontrol) and for the sample (ΔAsample) at 25°C. SOD activity = % inhibition × 3.75 (as % inhibition = ΔAcontrol – ΔAsample/ΔAcontrol × 100).
GPx activity was determined according to the methods described by Paglia and Valentine (Reference Paglia and Valentine1967). According to the kits’ protocol (GPx-2524; BioDiagnostic, Inc., Egypt), 10 µl of each sample was mixed well with the reaction mixture contained 1.0 ml of assay buffer (phosphate buffer and Triton X-100, pH 7.0), 100 µl of nicotinamide adenine dinucleotide phosphate (NADPH) reagent (glutathione, glutathione reductase and NADPH), and 100 µl of H₂O₂ (previously diluted 100 times). The decrease in absorbance at 340 nm per min (ΔA340) was recorded over a period of 3 min against deionized water. A convenient sample dilution was used to control the start of A340 at 1.5 and ΔA340 at 0.05 per min. The following equation was used to calculate the GPx activity: ΔA340/0.00622 × dilution factor.
mRNA expression analysis
At the end of embryo culture, 30 blastocysts from each group (three biological replicates containing pool of 10 hatched embryos each) were kept in cryogenic vials (Corning Incorporated, Corning, NY, USA) and directly plunged into liquid nitrogen (LN2) for later analysis. Total RNA isolation was performed using the Arcturs1 PicoPure1 RNA isolation kit (Applied Biosystems, Carlsbad, USA) per manufacturer’s instruction. Genomic DNA contamination was removed by performing column DNA digestion using RNase-free DNase (Qiagen GmbH, Hilden, Germany).
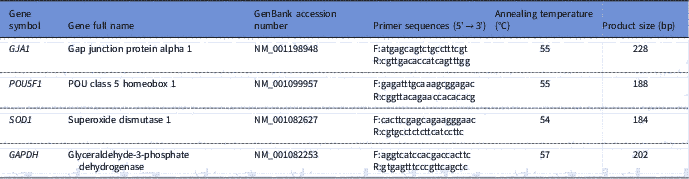
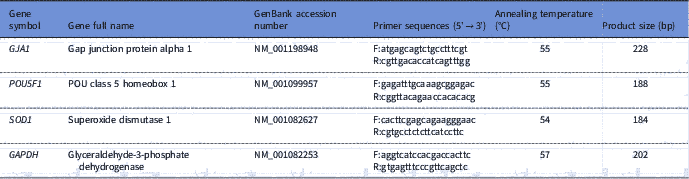
RNA was eluted in 11 μl of elution buffer. The RNA from each replicate was reverse transcribed using 1 mM oligo(dT) primers and the Rever-AidcDNA synthesis kit (Thermo Fisher Scientific, Heidelberg, Germany) per manufacturer’s recommendations. Sequence-specific primers (Table 1) for the real-time PCR were designed using the Primerblast web interface (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) and each pair of primers was tested to achieve efficiencies close to 1. qRT-PCR for expression analysis of two developmental GJA1 and POU5F1 genes, an antioxidant SOD1 gene, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene was performed in a 20 μl reaction volume containing Maxima SYBR Green qPCR Master Mixes with ROX (Thermo Fisher Scientific, Heidelberg, Germany), the cDNA samples and the specific forward and reverse primers in Mx3000P™ real-time PCR system (Stratagene). The thermal cycling parameter was set to 95°C for 3 min, 40 cycles at 95°C for 15 s and 60°C for 1 min. After the end of the last cycle, melting curve was generated by starting the fluorescence acquisition at 60°C and taking measurements every 7 s intervals until the temperature reached 95°C. According to the comparative cycle threshold (CT) method, fold changes in the relative gene expression of the target were determined using the equation 2– ΔΔCT (Livak and Schmittgen, Reference Livak and Schmittgen2001).
Table 1 Details of primers used for real-time PCR quantitative analysis
Statistical analysis
The experimental design was completely randomized with four groups of embryos treated with different levels of retinol (0, 10, 100 and 1000 nM). The variances in the data were homogeneous, as indicated by the HOVTEST option of the GLM procedure (SAS, 2004). Non-normally distributed data (parameters of in vitro development rates) were analyzed by using the CATMOD procedure (SAS, 2004). One-way analysis of variance (ANOVA) was used for statistical analysis of antioxidant enzymatic activity and gene expression parameters using GLM procedure. Tukey’s test was performed to determine differences among embryo groups for all traits. Results presented in the tables are least square means. The mathematical model included the main effect as follows:
where: Yij = An observation of individual ‘i’ of the treatment ‘j’; µ = Overall population mean; Tj = Treatment effect; and eij = Experimental error. The significance level was set at 5%.
Results
In vitro development rates
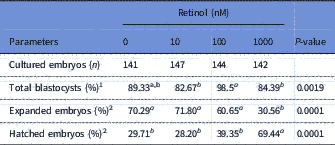
The overall data and in vitro developmental rates of embryos cultured with retinol are shown in Table 2. There was no significant difference in the total blastocyst rate between retinol groups and the control group. The expanded blastocyst rate was significantly (P<0.001) lower at the concentration of 1000 nM RT (30.56%) compared with the other groups (70.29, 71.80 and 60.65% for 0, 10 and 100 nM RT groups, respectively). In contrast, the hatchability rate was significantly (P<0.001) higher in the 1000 RT group (69.44%) than in the other groups (29.71, 28.20 and 39.35% for 0, 10 and 100 RT groups, respectively).
Table 2 In vitro development rates of rabbit embryos cultured with different levels of retinol
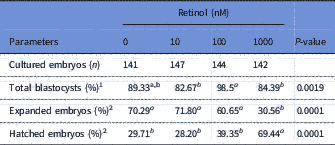
1 Calculated as a percentage of cultured embryos.
2 Calculated as a percentage of developed blastocysts.
a,bLeast squares mean (LSM) with different superscripts, within the same row, are significantly different (P<0.05).
Antioxidant biomarkers
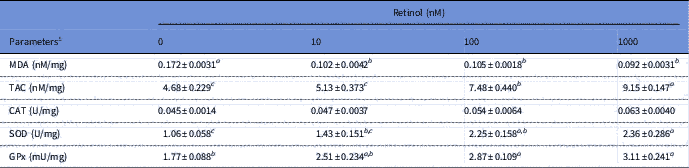
The results of antioxidant biomarkers in embryos treated or not treated with retinol are presented in Table 3. Retinol presence significantly (P<0.05) resulted in lower MDA levels when compared with the control group. The TAC levels were significantly (P<0.05) higher in the RT groups of 100 and 1000 nM than that in the 0 and 10 nM RT groups. Retinol at 100 and 1000 nM also induced a significant (P<0.05) increase in the activity of SOD and GPx antioxidant enzymes when compared with the control group. No significant differences were found in the CAT enzyme among RT groups.
Table 3 Antioxidant biomarkers of rabbit embryos cultured with different levels of retinol
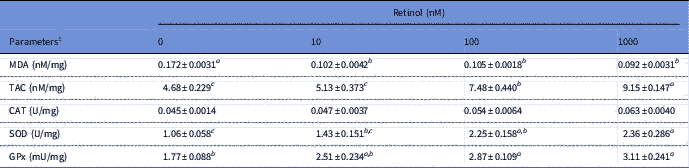
1 All parameters are presented as per mg of total protein. CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; TAC, total antioxidant capacity.
a,b,cLeast squared means (LSM)±standard error (SE) (n = 3) with different superscripts, within the same row, are significantly different (P<0.05).
mRNA expression
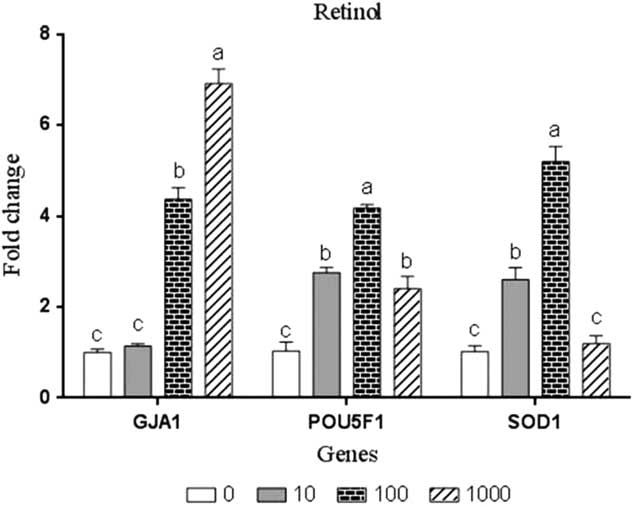
Results of quantitative real-time PCR for the examined developmental-related genes GJA1 and POU5F1 and antioxidant-related gene SOD1 in embryos treated or not treated with retinol are illustrated in Fig. 1. The concentrations of 100 and 1000 nM significantly (P<0.05) enhanced the expression of GJA1 gene by 4.37-fold and 6.92-fold, respectively, compared with the control group. Supplementing the culture medium with 10, 100 and 1000 RT significantly (P<0.05) upregulated the expression of POU5F1 by 2.76, 4.17 and 2.40 fold, respectively, compared with the controls. Furthermore, the expression of SOD1 gene was upregulated by retinol treatments, however this increase was significant (P<0.05) only at concentrations of 10 nM and 100 nM by 2.60- and 5.19-fold, respectively, compared with the control group.
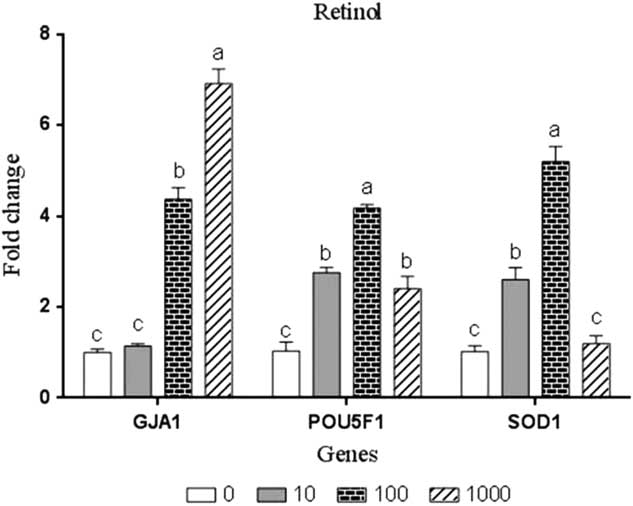
Figure 1 Gene expression analysis in rabbit embryos cultured with different levels of retinol (nM). Bars express the least squared means (LSM)±standard error (SE) (n = 3). a,b,cBars with different superscripts represent significant difference (P<0.05).
Discussion
Retinoid compounds are found in the female reproductive tract and they were suggested to be able to improve the in vitro development of preimplantation embryos in several species (Duque et al., Reference Duque, Diez, Royo, Lorenzo, Carneiro, Hidalgo, Facal and Gomez2002a,Reference Duque, Gómez, Hidalgo, Facal, Fernández and Diezb; Lima et al., Reference Lima, Oliveira, Goncalves, Montagner, Reichenbach, Weppert, Neto, Pina and Santos2004; Chiamenti et al., Reference Chiamenti, Aguiar Filho, Freitas Neto, Chaves, Paula‐Lopes, Lima, Goncalves and Oliveira2010, Reference Chiamenti, Filho, Moura, Paula-Lopes, Neves, Neto, Gonçalves, Lima and Oliveira2012). Retinoids are considered as a biological antioxidant network and an important regulators of redox signalling pathways (Ikeda et al., Reference Ikeda, Kitagawa, Imai and Yamada2005; Alminana et al., Reference Alminana, Gil, Cuello, Caballero, Roca, Vazquez, Gomez and Martinez2008). In the present study, over 574 rabbit embryos at morulae stages were used to evaluate the effect of retinol supplementation on the embryonic development to the blastocyst stage. The results show that the supplementation of retinol at the concentration of 1000 nM significantly increased the hatchability rate by 39.73% compared with the control group. In agreement with this finding, Vahedi et al. (Reference Vahedi, Zeinoaldini, Kohram and Farahavar2009) found that all-trans-RA supplementation at a concentration of 1 µM to the in vitro maturation (IVM) medium significantly increased bovine oocyte maturation. It was also reported (Kim et al., Reference Kim, Kim, Moon, Yoon, Lee and Lee2011) that RA could enhance the developmental competence of mouse oocytes through the suppression of apoptosis and the enhancement of cells differentiation during IVM. In addition, it was concluded that RA increased the number of oocytes attained the two-cell stage and also improved IVM quality (Abouzaripour et al., Reference Abouzaripour, Fathi, Daneshi, Mortezaee, Rezaie and Abdi2018).
Retinoids protect against oxidative damage by maintaining adequate endogenous competency and levels of antioxidants that are essential for oocyte maturation and embryonic development (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001; Alminana et al., Reference Alminana, Gil, Cuello, Caballero, Roca, Vazquez, Gomez and Martinez2008). Therefore, the current work investigated further roles of retinol in the activity of many antioxidant biomarkers in all experimental groups. The low MDA levels and high TAC levels in the RT-treated groups, particularly at concentrations of 100 and 1000 nM, indicated that retinol addition to the embryo culture medium reduces the degree of lipid peroxidation and oxidative stress damages in cultured embryos (Pasqualotto et al., Reference Pasqualotto, Agarwal, Sharma, Izzo, Pinotti, Joshi and Rose2004; Agarwal et al., Reference Agarwal, Gupta, Sekhon and Shah2008). In the present study, the activity of SOD and GPx antioxidant enzymes in the cultured embryos were significantly higher due to the high levels of retinol, while RT treatment did not affect CAT activity. It is well documented (Suzuki et al., Reference Suzuki, Sugino, Fukaya, Sugiyama, Uda, Takaya, Yajima and Sasano1999; Guerin et al., Reference Guerin, El Mouatassim and Menezo2001; Zelko et al., Reference Zelko, Mariani and Folz2002; Lasota et al., Reference Lasota, Ogonski, Blaszczyk, Seremak, Ligocki, Telesinski and Kopczynski2011; Wang, Reference Wang2012) that SOD enzyme has an important role in protecting cells against ROS reactions that may otherwise be toxic to the embryos. Also, GPx enzymes are essential for the glutathione redox cycle as a major source of protection against low levels of oxidant stress (Guerin et al., Reference Guerin, El Mouatassim and Menezo2001), whereas CAT enzymes become more significant in protecting against severe oxidant stress (Yan & Harding, Reference Yan and Harding1997; Mehaisen et al., Reference Mehaisen, Saeed, Gad, Abass, Arafa and El-Sayed2015). These observations could explain the higher competence of embryos cultured with retinol, showing higher hatchability rates for these embryos compared with controls.
Our results demonstrated that the embryo groups cultured with 10 and 100 nM RT expressed a higher mRNA for the SOD1 gene compared with the control group. The high expression in antioxidant- and oxidative stress-related genes was previously reported (Mehaisen et al., Reference Mehaisen, Saeed, Gad, Abass, Arafa and El-Sayed2015) in embryos cultured with other antioxidant materials like melatonin. In the present study, mRNA expressions of GJA1 and POU5F1 as important developmental-related genes were analyzed in all experimental groups. The results show a higher expression in these genes when embryos were cultured with 100 and 1000 nM RT compared with the control embryo group. GJA1 (also named Cx43) is one of intercellular coupling connexins that allows the embryonic cells to share low molecular weight metabolites and second messengers, thus facilitating homeostatic and developmental processes in the cultured embryos (Houghton et al., Reference Houghton, Barr, Walter, Gabriel, Grummer, Traub, Leese, Winterhager and Kidder2002). Other reports (De Sousa et al., Reference De Sousa, Valdimarsson, Nicholson and Kidder1993; Mehaisen et al., Reference Mehaisen, Saeed, Gad, Abass, Arafa and El-Sayed2015) diminish the role of GJA1 in embryo development because the possible presence of any of the other connexins, such as Cx30, Cx31, Cx40, and Cx45, is enough to start the developmental regulation process. Conversely, POU5F1 is essential for the regulation and differentiation of the preimplantation embryonic cells toward the inner cell mass (ICM) (Ovitt & Schöler, Reference Ovitt and Schöler1998; Kirchhof et al., Reference Kirchhof, Carnwath, Lemme, Anastassiadis, Scholer and Niemann2000; Kazemi et al., Reference Kazemi, Dashtizad, Shamsara, Mahdavinezhad, Hashemi, Fayazi and Hajarian2016). It was reported that POU5F1 is the early expressed factor, which has a critical role in cell differentiation and potential development of preimplantation embryos (Panda et al., Reference Panda, Pandey, Somal, Parmar, Bhat, Baiju, Bharti, Kumar, Chandra and Sharma2017). Furthermore, recent studies suggested that the expression of POU5F1 was reduced by the oxidative stress induced during the in vitro culture and vitrification of rabbit embryos (Mehaisen et al., Reference Mehaisen, Saeed, Gad, Abass, Arafa and El-Sayed2015). Similarly to the positive effects of retinol on rabbit embryos, in the present study, these authors found a positive effect of melatonin, as an antioxidant reagent, on the development and hatchability of embryos accompanied with high expression in the POU5F1 gene (Mehaisen et al., Reference Mehaisen, Saeed, Gad, Abass, Arafa and El-Sayed2015).
In conclusion, the current study shows that the addition of retinol at 100 or 1000 nM levels enhances the developmental capacity of rabbit embryos at the morula stage. The high expression of embryo-developmentally key genes, such as GJA1 and POU5F1 genes, appears to be one of the main mechanisms by which retinol improves the embryonic development. Furthermore, retinol treatment provides the cultured embryos with a high antioxidant capacity as presented by the high activity of SOD and GPx antioxidant enzymes. The antioxidant capacity of retinol seems to be another important pathway that can protect embryos from oxidative substrates produced during in vitro culture and, consequently, improves their potential development under such conditions.
Acknowledgements
The authors thank Prof. Dr Farid Stino (Professor of Poultry Breeding, Cairo University, Egypt) for the English editing and revision of this paper. We are very grateful to all the personnel from the Rabbit Research Unit and the Poultry Biotechnology Laboratory at the Faculty of Agriculture, Cairo University, for their assistance in rabbits’ monitoring and sample preparation throughout the experimental period.
Financial support
This work was supported by funds obtained from the Rabbit Embryo Cryobank Project, Department of Animal Production, Faculty of Agriculture, Cairo University (RECP, Code: 3-2 p11 GMKM), and from the Animal Production Research Institute (APRI AME).
Conflict of interest
There are no conflicts of interest.
Ethical standards
The method of sacrificing the rabbit does is slitting the neck for cutting the carotid arteries, jugular veins, oesophagus and trachea, without severing the spinal cord or the head, while the animal is alive. The authors assert that all procedures contributing to this work comply with the ethical standards of Cairo University Ethics Committee for the Care and Use of Experimental Animals in education and Scientific Research (CU-IACUC).