Introduction
The cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is a highly serious and major lepidopteran insect pests that producing severe damages to many economically main crops universal (Challa et al., Reference Challa, Sanivada and Koduru2013). H. armigera attacks causes US$ 17.7 billion financial damages every year. Because of its polyphagous nature, more than 200 plant species are not safe from its damages and control of this pest is problematic due to high fecundity and fast adaptation to synthetic insecticides (Ghosh et al., Reference Ghosh, Chatterjee and Roy2010; Devi et al., Reference Devi, Sharma and Rao2011). On the other hand, wide applying of insecticides, cause this pest resistance to most of them (Scott & Wen, Reference Scott and Wen2001; Heckel, Reference Heckel2012). Today the destructive effects resulting from the use of pesticide on human health by contaminating environments is clear completely (Dietz & van der Straaten, Reference Dietz and van der Straaten1992; Chikate et al., Reference Chikate, Dawkar, Barbole, Tilak, Gupta and Giri2016). Maybe one of the proven ways for controlling of H. armigera is transgenic plants producing Bt toxins, but because of large cultivation and wide usage, the resistance process is likely (Chikate et al., Reference Chikate, Dawkar, Barbole, Tilak, Gupta and Giri2016). So this is necessary to develop and use of new strategies for control of this pest and another pests without damages to the environment, natural enemies and humans.
As a modern influential and accurate tool, RNA interference (RNAi) has been widely applied in many aspects and powerfully accelerates the progress of life knowledge (Fire et al., Reference Fire, Xu, Montgomery, Kostas, Driver and Mello1998; Aravin et al., Reference Aravin, Naumova, Tulin, Vagin, Rozovsky and Gvozdev2001). Despite the fact that the RNAi pathways operate by highly conserved strategies in different organisms, they comprise different proteins and mechanisms. RNAi is a post-transcriptional regulator tool involving sequence-specific degradation of a target mRNA and now it is obvious that gene silencing is a conserved mechanism in numerous eukaryotes and it has been used in entomological investigation, too (Geley & Muller, Reference Geley and Muller2004; Ghanim et al., Reference Ghanim, Kontsedalov and Czosnek2007). RNAi is triggered by double-strand RNA (dsRNA) structures and the specificity is based on the sequence of one strand of these structures consistent to partial or complete sequence of a specific gene transcript (Siomi & Siomi, Reference Siomi and Siomi2009). This mechanism is interceded by the creation of small interfering RNAs (siRNAs) from the dsRNA. They are cleaved by dsRNA-specific endonucleases such as dicers (from the dicer gene) identified in Drosophila melanogaster. Wherein Dicer RNase III type enzymes bind and digest cytoplasmic dsRNAs and change them to siRNAs that have 21–23 nucleotides. These siRNA cleavage products then function as sequence-specific interfering RNA in transcript income, cleavage and translational inhibit (Yoo et al., Reference Yoo, Kragler, Varkonyi-Gasic, Haywood, Archer-Evans, Young, Lough and Lucas2004).
In recent years, RNAi technique has been used to study gene function in various organisms and also exhibited remarkable changes in pest control (Hannon, Reference Hannon2002; Gordon & Waterhouse, Reference Gordon and Waterhouse2007; Price & Gatehouse, Reference Price and Gatehouse2008; Huvenne & Smagghe, Reference Huvenne and Smagghe2010). However, successful RNAi is difficult to be achieved in some insects, especially for Lepidoptera (Terenius et al., Reference Terenius, Papanicolaou, Garbutt, Eleftherianos, Huvenne, Kanginakudru, Albrechtsen, An, Aymeric, Barthel, Bebas, Bitra, Bravo, Chevalier, Collinge, Crava, de Maagd, Duvic, Erlandson, Faye, Felföldi, Fujiwara, Futahashi, Gandhe, Gatehouse, Gatehouse, Giebultowicz, Gómez, Grimmelikhuijzen, Groot, Hauser, Heckel, Hegedus, Hrycaj, Huang, Hull, Iatrou, Iga, Kanost, Kotwica, Li, Li, Liu, Lundmark, Matsumoto, Meyering-Vos, Millichap, Monteiro, Mrinal, Niimi, Nowara, Ohnishi, Oostra, Ozaki, Papakonstantinou, Popadic, Rajam, Saenko, Simpson, Soberón, Strand, Tomita, Toprak, Wang, Wee, Whyard, Zhang, Nagaraju, French-Constant, Herrero, Gordon, Swevers and Smagghe2011), but in many studies acceptable outcome is achieved and maybe it need and this is a rationale reason for further study on this group of insects by this technique. In a new study, the effect of RNAi technique of selected candidate genes such as serine and cysteine proteases and another gene on growth and development of H. armigera was studied. The dsRNA-fed larvae showed significant reduction in the larval mass and abnormalities at the various stages of H. armigera development compared with their control diet (Chikate et al., Reference Chikate, Dawkar, Barbole, Tilak, Gupta and Giri2016). In another study, was showed that gene silence effect in insects with various dsRNA delivery methods consist mainly of injection, ingestion, soaking and feeding with transgenic plant, could be persuaded with most of these methods (Yu et al., Reference Yu, Christiaens, Liu, Niu, Cappelle, Caccia, Huvenne and Smagghe2013). The data of a study on effects of period RNAi on V-ATPase expression and rhythmic pH changes in the vas deferens of S. littoralis demonstrate that the upper vas deferens molecular oscillator involving the period gene have an crucial role in the regulation of rhythmic V-ATPase activity and periodic acidification of the lumen (Kotwica-Rolinska et al., Reference Kotwica-Rolinska, Gvakharia, Kedzierska, Jadwiga and Piotr2013). Wang et al. (Reference Wang, Dong, Desneux and Niu2013) screened H. armigera 3-hydroxy-3-methylglutaryl coenzyme A reductase and silenced it using systemic RNAi. This reduced the fecundity of the females, effectively inhibited oviposition and significantly reduced vitellogenin mRNA levels. Another studies can be referred that exhibited down regulation on the specific gene with dsRNA feeding such as silencing in chitin synthase in Spodoptera exigua (Chen et al., Reference Chen, Tian, Zou, Tang, Hu and Zhang2008) production of a transgenic tobacco that expressed ecdysone receptor encoding (EcR) dsRNA from H. armigera (Zhu et al., Reference Zhu, Liu, Ma, Zhang, Qi, Wei, Yao, Zhang and Li2012) and RNAi on acetyl cholinesterase in H. armigera (Kumar et al., Reference Kumar, Gupta and Rajam2009). The effects of siRNA on seven target genes on Bombyx mori embryos were determined. Results show that injection of siRNA is an operative reverse-genetics instrument for the study of embryogenesis in vivo in insects (Yamaguchi et al., Reference Yamaguchi, Mizoguchi and Fujiwara2011). In a control pest study through RNAi on western corn rootworm Diabrotica virgifera virgifera was surveyed 290 possible target genes in by feeding of artificial diet (AD) with dsRNA and the results displayed that only 14 genes were established specific down regulation and caused in notable larval stunting and death (Baum et al., Reference Baum, Bogaert, Clinton, Heck, Feldmann, Ilagan, Johnson, Plaetinck, Munyikwa and Pleau2007). A previous study in the Lepidopteran Spodoptera litura found that injection of dsRNA could competently down regulate the amino peptidase-N expression level in larval midgut, but feeding bioassay of dsRNA did not induce any RNAi effect (Rajagopal et al., Reference Rajagopal, Sivakumar, Agrawal, Malhotra and Bhatnagar2002).
Maybe the first step for a successful RNAi for control of a pest is to find a target gene (Jing & Zhao-jun, Reference Jing and Zhao-jun2014). This gene can be an enzyme such as α-amylase and Juvenile hormone esterase (JHE). Alpha-amylases (α−1,4-glucan-4-glucanohydrolases; E.C. 3.2.1.1) belong to an important category of digestive enzymes, in some of substrate such as starch, glycogen and various other related carbohydrates catalyze the hydrolysis of α-D-(1,4)-glucan linkages (Franco et al., Reference Franco, Riggen, Melo, Bloch, Silva and Grossi2000). At the end, they convert starch to oligosaccharides, which are supplementary hydrolyzed to glucose by α-glucosidase and make a resource full of energy (Zeng & Cohen, Reference Zeng and Cohen2000). In insects, α-amylases improvement insect's digestive capability, help them to survive in varied situations and upsurge their fitness rate. Even throughout non-feeding phases such as pupa, amylases are also active that refers to the importance of these group of enzymes for development stages of insects (Zhu et al., Reference Zhu, Liu, Maddurb, Oppert and Chen2005).
JHE is a carboxylesterases (COEs) that has got much attention for its crucial role in metamorphosis regulating in arthropods and insects specially. The key hormone, juvenile hormone (JH) is hydrolyzed by JHE and titer of it is controlled by JHE, too. Growth, development, metamorphosis, diapause and reproduction in insects are critical options that are controlled by JH (Denlinger, Reference Denlinger, Kerkut and Gilbert1985; Riddiford et al., Reference Riddiford, Himura, Zhou and Nelson2003). Before the beginning of metamorphosis, a reduction in the biosynthesis of JH and an increase in JHE activity happens (Roe & Benkatesh, Reference Roe, Benkatesh and Gupta1990). This then creates the step for the promotion of ecdysteroid level (Mizoguchi, Reference Mizoguchi2001). At the time of elevation in ecdysteroid levels for larval molts, JH typically exists and guarantees larval molt and going them to next instar although, at the time of the last instar molt, JH fades and ecdysone induces metamorphosis (Riddiford, Reference Riddiford, Gilbert, Tata and Atkinson1996). All of these proven information show that α-amylase and JHE are critical enzymes and can be the excellent targets for RNAi.
In this study, for the first time we focused on this two enzymes and selected them as a target for RNAi on H. armigera to elucidate the effect of the direct injection and feeding bioassay on this pest. The results indicated that feeding and injecting bioassay of both α-amylase and JHE dsRNA induced RNAi in H. armigera. Effects of RNAi also elucidated on the enzyme activity level of α-amylase and malformation and abnormality on pupa and adults, and on expression of the mRNA level.
Materials and methods
Insect culture
The cotton bowllworms, H. armigera were collected from tomato plants at Manujan, Kerman Province, Iran in 2015 (27°26′N, 57°30′E). H. armigera larvae were reared in controlled chambers at 26±°C at 75 ± 5% relative humidity under a photoperiod of 14/10-h light/dark using an AD (Kotkar et al., Reference Kotkar, Bhide, Gupta and Giri2012). Before to the 3rd instar, every five larvae were placed on a piece of the diet in the same container, and subsequently because of cannibalism the larvae were reared separately.
Sequences and phylogenetic analysis
The nucleotide sequences of the α-amylase and JHE were detected from GeneBank database of National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/). Within Blast Program available on this website the sequences was blasted as blastn. Then amino acid residues were translated within Lasergene, EditSeq builder software. Sequences were aligned by using Clustal W method using the MegAlign Software and phylogenetic tree was drawn with MEGA 6.0 software.
RT–PCR of α-amylase and JHE genes
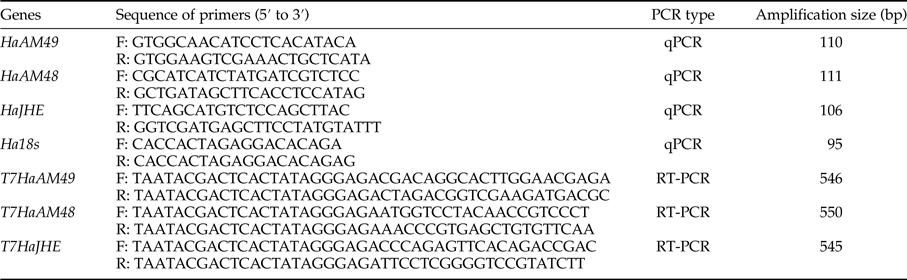
Total RNAs from whole body of 1st to 5th instar larvae, pupae, adult moth and midgut, epidermis, fat body, hemolymph of 5th instar larvae were extracted with GeneAll RibospinTM total RNA purification Kit (GeneAll). First strand cDNA was produced from 1 µg RNA using HyperScriptTM First strand Synthesis Kit (GeneAll) with oligo dT18 primer. The synthesized single-stranded cDNA was used as a template for PCR amplification with gene-specific primers (table 1) with 35 cycles under conditions of 1 min at 95°C for denaturation, 1 min at 54°C for annealing and 1 min at 72°C for extension. PCR of β-actin was used as a control with specific primers (table 1).
Table 1. Nucleotide primers used for RT–PCR, to synthesize dsRNA, and to perform qRT–PCR.
Synthesis of dsRNA
Total RNAs from whole body and midgut for JHE and α-amylase, respectively, were extracted with GeneAll RibospinTM total RNA purification Kit (GeneAll). First strand cDNA was produced from 1 µg RNA using HyperScriptTM First strand Synthesis Kit (GeneAll) with oligo dT18 primer. The dsRNAs were designed targeting at α-amylase and JHE genes, which both encoded vital functional enzyme for H. armigera. Sequence analyses were done by CLUSTAL W (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Green fluorescent protein (GFP) was applied as a negative control. Primers were designed by NCBI's primer-BLAST web and using gene-specific sequences that were adapted to T7 promoter sequence at 5′ end that the primer sequences and amplicon length for production of dsRNA of each mRNA are given in table 1. cDNAs synthesized from mRNA of treated larvae, were used for amplification of target regions. DsRNA was synthesized using the MEGAScript dsRNA synthesis kit (Ambion, Huntingdon, UK) according to the manufacturer's instructions. After produced, dsRNAs were purified and determined concentration for quality and quantity by Nanodrop 1000 spectrophotometer (Thermo scientific, Waltham, MA, USA) and agarose gel electrophoresis 1%. DsRNAs amplicon from this step were used for injecting and feeding bioassays of H. armigera.
Ingestion of dsRNA
To assess the insecticidal activity of the dsRNA specific on the growth and development of H. armigera, surface of AD cubes (~1 cm) were covered with 60 µg dsRNA solution and after diffusing into cube it was delivered to 4th-instar larvae for all genes. As non-dsRNA control, AD covered with DEPC-treated water was applied. DsRNA targeting GFP was used as negative control. DsRNA for all the target genes were fed larvae three times with interval of 24 h (larvae were allowed to feed for 72 h). After this time, rearing was continued on normal AD (without dsRNA) to observe for larval growth and development. 24, 48, 72 and 96 h post-feeding on normal AD, silencing effects were studied. Fifty insects were treated in each treatment, and the experiment was replicated for three times. After each time 20 larvae from each of groups were picked up randomly and were placed in liquid nitrogen for another experiments.
Injection of dsRNA
The 4th-instar larvae of cotton bollworms were ice anesthetized and 4 µl of the dsRNA solution was injected into larval hemocoel through the penultimate abdominal segments (Jing & Zhao-jun, Reference Jing and Zhao-jun2014). Like the section ‘Ingestion of dsRNA’ dsRNA for all the target genes were injected to larvae three times with interval of 24 h (each treated larvae received 10 µg dsRNA). In the two control groups, the same volume of DEPC and dsgfp were injected. After injection AD was delivered and rearing continuous was on normal AD to observe for larval growth and development. Fifty insects were treated in each treatment, and the experiment was replicated for three times.
Quantitative real-time PCR analysis
Ten larvae were selected from each group in digestion and injection bioassay. The RNAs from each group were extracted with GeneAll RibospinTM total RNA purification Kit (GeneAll) from whole body for Ha-JHE and midgut (for Ha-AMY48 & 49). For the elimination of genomic DNA contamination, the total RNA was treated with DNase I, RNase-free (Fermentas, Thermo Scientific). The quality and quantity of RNA were determined by spectrophotometric analysis using Nanodrop (Thermo Scientific) and agarose gel electrophoresis. First strand cDNA was produced from using HyperScriptTM First strand Synthesis Kit (GeneAll) according to the manufactures instruction. The relative transcript level of target genes was calculated according to the 2−ΔΔCT method (Livak & Schmittgen, Reference Livak and Schmittgen2001). The primers were designed by using of IDTDNA web, https://sg.idtdna.com/PrimerQuest (table 1). For normalization of relative expression was used of 18srRNA gene (Ha18s), GenBank: AJ577253.1.
Alpha-amylase-specific activity
The dsRNA treated and control larvae were cold-immobilized, dissected under a stereoscopic microscope, and their midguts were removed. The midguts were then cleaned of adhering unwanted tissues and homogenized with a hand-held glass grinder on ice. The homogenates were centrifuged at 15,000 × g at 4°C for 15 min. The resulting supernatants were used as enzyme source for calculating α-amylase activity. Alpha-amylase activity was assayed according to Bernfeld (Reference Bernfeld1955) using dinitrosalicylic acid (DNS) as the visualizing reagent and 1% soluble starch as the substrate. Briefly, 10 µl enzyme extract was incubated for 30 min at 35°C with 100 µl buffer and 15 ml soluble starch. The experiment was stopped with the addition of 75 µl DNS and heating in boiling water for10 min. The absorbance of the reaction mixture was read at 540 nm using a microplate reader. In the blanks, denatured enzyme extract (5 min heating in boiling water) was used instead of enzyme extract. One unit of α-amylase activity was defined as the amount of enzyme required to produce 1 mg maltose in 30 min at 35°C. All assays were done in triplicate.
Visualization of α-amylase activity
In-gel α-amylase assay was performed using native-polyacrylamide gel electrophoresis (PAGE) for visualizing α-amylase activity. Enzyme extract was diluted in an electrophoresis sample buffer (62.5 mM Tris–HCl, pH 6.8, 10% (v/v) glycerol, 0.01% (w/v) bromophenol blue) and loaded in a gel of 5% stacking and 10% separating polyacrylamide gels. To observe α-amylase activity bands after native PAGE, there solving gel was incubated in a 1% (w/v) starch solution for 60 min with gentle shaking. Finally, it was stained with a solution of 14 mM KI and 10 mM I2. The bands of α-amylase activity appeared as clear areas in the field of black background of the gel.
Statistical analysis
Data were subjected to analysis of variance (ANOVA), and the means were compared by Student's t-test. Statistical analyses were performed using the software Statgraphics Plus 5.1.
Results
Sequences properties and phylogenetic analysis
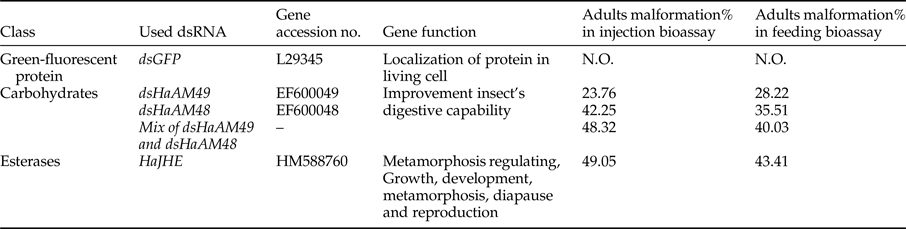
A blast search using NCBI database determined three mRNA sequence for H. armigera α-amylase gene, Ha-AMY (Accession number: EF600048, EF600049 and EU325552) and one mRNA candidate genes with complete open reading frames (ORFs) for JHE gene, Ha-JHE (Accession no.: HaHM588760). Through this study for every gene one abbreviation was selected as following (EF600048: Ha-AMY48); (EF600049: Ha-AMY49); (EU325552: Ha-AMY52); (HM588760: Ha-JHE). In the case of Ha-AMY, two sequences, Ha-AMY52 and Ha-AMY49, had 98% identity in Blast P when highly similarity option was selected. Between two this sequences Ha-AMY49 was chosen. Ha-AMY48 has 1485 bp in length and 1262 bp in ORFs. This sequence has three conserved domain. Ha-AMY49 is some bigger than Ha-AMY 48, as 1502 bp located in it ORF region. Ha-JHE has 3011 bp of nucleotides about twofold rather than Ha-AMY48 sequence and a long conserved domain with 512 bp. Each dsRNA were named with specific name related to their gene; dsHa-AMY48 for HaEF600048, dsHa-AMY49 for HaEF600049 and dsHa-JHE for HaHM588760 mRNA. Translated amino acids of candidate genes were detected, and then the schematic pictures of amino acid sequences were prepared (fig. 1).
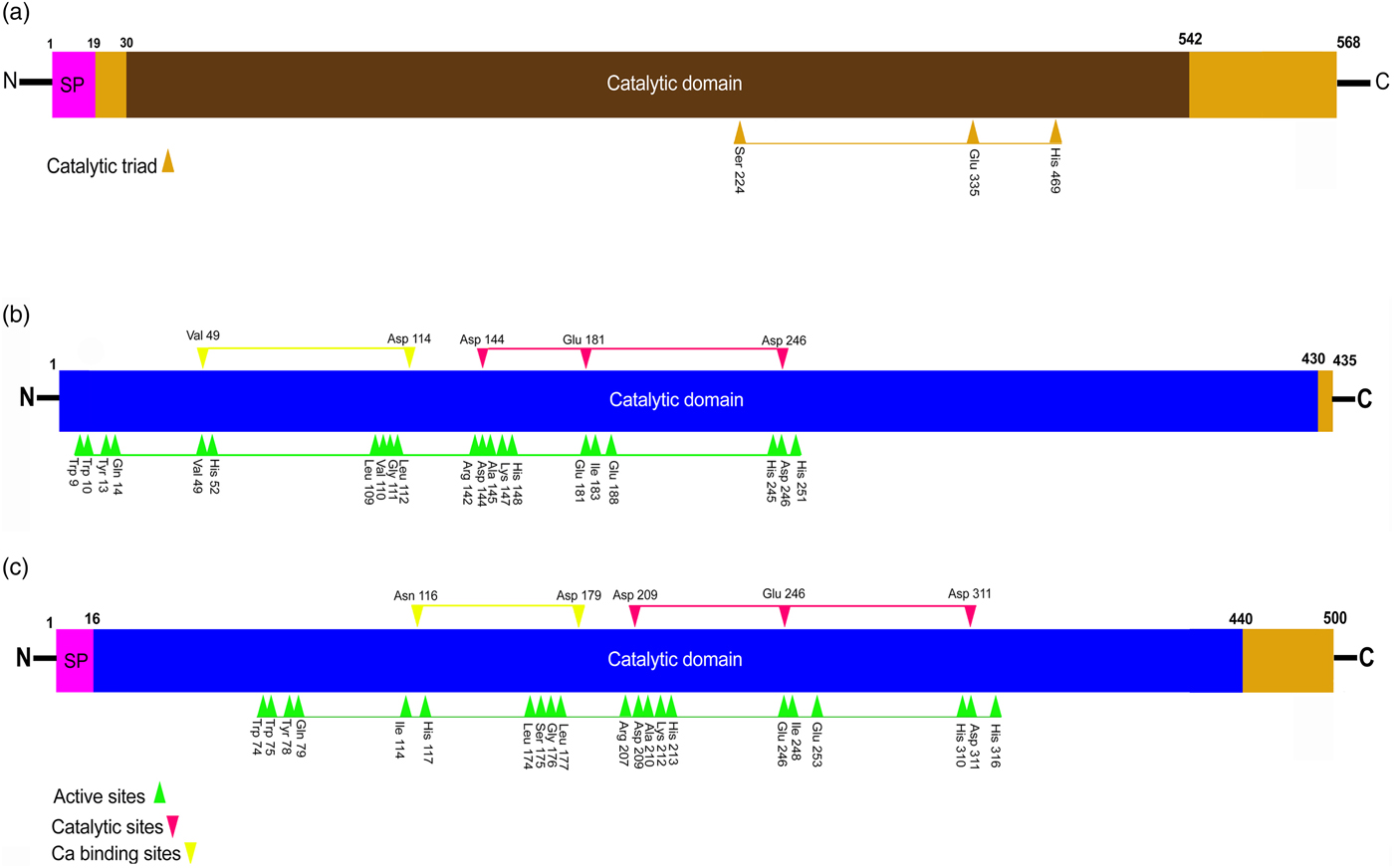
Fig. 1. Diagrams showing analysis of candidate enzymes amino acid sequences (a) H. armigera JH esterase protein (AEB77712), (b, c) H. armigera α-amylase proteins (ABU98613 & ABU98614) (Ha-AMY1 & 2), (SP; Signal peptide).
Alignment and phylogenetic analysis tree for amino acid residues of candidate genes in H. armigera and another well-known animal proteins were drawn separately (fig. 2a, b). The GenBank numbers or NCBI reference sequence of alignment α-amylase proteins are following: (H. armigera: ABU98613, ABU98614, ACB54942) (S. litura: AGV76304); (Spodoptera frugiperda: AAO13754); (Plutella xylostella: XP_011548154); (Papilio xuthus: XP_013164404); (Papilio polytes: XP_013143960); (Papilio machaon: XP_014357550); (Oatrinia nubilalis: AAA03640); (Mamestra configurata: AEA76309); (B. mori: ACJ49024); (Anagasta kuehniella: ACL14798); (Scirpophaga incertulas: ABV24963); (Apis mellifera: BAA86909); (D. melanogaster: CAA59126); (Diatraea saccharalis: AAP92665); (Homo sapiens 1: AAA51724); (H. sapiens 2: BAA14130) and (Mus musculus: NP_001103975). The GeneBank number or NCBI reference sequence of JHE are provided: (H. armigera: AEB77712); (Sesamia nonagrioides: ABW24129); (Spodoptera littoralis: ACV60229); (P. xylostella: XP_011563398); (P. machaon: XP_014365132); (Omphisa fuscidentalis: ACB12192); (Manduca sexta: AAG42021); (Mythimna separate: AJR27471); (Heliothis virescens: AAB96654); (Heliothis viriplaca: AGB93712); (Chilo suppressalis: AIY69071); (Choristoneura fumiferana: AAD34172); (B. mori: AAR37335); (Amyelois transitella: XP_013197467); (Agrotis ipsilon: AGS49136); (D. melanogaster: AAK07833); (A. mellifera: AAU81605) and (Adoxophyes honmai: BAR88201.1). Neighbor-joining trees were constructed a Poisson-corrected distance with bootstrap test of 1000 replications. This analysis showed two α-amylases from H. armigera (ABU98614 and ACB54942) were closely related together but had not high relation with neighbor sequences. Our finding showed a neighbor relatively between A. mellifera and H. armigera amino acid seq. (ABU98613). All studied sequences divided into six groups that mammalian animals such as human and mice were in one group and near the two H. armigera seq.s. (fig. 2c). JHE amino acids divided into ten groups based on phylogenetic tree in (fig. 2d). JHE amino acid of our target pest is near to Heliothis genus amino acids.
Fig. 2. Alignment and phylogenetic analysis of Ha-JHE and Ha-AMY48 & 49 with its homologs from other insect species. (a, b) The identical amino acid residues that are conserved among all of the sequences are indicated by white letters in black background. The conserved α-amylase and JHE signal peptide (as indicated by a dotted line at the top of the upper panel) active site is indicated with arrowheads. The calcium-binding sites in α-amylase (AMY) seq.s are indicated with a black circle. The catalytic site is indicated with a gray diamond (a) alignment of JHE amino acid, (b) alignment of AMY amino acids). (c, d) The phylogenetic analysis is performed using the Seqman Clustal W program (DNAstar package, DNASTAR Inc., Madison) with 1000 bootstrap replicates at a Gap Penalty of 10 and a Gap Length Penalty of 0.2. (c) Alignment of AMY amino acids. (d) Alignment of JHE amino acid.
Expression of candidate genes
We study on the expression of all candidate genes in development stages and some tissues of H. armigera. All target genes expressed in all larval stages from 1st to 5th and in pupa and adult, too. In the study of gene expression at some tissues, we found that all genes were expressed in midgut. Also Ha-AMY49, Ha-AMY52 and Ha-JHE were expressed in epidermis and fat body (fig. 3).

Fig. 3. Expression profiles of Ha-AMY48, Ha-AMY49, Ha-AMY52 and Ha-JHE at different developmental stages from 1st-instar larvae to adult and in different tissues: midgut (G), epidermis (Ep), fat body (FB) and hemolymph (H).
Silencing of related mRNAs of H. armigera
Gene silence effect induced by dsRNA ingestion
For this experiment larvae were treated with poisoned AD to dsRNA. Gene expression analysis in most cases showed significant RNAi effects of target sequences in fed larvae compared with adjacent larvae in the control group. After 24 h of third cube feeding, was observed decreasing in HaAMY48, HaAMY49 and Ha-JHE expression 56, 59.7 and 14.4%, respectively. This slaking continued and were more after 48, 72 and 96 h after feeding last smeary cube to dsRNA. So that data after 96 h showed more than 96% RNAi effect on HaAMY49 and a high suppression on HaAMY48 (80%). Down regulation of Ha-JHE expression after 24, 48, 72 and 96 h had significant differences with control group. These results showed that dsRNA feeding induced interference effects on the expression of selective candidate with variable efficacy and time have an important role (fig. 4a–c).

Fig. 4. Knockdown of Ha-AMY48 (a), Ha-AMY49 (b) and Ha-JHE (c) expression by ingestion of dsRNA. The larvae samples of each group were collected post after 24, 48, 72 and 96 h feeding of last treated AD cube. Non-dsRNA (DEPC) and ds gfp were used as control. Relativity was calculated basis of DEPC groups. All the experiments were triplicated. Asterisk indicates significant difference of relative expression (P < 0.05, Student's t-test).
Gene silence effect induced by dsRNA injection
In injection bioassay, three injections were done and the next experiment after third injection in three times, 24, 48 and 72 h, same ingestion bioassay, was done. All of gene expression results from Real-Time PCR analysis for these three candidates genes, showed significant suppression. HaAMY48 mRNA expression was suppressed after 24, 48 and 72 h at a rate of 61.5, 71.5 and 74.3%, respectively. Same these results was happened for HaAMY49 that was observed 70.5, 87.7 and 91.5% at 24, 48 and 72 h, respectively, after third injection of dsRNA compared with control groups. An impressive down regulation was observed about Ha-JHE expression, so that after 72 h, 75% of this gene was interfered by RNAi effects (fig. 5a–c). Figure 6 shows some phenotypic effects in larvae, pupa and adult that treated in the larval stage with Ha-AMY dsRNAs as injection.
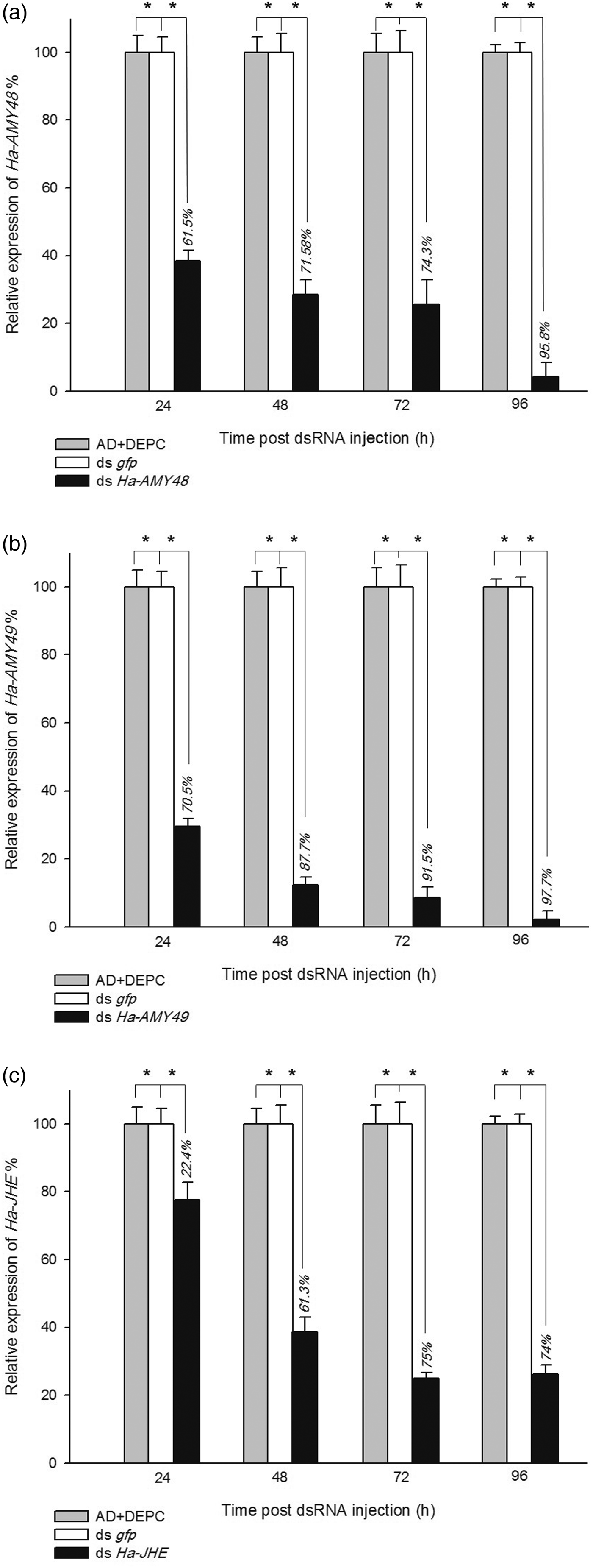
Fig. 5. Relative mRNA expression of Ha-AMY48 (a), Ha-AMY49 (b) and Ha-JHE (c) expression by injection of dsRNA. The larvae samples of each group were collected post after 24, 48, 72 and 96 h last dsRNA injection. Non-dsRNA (DEPC) and ds gfp were used as control. Relativity was calculated basis of DEPC groups. All the experiments were triplicated. The percentages on bars show knockdown % of gene. Asterisk indicates significant difference of relative expression (P < 0.05, Student's t-test).
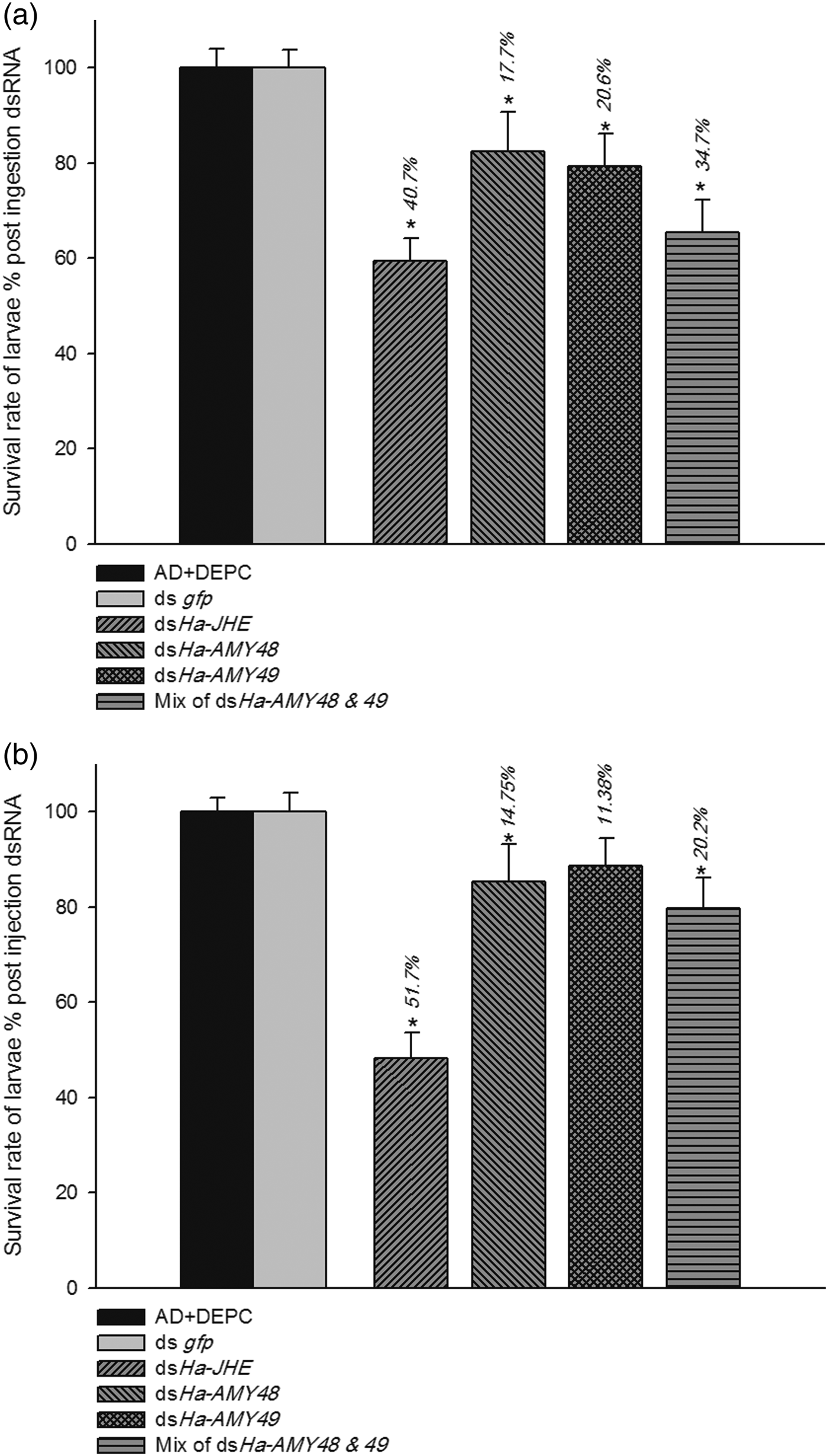
Fig. 6. Survival rate % of cotton bollworm larvae: after rearing on AD containing dsRNA (a) and after dsRNA injection (b). In feeding experiment dsRNA for each gene was applied thrice onto surface of AD cube after interval of 24 h and in injection case, dsRNA thrice injected to body cavity interval of 24 h and then larvae were reared on normal AD and their further growth and development and was observed until larvae were change to pupa. Non-dsRNA (DEPC) and ds gfp were used as control. Relativity was calculated basis of DEPC groups. The percentages on bars show mortality %. Asterisk indicates significant difference of relative expression (P < 0.05, Student's t-test).
Effects of ingestion and injection of candidate's genes dsRNA on survival, mass, growth performance and development of H. armigera
The effects of feeding bioassay on some biology aspects of H. armigera were studied. Among of these three genes, larvae that were reared on AD containing dsHa-JHE, showed the lowest survival (60%), while this percent for larvae were fed with dsRNAs related to α-amylase, were about 80% for both HaAMY48 and 49. The larvae growth on AD cubes containing an equal mix of these two dsRNAs with near 30% mortality, did not show significant effects. Same results were observed in injecting bioassay. About 50% of larvae after three injection of HaJHE dsRNA, were survived and were able change to pupa. Injection of dsHaAMY48, 49 and mixing of them (dsHa-AM48 + 49), had not high effects on mortality of larvae (about 10%) (fig. 7a, b).
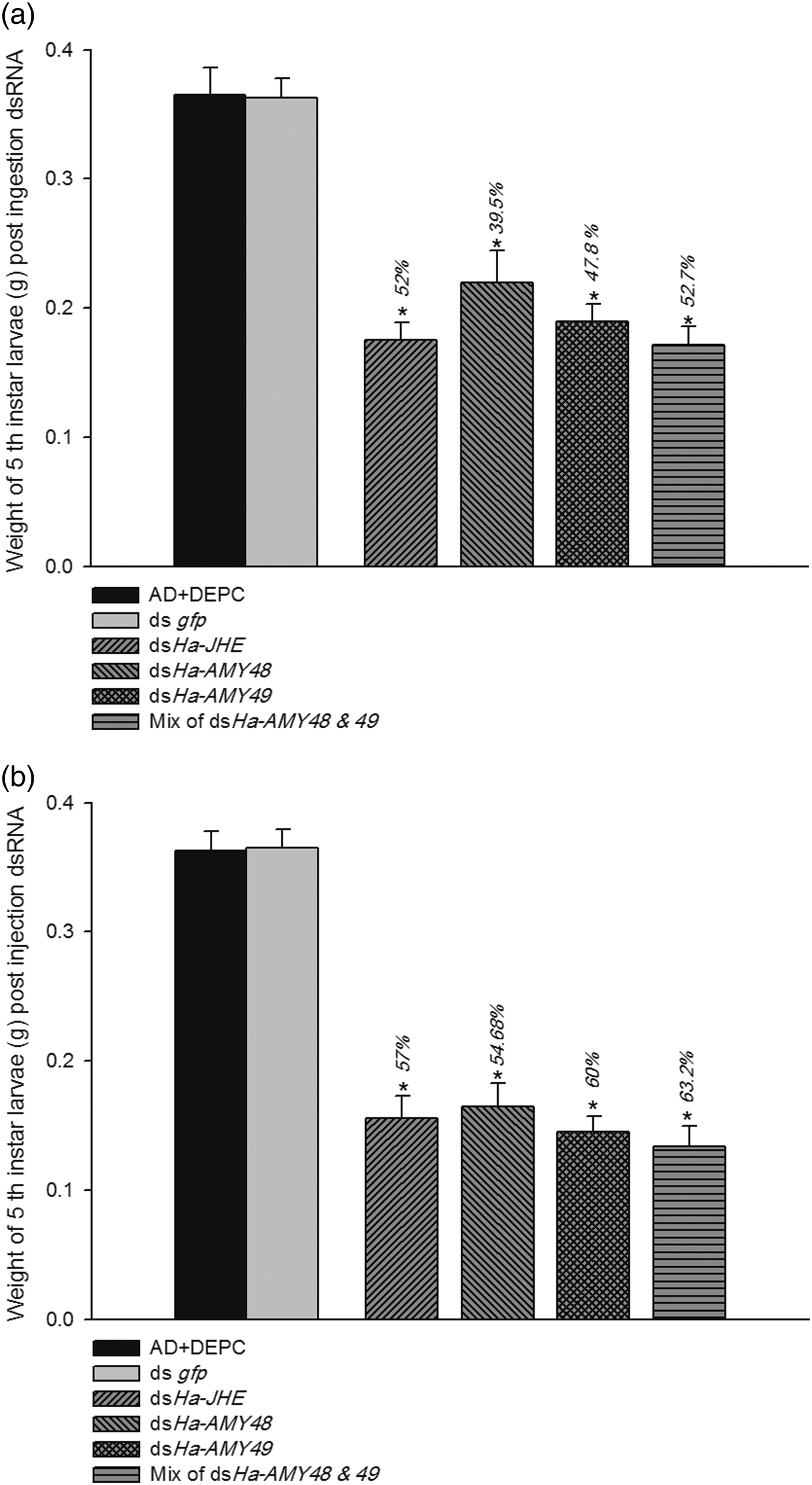
Fig. 7. Silencing effects of Ha-AMY48, Ha-AMY49 and Ha-JHE on larval mass. After rearing on AD containing dsRNA (a) and after dsRNA injection (b). In feeding experiment dsRNA for each gene was applied thrice onto surface of AD cube after interval of 24 h and in injection case, dsRNA thrice injected to body cavity interval of 24 h and then larvae were reared on normal AD. The larvae of 5th day of 5th instar were weighted. Non-dsRNA (DEPC) and ds gfp were used as control. The percentages on bars show weight loss % that were calculated basis of DEPC groups. Asterisk indicates significant difference of relative expression (P < 0.05, Student's t-test).
There were found impressive results for effects of ingestion and injection dsRNA. The findings suggest these candidate genes have high effects on reduction of larval weight. Fifth instars of H. armigera that had 72 h feeding on AD cubes containing dsHa-JHE during their developmental period, lost about 53% of weight. Three groups of larvae that were fed with HaAMY48, 49 dsRNA and an equal mix of these two dsRNAs were weight loss (40, 48 and 53%, respectively) (fig. 8a, b). Effects of RNAi on some larvae, pupa and adult were showed in (fig. 8c). Some malformations on adult moths were observed in both ingestion and injection of dsRNAs in compare with control groups. The highest malformations of moths happened for dsHa-JHE injection, about 49% (table 2).
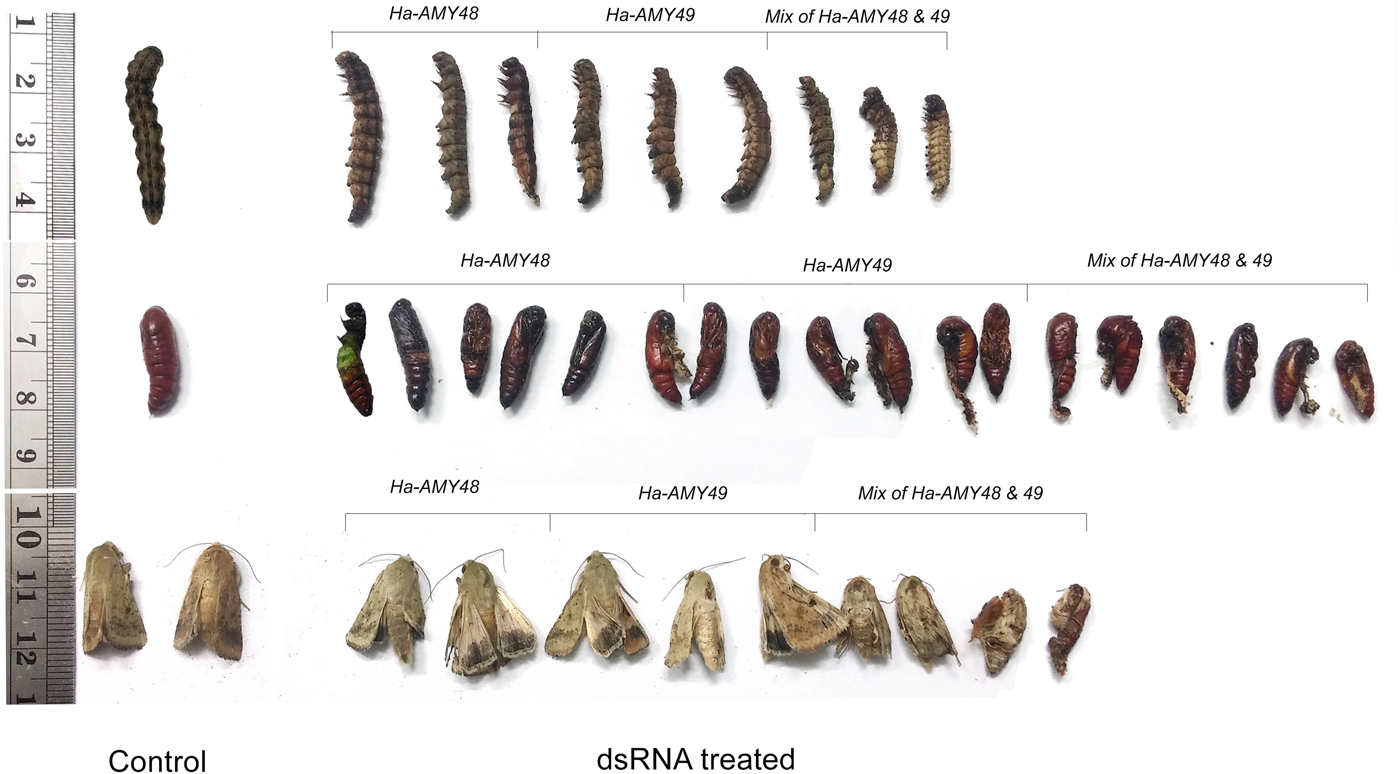
Fig. 8. RNAi of candidate genes triggered some malformation and abnormality on larvae, pupa and moth of H. armigera in injecting bioassay. These insects are the representative of all treated insects.
Table 2. Malformation % on moth after feeding and injecting bioassay of candidate genes.
Silencing effects of α-amylase candidate genes on midgut enzyme activity
Midgut crude extract of the larvae that fed with cubes containing dsHa-AMY48, 49 and equal mix of them showed significant reduction of α-amylase-specific activity on specific substrate, starch, to their respective control, after 24, 48, 72 and 96 h of the latest feeding and ingestion bioassay. Specific activity of α-amylase decreased about 35% after 72 h of feeding dsRNA. But after 96 h insects recovered the level of α-amylase enzyme about 14% in maximum condition. Enzyme inhibition quantity arrived more than 87% in injection bioassay after 96 h. In the feeding bioassay significant difference was not observed between effects of dsHaAMY48 to dsHaAMY49 and dsHaAMY48 + 49 on enzyme activity if compared together. In injection assay, these results were impressive. The high reduction of enzyme activity was observed after 96 h, for dsHaAMY49, dsHaAMY48 and equal mix of them together in the treated group (59, 79 and 87.5%). In both, group of bioassay was recorded significant reduction to their respective controls in all tested times (fig. 9a, b)
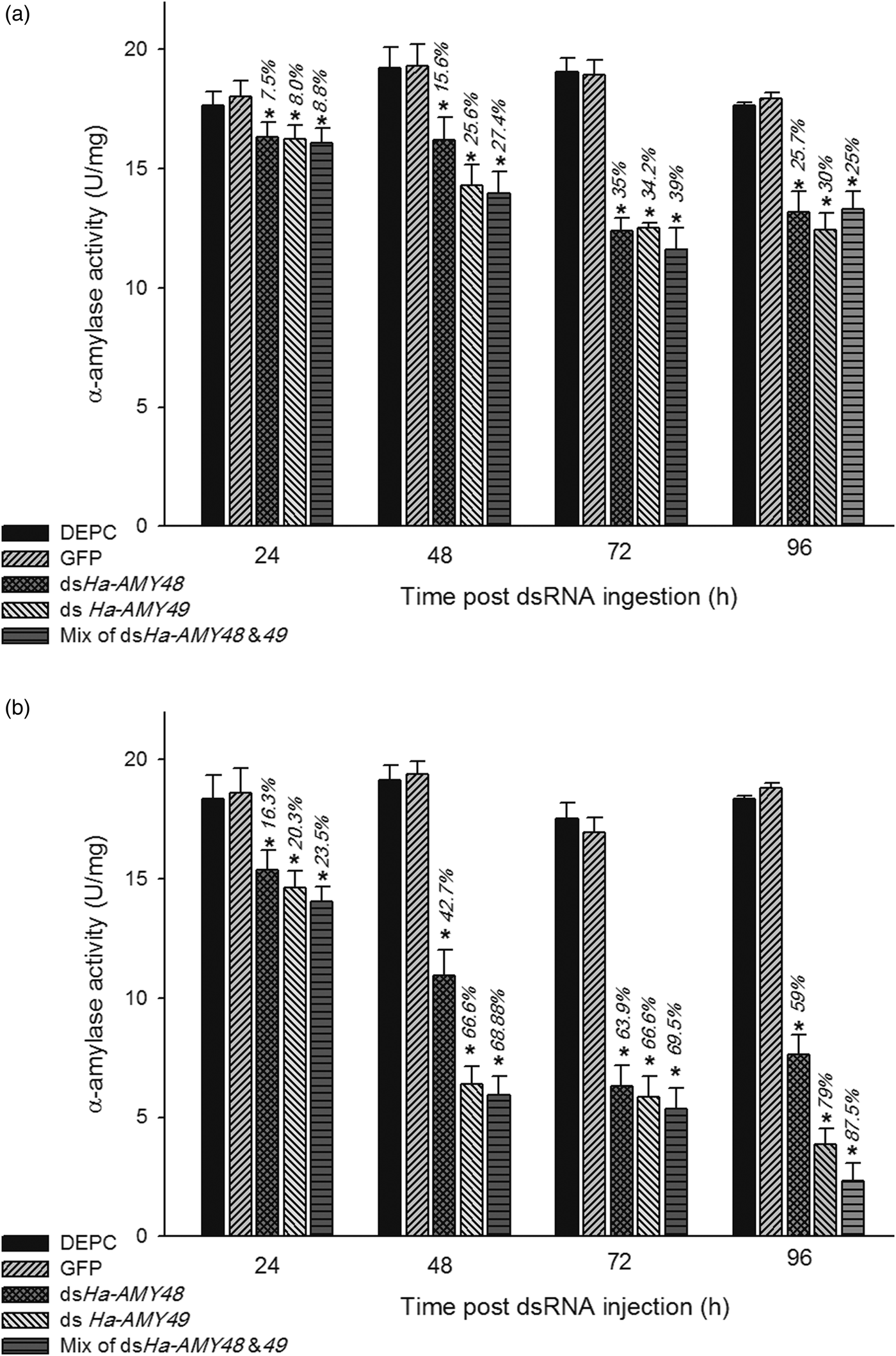
Fig. 9. RNAi effects on α-amylase-specific activity post 24, 48, 72 and 96 h of last treatment; midgut enzyme activity was calculated after dsRNA treated as feeding (a) and injecting (b) on specific substrate, starch. The percentages on bars show inhibition % of enzyme activity that were calculated basis of DEPC groups. Asterisk indicates significant difference of relative expression (P < 0.05, Student's t-test).
Effects of injection bioassay on visualization of midgut α-amylase activity
The zymogram analysis showed that after DEPC injection, three bands of α-amylase activity in the midgut of cotton bowl worm larvae were active. However after 24 h, the third band was weak and after 48 and 72 h in addition to the third band, the second band was also fade (fig. 10).
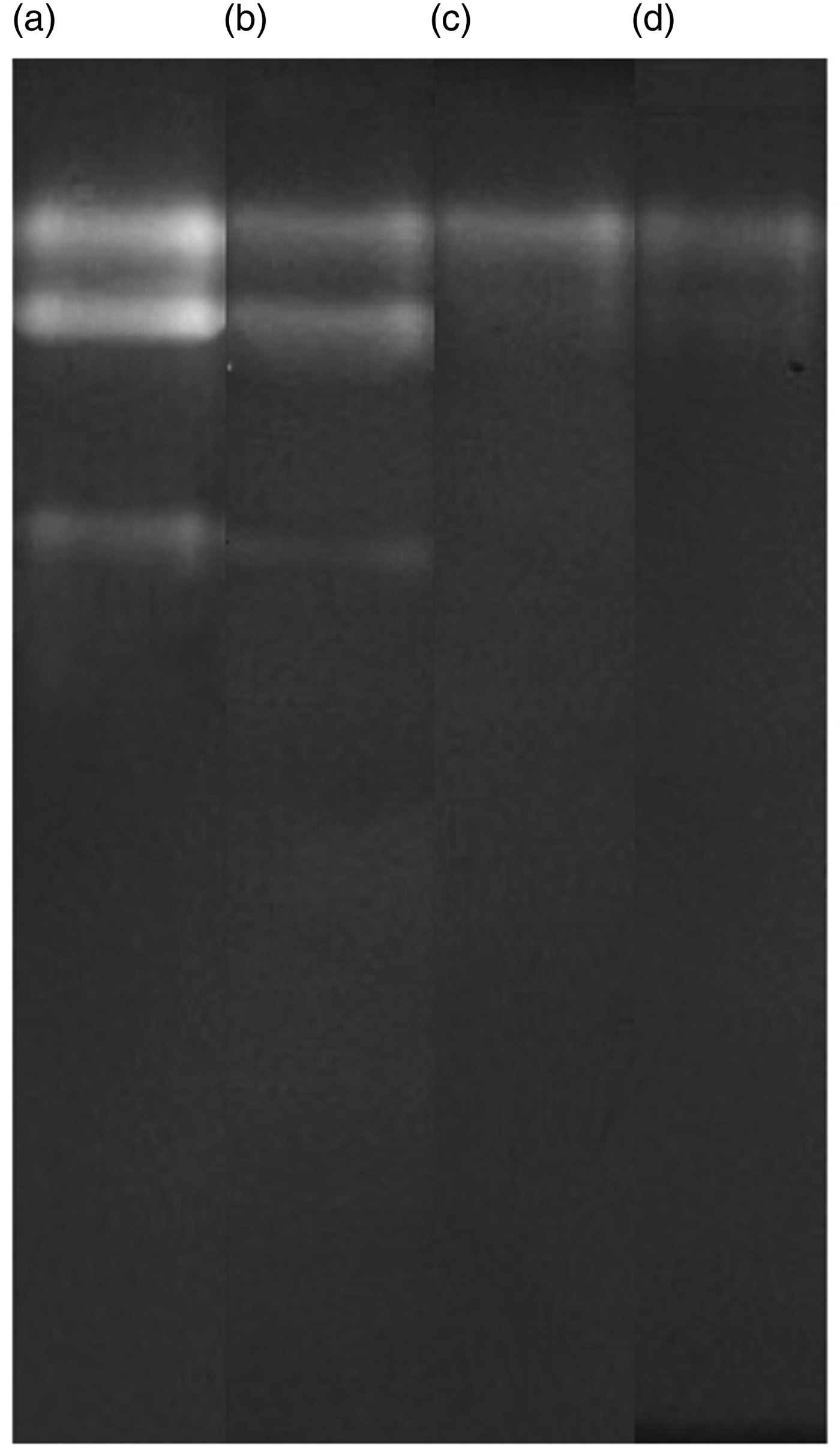
Fig. 10. Visualization of midgut α-amylase activity undergoes RNAi effects as injection bioassay. After 3rd injection in the treated larvae silencing effect of midgut α-amylase activity was studied by native PAGE profile after 24, 48 and 72 h in compare with the control group. Dsgfp (lane a), α-amylase activity in larval midgut after 24 h (lane b), after 48 h (lane c) and post 72 h (lane d).
Discussion
In the present paper, α-amylase and JHE genes were chosen as candidate for RNAi. One of the important reasons for this selection is critical roles of these proteins in insects. In recent years, varying RNAi studies were done on insects, especially on lepidopteran pests such as H. armigera. Considering the importance of this pest, various genes that are responsible for varied functions such as digestion, detoxification, metamorphosis, lipid transport, etc. were selected to silencing (Chikate et al., Reference Chikate, Dawkar, Barbole, Tilak, Gupta and Giri2016).
In this study, two dsRNAs were synthesized for interfering of midgut α-amylase expression and one more related dsRNA was designed for whole-body JHE in cotton bollworm larvae, too, that to achieve these goals, were used feeding and injecting bioassay. After feeding bioassay, the results of qPCR showed that significant down regulation of transcripts was happened after 24 (except Ha-JHE), 48, 72 and 96 h. Suppression of mRNA expression was also observed in injecting bioassay results. We found that the injection of dsRNA to hemocoel is more effective to outbreak RNAi than when it is fed to larvae. Down regulation of Ha-JHE after dsRNA injection was about three times, than feeding bioassay (75 and 23%, respectively). The delivery methods of dsRNA are effective for RNAi.
In a previous study in S. exigua, it was observed that ingestion of dsRNA did not induce any silencing effects on amino peptidase-N gene in midgut of larvae but micro injection could down regulate this gene very well (Rajagopal et al., Reference Rajagopal, Sivakumar, Agrawal, Malhotra and Bhatnagar2002). Jing & Zhao-jun, Reference Jing and Zhao-jun2014 studied on efficiency of different method to delivery of dsRNA in cotton bollworm and in they concluded that ultraspiracle protein gene (USP) could be suppressed by single injection of in vitro synthesized dsRNA but no phenotypic effect was observed by single ingestion. Considering of RNAi studies in H. armigera illustrated that dsRNA applied dose, type of delivery method of dsRNA and exposure time of dsRNA with site of expression are effective on triggering silencing effects on target genes. A review on RNAi in lepidopteran order showed positive correlation between amount of applied dsRNA and silencing efficiency (Terenius et al., Reference Terenius, Papanicolaou, Garbutt, Eleftherianos, Huvenne, Kanginakudru, Albrechtsen, An, Aymeric, Barthel, Bebas, Bitra, Bravo, Chevalier, Collinge, Crava, de Maagd, Duvic, Erlandson, Faye, Felföldi, Fujiwara, Futahashi, Gandhe, Gatehouse, Gatehouse, Giebultowicz, Gómez, Grimmelikhuijzen, Groot, Hauser, Heckel, Hegedus, Hrycaj, Huang, Hull, Iatrou, Iga, Kanost, Kotwica, Li, Li, Liu, Lundmark, Matsumoto, Meyering-Vos, Millichap, Monteiro, Mrinal, Niimi, Nowara, Ohnishi, Oostra, Ozaki, Papakonstantinou, Popadic, Rajam, Saenko, Simpson, Soberón, Strand, Tomita, Toprak, Wang, Wee, Whyard, Zhang, Nagaraju, French-Constant, Herrero, Gordon, Swevers and Smagghe2011). In a same study, when was applied 15 µg g−1 of a 667 bp dsRNA of transferrin gene with AD, after 12, 24, 36 and 48 h was observed 0, 95, 4 and 0% mRNA decrease but changing amount of dsRNA to 35 µg g−1 caused 99, 99, 99 and 45% reduction of the mRNA level, respectively (Zhang et al., Reference Zhang, Shang, Lu, Zhao and Gao2015). Briefly 8.5, 4.4, 62.2 and 59.5% down regulation of chitinase gene with applying 0.425, 21.25, 10.625 and 53.125 µg g−1, respectively, was observed by feeding of transformed bacteria that expression related dsRNAs (Yang & Han, Reference Yang and Han2014). Feeding of transplastomic leaves that expressed a 19 bp dsRNA for chitin synthase, cytochrome P450 and v-ATPase, showed phenotypic effects on H. armigera such as mortality and growth inhibition (Jin et al., Reference Jin, Singh, Li, Zhang and Daniell2015). Lim et al., Reference Lim, Robinson, Jain, Chandra, Asokan, Asgari and Mitter2016 published comprehensive review paper that in this paper they mentioned some study about progresses and challenges related to applying of diet delivery of dsRNA in H. armigera through dsRNA solution, transgenic plant carrying a hairpin construct and bacterial suspension. Some phenotypic effects of candidate genes silencing was observed such as abnormality at larval, pupal and adult stages. These effects were occurred as impressive weight losing in larvae, too. Ha-JHE selected gene trigger more malformation, mortality and abnormality to cotton bollworm metamorphosis stages than each Ha-AMYs genes candidate. JHE is crucial importance in the regulation of development, metamorphosis and reproduction (Gilbert et al., Reference Gilbert, Granger and Roe2000). In a same study, RNAi of JH acid methyl transferase at H. armigera made some phenotypic effects such as growth inhibition and decreased pupation rate (Asokan et al., Reference Asokan, Sharath, Manamohan and Krishna2013). Suppression of amylase candidate genes, had phenotypic effects as well as Ha-JHE even though when larvae were fed or were injected with mixing of dsRNA (Ha-AMY49:48(1:1)) most abnormality was observed. Zhu et al. (Reference Zhu, Liu, Maddurb, Oppert and Chen2005) mentioned that α-amylases presence in insect's crucial processes such as digestion and help them to survive in varied situations and increase their fitness rate, and this enzyme is active in non-feeding phases such as pupa. Undoubted, considering to this important roles, each interfering in α-amylases pathway causes negative effects on insect's fertility and fecundity. We found significant reduction of α-amylases activity on the larvae that fed on AD cubes containing dsHaAMY48, 49 and dsHa-AMY49:48(1:1) and treated larvae with these dsRNA as injection. Output of this experiment confirmed RNAi effect on α-amylases in this paper. Interestingly, silencing effects on decreasing of catalysis effects of this enzyme on a specific substrate in the treated larvae with injection bioassay was appeared more than the dietary introduction in compare with control groups. Jing & Zhao-jun (Reference Jing and Zhao-jun2014) mentioned that dsRNA of USPs was degraded faster in midgut juice of H. armigera than hemolymph. Microinjection assay had 75% reduction on target gene in Rhodnius prolixus (Hemiptera), but dsRNA feeding only showed 42% reduction (Araujo et al., Reference Araujo, Santos, Pinto, Gontijo, Lehane and Pereira2006). Zymogram analysis showed three isozyme activity bands for α-amylases in control population of this species, while it was observed only two weak bands outcome of this enzyme activity of midgut crude extract from the larvae that were injected by Ha-AMY49:48(1:1) dsRNA, actually expression of one isozyme was suppressed 100%.
Conclusions
Our studies showed that both diet and injected delivered dsRNA effectively silenced candidate gene. But injection bioassay was more effective than ingestion method. Findings of this paper showed exposure time of dsRNA have a positive correlation with expression of interfering effects such as lose weight, adult malformations and specific enzyme activity. Certainly employed dose of dsRNA has a fundamental role on RNAi that it is better to considered. We understood that two candidate genes for α-amylase are different compare together because applying of their dsRNA showed an accumulative effect. Due to the mortal and destructive phenotypic effects of looking for silencing of Ha-AMY and Ha-JHE genes, we found that these genes can be candidates for new strategies to control of H. armigera.
Acknowledgements
We are sincerely grateful to the Hamedan Science and Technology Park, Biotechnology laboratory, for all of helps to us.














