INTRODUCTION
Tropical montane cloud forests (TMCF) are among the most structurally complex of all terrestrial ecosystems, due in part to the abundance and diversity of epiphytes (Vareschi Reference VARESCHI and Huber1986). Epiphytes contribute to complex arboreal communities, which consist of vascular and non-vascular plants, decomposing litter from the host tree and epiphytes, and canopy soil, which we refer to as epiphytic material (hereafter, EM) (Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2004). These canopy communities have attracted the attention of tropical botanists and ecologists for over 125 y (Schimper Reference SCHIMPER1888). Vascular epiphytes can account for up to one-third of the species and one-half of the individual plants at the stand level in TMCFs (Gentry & Dodson Reference GENTRY and DODSON1987a). Because epiphytes are limited in their ability to gain access to terrestrial soil resources, many of them rely on morphological and physiological adaptations and biotic interactions to acquire nutrients and water, including litter-impounding pools, foliar trichomes, foliar water storage, foliar uptake capacity, insectivory, myrmechochory and poikilohydric foliage (Benzing Reference BENZING1990, Gotsch et al. Reference GOTSCH, NADKARNI, DARBY, GLUNK, DIX, DAVIDSON and DAWSON2015). The species composition, physiology, distribution and conservation of EM in TMCF pools have been summarized elsewhere (Bruijnzeel et al. Reference BRUIJNZEEL, SCATENA and HAMILTON2010, Coxson & Nadkarni Reference COXSON, NADKARNI, Lowman and Nadkarni1995, Gentry & Dodson Reference GENTRY and DODSON1987b, Ingram & Nadkarni Reference INGRAM and NADKARNI1993, Sugden & Robins Reference SUGDEN and ROBINS1979, Zotz & Hietz Reference ZOTZ and HIETZ2001), but we lack a comprehensive understanding of the ecosystem roles played by EM.
Many studies in TMCFs have suggested that EM contributes to ecosystem function by increasing cloud water and nutrient interception and retention in the canopy, affecting the amounts and dynamics of inputs of resources to the forest floor, and creating or enhancing habitat for animals (Figure 1). In some TMCFs, epiphytic foliage provides a large fraction of the leaf area and foliar biomass of tree crowns (Hofstede et al. Reference HOFSTEDE, WOLF and BENZING1993), which enhances wet and dry deposition by increasing the total physical area of canopy surfaces (Nadkarni Reference NADKARNI1984a). The dynamic nature of EM – which turns over rapidly due to short life spans relative to trees – allows it to move the allochthonous nutrients (ions derived from outside the ecosystem, e.g. in rain or mist) it impounds from the canopy to ground-dwelling members of the ecosystem via throughfall, stemflow and litterfall (Figure 2; Nadkarni & Matelson Reference NADKARNI and MATELSON1991). EM components – particularly non-vascular epiphytes and canopy soils – have a high water-storage capacity and can retain water intercepted by vascular plants in the canopy (Hölscher et al. Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004, Köhler et al. Reference KÖHLER, TOBÓN, FRUMAU and BRUIJNZEEL2007). EM also supports organisms that fix nitrogen (N) (Forman Reference FORMAN1975), which enhances the total nutrient pool available to components of EM and to the forest as a whole. The micro-environment of canopy soils in TMCFs can be highly acidic, which suppresses microbially mediated activities such as nitrification, creating less mobile forms of ions, and thus a more nitrogen-retentive environment (Vance & Nadkarni Reference VANCE and NADKARNI1990). Both live and dead EM components provide food and habitat resources for a great number of birds and arboreal mammals (Nadkarni & Matelson Reference NADKARNI and MATELSON1989, Remsen & Parker Reference REMSEN and PARKER1984). All of these elements, then, create a canopy subsystem that is independent but related to the forest as a whole (Carroll Reference CARROLL and Waring1980).
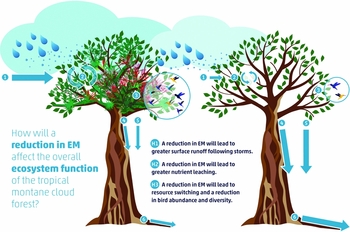
Figure 1. Ecosystem roles of epiphytic materials (EM, left tree) and the hypothesized reduction in ecosystem services due to a loss of EM (right tree). Water and nutrient deposition (1), water cycling and nutrient retention (2), and food resources and habitat are all reduced in the absence of EM. Decreases in interception and cycling due to a loss of EM will lead to an increase in stem flow (4, right) and throughfall (5, right) in disturbed EM communities and eventually lead to increases in surface run-off (6, right). These changes in ecosystem function can have large-scale impacts on the tropical montane cloud forest ecosystem.

Figure 2. Pathways of water and nutrients through EM. Water and nutrients enter EM largely through the interception of wet deposition of water and nutrients (1). Intercepted moisture and nutrients enter EM via epiphytic throughfall and stemflow of vascular epiphytes (2), which can then be immobilized or recycled within the dead components of EM or taken up and stored in live EM components (3) or travel over or within the EM substrate as stemflow of host trees (4). Intercepted materials can also be moved to other ecosystem components via host tree throughfall (5) or through litter fall of EM that falls on its own or rides down on falling branches and trunks (6).
TMCFs are dynamic habitats that are subject to natural disturbances such as wind and hurricanes (Gannon & Martin Reference GANNON and MARTIN2014, Lawton et al. Reference LAWTON, NAIR, PIELKES and WELCH2001, Pounds et al. Reference POUNDS, FOGDEN and CAMPBELL1999). Increasingly, disturbance is caused by human activities, such as harvesting of trees and secondary forest products, conversion to agriculture and climate change. These activities can also cause a reduction in or loss of the important ecosystem functions that the EM provide to the TMCF ecosystem. The rate of recovery of diversity and biomass of epiphytes following clearing on canopy-level branches is very slow. A study in Monteverde, Costa Rica documented that recovery of the EM community took on the order of two to three decades, indicating that even though canopy plant communities appear to be lush and resilient, physical disturbance can have extremely long-term impacts (Nadkarni Reference NADKARNI2000). Without healthy communities of EM, or with reduced EM vitality, these inputs and resources could be lost or reduced, resulting in less productive and efficient systems that are biologically less active and resilient (Figure 1).
Epiphytic communities in old-growth forests also tend to be far more abundant and diverse than those in recovering or secondary forests following disturbance. One study that directly compared epiphytic communities in old growth and 40-y-old secondary forests in the Dominican Republic found epiphyte life-form diversity, species richness, and abundance were strikingly higher in old-growth forests (e.g. arborescent ferns, palms, epiphytic bromeliads, orchids and bryophytes) (Martin et al. Reference MARTIN, SHERMAN and FAHEY2004).
EM and the functions they provide are likely to be vulnerable to projected changes in climate, as they are largely disassociated from resources on the ground. Nadkarni & Solano (Reference NADKARNI and SOLANO2002) used experimental transplants of upper cloud-forest epiphyte mats to tree canopies at slightly lower altitudes that experience longer dry-season conditions, and documented that vascular epiphytes exposed to drier conditions experienced greater mortality and lower leaf production than control conspecifics exposed to moister conditions. Other researchers have found that epiphytes, despite adaptations to store water and nutrients, are not particularly resistant to the leaf water potentials associated with extended dry periods (Gotsch et al. Reference GOTSCH, NADKARNI, DARBY, GLUNK, DIX, DAVIDSON and DAWSON2015, S. Gotsch, unpubl. data). As the global atmosphere continues to change and as human activities continue to negatively affect epiphytes, EM may be the first biotic indicators of change.
In this paper, we review the literature on the ecological significance of EM in TMCFs. We also include studies from subtropical and temperate forests that receive significant inputs of low-lying clouds or fog as a point of comparison (herein, cloud-affected forests, Mulligan Reference MULLIGAN, Bruijnzeel, Scatena and Hamilton2010). We focus on three main ecosystem functions: water interception and storage, nutrient interception and storage, and food, and habitat use by animals (Figure 1). We synthesize our current understanding of the importance of EM to ecosystem function and place that in the context of possible outcomes of changes in EM ecosystem function due to changes in land use and climate.
BIOMASS OF EM
Quantifying the total amount of EM biomass is critical to understanding its role in ecosystem processes. In most mature TMCFs where EM loads are high, canopy soils constitute a large proportion of the dead biomass and nutrient capital that accumulates on nearly all stem surfaces held within the canopy (Hofstede et al. Reference HOFSTEDE, WOLF and BENZING1993, Nadkarni Reference NADKARNI1984a, Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2004). In the Monteverde TMCF, canopy soils constituted 63% of total EM biomass in the primary forest, and 3% of EM biomass in the secondary forest.
The biomass of epiphytes and their associated organic matter varies greatly across sites. The lowest estimate of EM (0.5 kg ha−1) was documented for an 8-y-old regeneration site in subtropical cloud forest in the Canary Islands; the greatest EM biomass (44000 kg ha−1) was recorded for a TMCF in Colombia (Hofstede et al. Reference HOFSTEDE, WOLF and BENZING1993, Patiño et al. Reference PATIÑO, GONZÁLEZ-MANCEBO, FERNÁNDEZ-PALACIOS, ARÉVALO and AND BERMÚDEZ2009). TMCF old-growth forest sites generally had the highest biomass (mean±SE = 25670±5730 kg ha−1, n = 7) although there was an order of magnitude of variation across the sites (Table 1). Studies that determine biomass per hectare do so by measuring biomass on a number of branches or trees and then scale up values based on stand basal area. Such scaling may introduce potentially large errors into these estimates.
Table 1. Biomass estimates (kg ha−1) of epiphyte communities in cloud-affected forests. Biomass was measured on dried subsamples from dominant canopy trees in the study sites and these values were then scaled up to the stand level (numbers of samples per tree and trees varied across sites). In most cases, all dominant plant functional groups (vascular plants, bryophytes, ferns) were considered as well as dead organic matter or DOM (canopy litter, detritus and arboreal soil). In two cases, only bryophyte groups were considered. In these cases (McCune Reference MCCUNE1993, Patiño et al. Reference PATIÑO, GONZÁLEZ-MANCEBO, FERNÁNDEZ-PALACIOS, ARÉVALO and AND BERMÚDEZ2009) the dominant members of the canopy are bryophytes. The extent to which canopy soil is present in these systems was not reported. We limit our synthesis to those studies that quantify biomass at the stand level.

This synthesis also indicates that the EM takes decades to accumulate after disturbance and that the forests with higher EM biomass are likely to take even longer to reach such levels. For example, in the Canary Islands, only 0.5 kg ha−1 of EM accumulated after 8 y of recovery and that number rose to 205 kg ha−1 after 25 y. In the span between 25–60 y of regeneration, EM biomass accumulated exponentially (from 205 to 1253 kg ha−1, Patiño et al. Reference PATIÑO, GONZÁLEZ-MANCEBO, FERNÁNDEZ-PALACIOS, ARÉVALO and AND BERMÚDEZ2009). In the wetter TMCF of Monteverde Costa Rica, EM biomass in a ~40-y secondary forest was 200 kg ha−1, whereas EM in the old-growth forest was over two orders of magnitude greater (Table 1, Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2004).
WATER-HOLDING CAPACITY OF EM
The large surface area of canopy epiphytes and the high water-storage capacity of bryophytes and canopy soils in the TMCF can lead to overall high canopy water storage. For example, in Costa Rica, mosses in the TMCF have been documented to have a water-holding capacity of over 400% of their dry weight (Hölscher et al. Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004, Köhler et al. Reference KÖHLER, TOBÓN, FRUMAU and BRUIJNZEEL2007). In an extreme case, a tropical cushion moss species, Octoblepharum pulvinatum, has a water-holding capacity of ~7000% its dry weight (Wagner et al. Reference WAGNER, BADER and ZOTZ2014). At the stand level, the average of point estimates of water-storage capacity across all studies is 2.2±0.8 mm. The maximum water-storage capacity of EM varies from 0.81 mm in a tropical montane rain forest in the Talamanca Mountains of Costa Rica, to 5.0 mm in a mossy elfin forest in Tanzania (Hölscher et al. Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004, Pócs Reference PÓCS1980) (Table 2).
Table 2. Water retention and maximum water content of epiphyte communities in cloud-affected forests. In the Pócs (Reference PÓCS1980), Zotz et al. (Reference ZOTZ, BUDEL, MEYER, ZELLNER and LANGE1997), Wagner et al. (Reference WAGNER, BADER and ZOTZ2014) and Pypker et al. (Reference PYPKER, UNSWORTH and BOND2006) studies, maximum water content was determined as the difference between the saturated epiphyte mat weight and the fresh weight of the mat. In the Hölscher et al. (Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004) and Köhler et al. (Reference KÖHLER, TOBÓN, FRUMAU and BRUIJNZEEL2007) experiments, the fresh and dry weight of moss mats were determined at different times of the year. In these two cases, the maximum water content was the greatest percentage of fresh to dry weight measured throughout the year.

The importance of EM to total canopy interception is likely to vary greatly depending on the dominant components of EM (bryophytes versus vascular epiphytes) and rainfall patterns (Hölscher et al. Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004, Pócs Reference PÓCS1980). Only one study quantified the water-storage capacity of vascular and non-vascular components of the EM (Pócs Reference PÓCS1980). Water-storage capacity was within the range of that documented in bryophyte-dominated systems (Table 2). Future work is needed to quantify the water storage of different components of the EM, especially in wetter TMCFs, which have high biodiversity and biomass. These sites tend to have vascular epiphytes with specialized leaf and stem adaptations for water storage, which may play an important role in canopy water storage (Gotsch et al. Reference GOTSCH, NADKARNI, DARBY, GLUNK, DIX, DAVIDSON and DAWSON2015, Ogburn & Edwards Reference OGBURN and EDWARDS2010).
Although the capacity for water storage by EM may be high, the realized water storage will depend on rainfall dynamics and canopy microclimate at each site. If the EM remains close to saturation at the time of a rainfall event, then little to no additional water storage will be realized. In the Talamanca region of Costa Rica, Hölscher et al. (Reference HÖLSCHER, KÖHLER, VAN DIJK and BRUIJNZEEL2004) found that despite a considerable maximum storage capacity, modelling results suggest that mosses contribute just 6% of total interception due to their generally high saturation. While consistent rainfall inputs may be the case in some sites, microclimate in the TMCF is often variable (Holwerda et al. Reference HOLWERDA, BRUIJNZEEL, MUÑOZ-VILLERS, EQUIHUA and ASBJORNSEN2010, Ritter et al. Reference RITTER, REGALADO and ASCHAN2009). Köhler and colleagues found that during a 3-d period without rainfall, maximum water loss was 251% of dry weight for bryophytes and 117% for canopy humus (Köhler et al. Reference KÖHLER, TOBÓN, FRUMAU and BRUIJNZEEL2007). In TMCFs with more variability and seasonality in precipitation, EM will have a larger role in forest water-holding capacity.
Changes in climate such as increases in cloud-base heights and precipitation patterns may increase dry periods and thereby alter water storage dynamics of EM. In the TMCF of Monteverde, Costa Rica, over the last 40 y, researchers have documented changes in climate that include increased variability in annual precipitation and total rainfall and an increase in the number of days without rain on an annual basis, and the number of 3–5-d periods without precipitation (Pounds et al. Reference POUNDS, FOGDEN and CAMPBELL1999, A. Pounds, unpubl. data). In this region, storm size is increasing. Large rain events may quickly saturate the EM and terrestrial soils and less of the total water in each event will be available to the forest as a whole since more will be lost as run-off. Increases in drought events may also lead to water stress in EM, leading to changes in species composition and biomass, which will in turn also affect water-storage capacity.
NUTRIENT POOLS AND FLUXES OF EM
Nutrient pools
Nutrients within the EM have only rarely been quantified, due to difficulties with canopy access and because the spatial complexity (three-dimensional irregular topography of tree surfaces) of EM makes statistical analysis difficult (Affeld et al. Reference AFFELD, SULLIVAN, WORNER and DIDHAM2008, Gradstein et al. Reference GRADSTEIN, NADKARNI, KRÖMER, HOLZ and NÖSKE2003). Several studies have compared nutrient content of terrestrial and epiphytic vegetation (Cardelús & Mack Reference CARDELÚS and MACK2010, Hietz et al. Reference HIETZ, WANEK, WANIA and NADKARNI2002, Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2004). One study assessed the biomass and nutrient capital of EM and terrestrially rooted components of a primary and an adjacent secondary montane forest in Monteverde, Costa Rica (Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2004, Table 3). The latter study also compared the nutrient capital held in labile portions (i.e. those elements that are rapidly decomposed, including foliage and small stems) vs. non-labile elements (large stems, bark, trunk) of the EM vs. the terrestrially rooted material in the primary and secondary forest. The ratios were 4.4:1 in the primary forest and 0.05:1 in the secondary forest, indicating a much larger proportion of available nutrients are held in the primary-forest EM than in the secondary-forest EM.
Table 3. Nutrient capital of epiphytes in TMCFs. Amounts of mineral capital in epiphytes in proportion to ecosystem components of cloud forest ecosystems. Amounts of mineral capital of total above ground and of foliar components are given in kg ha−1. Proportions of stand-level epiphyte mineral capital estimates (in parentheses) are expressed as a percentage of ecosystem components.

Canopy soil in TMCFs is of special interest in assessing the functional roles of EM because of its capacity to capture incoming nutrients by storing them on its negatively charged sites and its large pool of organically bound nutrients (Lesica & Antibus Reference LESICA and ANTIBUS1991, Nadkarni & Longino Reference NADKARNI and LONGINO1990, Vance & Nadkarni Reference VANCE and NADKARNI1990, Veneklaas Reference VENEKLAAS1992). These arboreal soils are histosols (dominated by organic materials, Bohlman et al. Reference BOHLMAN, MATELSON and NADKARNI1995, Nadkarni et al. Reference NADKARNI, SCHAEFER, MATELSON and SOLANO2002) and are derived from decomposing live epiphytes and canopy biota and ions intercepted from wet and dry deposition and throughfall (Coxson & Nadkarni Reference COXSON, NADKARNI, Lowman and Nadkarni1995).
Intercanopy nutrient fluxes
Nutrient inputs for EM may be autochthonous (intercepted host tree litter, throughfall and stemflow) or allochthonous (ions derived from outside the ecosystem, e.g. in rain or mist). From the standpoint of ecosystem function, it is important to distinguish between those two source types. If epiphytes obtain all of their nutrients from autochthonous sources, then they would simply be diverting nutrients from the tree to the ground flux pathway for some length of time and not increasing the total nutrient pool. Alternatively, if they were to sequester nutrients from outside the system, this would potentially increase the total nutrient input to the ecosystem in addition to altering the form and location of these new nutrients (Nadkarni & Matelson Reference NADKARNI and MATELSON1991, Figure 2). However, we have found no studies that have documented the degree to which nutrient input to EM derives from these two sources.
However, atmospheric deposition has been documented as one of the major avenues for input of new nutrients in TMCF ecosystems (Clark et al. Reference CLARK, NADKARNI, SCHAEFER and GHOLZ1998a, Stewart et al. Reference STEWART, SCHMIDT, TURNBULL, ERSKINE and JOLY1995). Tobón et al. (Reference TOBÓN, KÖHLER, FRUMAU, BRUIJNZEEL, BURKARD, SCHMID, Bruijnzeel, Scatena and Hamilton2010) noted relatively long residence times of ions in cloud water and mist captured by epiphytic bryophyte mats, facilitating nutrient retention. In the TMCF of Monteverde Costa Rica, per cent net retention of nitrate and ammonium by the canopy was 80% and 61%, respectively, indicating that a large majority of the inorganic N in atmospheric deposition was retained by the canopy for some length of time, although the intervals of this dynamic have not been measured (Clark et al. Reference CLARK, NADKARNI and GHOLZ1998b). Clark et al. (Reference CLARK, NADKARNI and GHOLZ2005) developed a canopy model driven with hourly meteorological and event-based atmospheric deposition data to estimate inorganic N retention from atmospheric deposition by canopy components. They estimated net retention of inorganic N by samples of four components of canopy communities: (1) epiphytic bryophytes, (2) epiphyte assemblages (small vascular epiphytes, intercepted litter and canopy soils), (3) large vascular epiphyte foliage and (4) host-tree foliage. Samples of each component were exposed in situ to artificial cloud water and precipitation solutions that mimicked field concentrations. Leaching experiments indicated that NO3 − was strongly retained by and incorporated into epiphytic bryophytes, but not by the foliage of large vascular plants. Net retention of NH4 + by epiphytic bryophytes and epiphyte assemblages was somewhat lower, and reflected the internal cycling of NH4 + in canopy soils. With a multi-layered model of the canopy, they determined epiphytic assemblages retained 33–67% of the inorganic N deposited in cloud water and precipitation (annually, 3.4 kg N ha−1 y−1, c. 50% of the inorganic N in atmospheric deposition, 6.8 kg N ha−1 y−1). Thus, in this TMCF, epiphytic bryophytes play a major role in N retention and cycling in the canopy by transforming highly mobile inorganic N (c. 50% of atmospheric deposition is NO3 −) to less mobile (exchangeable NH4 +) and recalcitrant forms in biomass and remaining litter and canopy humus. Nitrogen fixation may be an important allochthonous source of N for TMCF epiphytes, either by above-ground adventitious roots (e.g. Alnus sp.) (Murcia Reference MURCIA1997) or, more commonly, by asymbiotic and lichenized N fixation (Forman Reference FORMAN1975).
A potential autochthonous source to epiphyte communities is abscised plant litter that is intercepted within the tree canopy by inner branches and their epiphytes. The decomposition and mineralization of nutrients from abscised canopy foliage may differ from that on the forest floor. For example, in the Monteverde TMCF, Nadkarni & Matelson (Reference NADKARNI and MATELSON1991) quantified the amounts of nutrients in litterfall that are stored within the canopy and then released via decomposition and mineralization. Decomposition rates of tethered leaves within the canopy were half that of leaves on the forest floor (turnover time in the canopy = 2.7 y vs. 1.7 y on the forest floor). Inputs from intercepted host-tree litterfall appeared to be small relative to the productivity of epiphytes, which suggested that epiphytes rely on other sources of nutrients.
Nutrient release and uptake
Nutrients from live and dead EM are released into the nutrient cycles of whole forests when: (1) EM falls to the forest floor (via senescence, wind, disruption by birds and mammals), dies and decomposes; (2) EM is leached by precipitation and moved via stemflow and throughfall; and (3) epiphyte and host tree canopy roots take up nutrients within the canopy (Figure 2). Nearly all litterfall studies have focused on the biomass and nutrient composition of fine litter derived from terrestrially rooted plants, but in TMCFs, EM litterfall can constitute a potentially significant nutrient transfer pathway (Songwe et al. Reference SONGWE, FASEHUN and OKALI1988, Tanner Reference TANNER1980). In Monteverde, for example, EM input constituted c. 15% of total litterfall biomass and nutrient input to the forest floor, but it was deposited more sporadically in time and less regularly in space than terrestrially rooted litterfall (Nadkarni & Matelson Reference NADKARNI and MATELSON1992). Major disturbance events such as hurricanes (which occur in some TMCFs) may be critical. Future research should differentiate the sources of litterfall (epiphytic vs. host tree foliage) in TMCFs that are affected by hurricanes and other major storms.
Direct uptake of nutrients can also occur from adventitious roots from dead organic matter in the canopy. Canopy rooting is a widespread phenomenon in diverse taxa, including common neotropical TMCF families Lauraceae, Flacourtiaceae and Cunoniaceae (Herwitz Reference HERWITZ1986, Nadkarni Reference NADKARNI1981). In the Monteverde Cloud Forest, fine-root biomass in canopy soils of canopy branch junctions was 20% higher than comparable organic soil horizons on the forest floor, although the amount of host tree canopy roots relative to epiphytic roots was not reported (Vance & Nadkarni Reference VANCE and NADKARNI1992). The importance of mycorrhizal fungi to plant nutrition and seedling establishment in low-nutrient environments has been demonstrated in TMCF canopy environments (Maffia et al. Reference MAFFIA, NADKARNI and JANOS1993, Rains et al. Reference RAINS, NADKARNI and BLEDSOE2003). Hertel et al. (Reference HERTEL, HOHLER and RILLIG2011) suggested that canopy roots of trees in an upper TMCF oak forest, though common and showing an abundance of endophytic fungi, lacked mycorrhizal colonization. These canopy roots appeared not to be important, as they showed a reduced ability for nutrient acquisition relative to terrestrial roots. Surveys of canopy plants are needed to ascertain the extent of mycorrhizas and other symbionts in canopy microhabitats.
Epiphytes move physically from the canopy to the forest floor when they are dislodged by wind or animals, or because their associated branches break and fall (Strong Reference STRONG1977). Some epiphytes die, decompose and vanish quickly, while others can survive for months or even years (Matelson et al. Reference MATELSON, NADKARNI and LONGINO1993). Implications for nutrient cycling are that before nutrients can be released, the live plants must die, so fallen EM affects nutrient cycling differently than tree and shrub litterfall, which has already senesced (and has often undergone retranslocation) before arriving to the forest floor, ready for nutrient release. Little has been quantified concerning epiphyte decomposition, but results from one study indicate that certain components of EM decompose very rapidly (e.g. bryophytes, turnover = 0.23 y), while others decompose very slowly (e.g. canopy soils, turnover = 3.6 y) (Nadkarni & Matelson Reference NADKARNI and MATELSON1991).
Leaching and throughfall
An extensive literature on nutrient transfer via crown-wash in whole forests exists (reviews in Bruijnzeel & Proctor Reference BRUIJNZEEL and PROCTOR1995, Cavelier et al. Reference CAVELIER, JARAMILLO, SOLIS and DE LEÓN1997). For canopies where vascular plants dominate, nutrient loss from the canopy is largely due to the re-suspension of surface-deposited nutrients (dry deposition) and from exchange processes between surface water films and mesophyll cells (Scott & Hutchinson Reference SCOTT and HUTCHINSON1987). In TMCF, where non-vascular and vascular epiphytes co-occur, both processes may be co-occurring, but more research is needed on these processes (Figure 2).
EM RESOURCES FOR ANIMALS
The Food and Agriculture Organization of the UN describes TMCFs as comprising only a portion of the 1.6% of the world's closed tropical forests, yet they provide habitat for numerous vertebrate and invertebrate species, many of which are endemic (Aldrich et al. Reference ALDRICH, BUBB, HOSTETTLER and VAN DE WEIL2000, https://portals.iucn.org/library/node/7809). EM facilitates the existence of animal diversity because it provides food, water and habitat resources (Ackerman Reference ACKERMAN1986, Céréghino et al. Reference CÉRÉGHINO, LEROY, DEJEAN and CORBARA2010, Hietz & Winkler Reference HIETZ and WINKLER2006, Wilson Reference WILSON1988). With the increasing pressure of land-use and changes in climate, it is critical to identify the relationships between EM and the animals that interact with them. Little research on invertebrate use of EM in TMCFs exists, with some exceptions (Nadkarni & Longino Reference NADKARNI and LONGINO1990). We focus here on the interactions of birds and mammals with EM in the TMCFs of Costa Rica to illustrate the significance of EM for animals, which provide the most complete set of relevant data (Nadkarni & Matelson Reference NADKARNI and MATELSON1989, Nadkarni & Wheelwright Reference NADKARNI and WHEELWRIGHT2000, Sillett Reference SILLETT1994).
Epiphyte foraging and fruit dispersal
Research on bird use of EM has been studied by Nadkarni & Matelson (Reference NADKARNI and MATELSON1989) and Sillett (Reference SILLETT1994), and provides the most complete studies exploring the importance of EM for survival of animal populations. In Monteverde, of the 33 bird species studied, 59% used epiphyte food resources, which were primarily hummingbirds, tanagers and flycatchers (Nadkarni & Matelson Reference NADKARNI and MATELSON1989). In the Talamanca Mountains, birds used EM and epiphytes by foraging on the undersides of branches, probing epiphytes and bark surfaces, and feeding on epiphytic fruits (Sillett Reference SILLETT1994). Many bird species were documented as epiphyte-specialists, species using epiphytes for at least 75% of their foraging time (Sillett Reference SILLETT1994). For example, the buffy tuftedcheek (Pseudocolaptes lawrencii) spent 74% of its time foraging in bromeliads, often pulling out and tossing leaf litter to reveal food items. For birds foraging in flowers, epiphytic flowers were used more frequently then flowers of the host trees (Nadkarni & Matelson Reference NADKARNI and MATELSON1989). Because of this frequent use of epiphyte resources by birds, the resource pool available to birds in tropical systems might be underestimated. Further studies are needed to estimate the resource pool for birds in TMCF ecosystems (Nadkarni & Matelson Reference NADKARNI and MATELSON1989).
In a Costa Rican TMCF, pollinating and dispersing animals appear to provide critically important vectors for epiphyte pollen and seed dispersal accounting for 62% of the epiphyte species studied (Figure 3), thereby contributing to sustaining healthy epiphyte populations. While these species do not take into account abundant species such as ferns and orchids, which do not use animal dispersers, fruiting species are also common and abundant and clearly provide an important resource to animals in the TMCF. Many epiphyte species and their pollinators and dispersers have highly specific relationships with animal dispersers (Fontoura et al. Reference FONTOURA, CAZETTA, NASCIMENTO, CATENACCI, DE VLEESCHOUWER and RABOY2010). Additionally, epiphytes and EM provide critically important habitat and food resources, which are especially important during times of the year when trees and shrubs are not flowering and fruiting. Research in other TMCFs on the relationships between EM and vertebrates are needed to understand the impacts and importance of EM.

Figure 3. Proportion of determined epiphyte species and their vertebrate and invertebrate pollinators and dispersers studied in TMCF of Costa Rica. The upper graph represents pollinator modes and the lower graph represents fruit and seed disperser modes. Insects, birds and bats were the three most important pollinator modes, whereas, birds, wind and mammals were found to be the three most important dispersal modes. Sample sizes (number of epiphyte species) for each pollinator and dispersal mode are shown in the graph. While these species do not take into account abundant species such as ferns and orchids, which do not use animal dispersers, fruiting species are also common and abundant and clearly provide an important resource to animals in the TMCF. This data was drawn from three publications, including Cascante-Marín et al. (Reference CASCANTE-MARÍN, OOSTERMEIJER, WOLF and DEN NIJS2005), Appendix 1 (Haber) in Nadkarni & Wheelwright (Reference NADKARNI and WHEELWRIGHT2000), and Bush & Beach (Reference BUSH and BEACH1995).
PRIORITIES FOR FUTURE RESEARCH
We suggest six areas for future research on EM in TMCF which include: (1) more process-oriented studies, particularly with respect to water interception and uptake by epiphytes, and retention by canopy soils; (2) the development of standardized measurements of canopy nutrient and water cycling so that measurements can be compared between sites and studies; (3) define the limits of scaling from within-tree to ecosystem- and landscape-level spatial scales, using statistically sound techniques and an understanding of the distribution of variability within and across sites; (4) additional experimental work (drought experiments, fertilization studies, removal experiments) to determine water and nutrient limitation as well as vulnerability to projected changes in climate; (5) establish long-term studies to quantify epiphyte growth, disturbance, death and recolonization rates; and (6) initiate simultaneous studies in a wide range of TMCF to generate a broadly based and comparable pool of data on EM and its effects in TMCF systems.
CONCLUSIONS
Epiphytes flourish in TMCFs. In the past three decades, exciting changes in canopy research approaches have informed our knowledge of canopy communities and their roles in ecosystem function. Research has shifted from autecological studies on individual organisms to ecosystem-level studies on the complex ways by which the canopy influences the processing of nutrients, water and light, enhancing our understanding of dynamics in primary forests and identifying potential effects of canopy disturbance or removal due to natural and human activities.
EM derives a significant fraction of water and nutrition from atmospheric sources, and provides animals with considerable resources including food, water, nesting materials and habitat. Evidence has demonstrated that the presence of EM can result in the interception and retention of atmospheric nutrients, which are subsequently cycled to other ecosystem members via multiple dynamic pathways. This enhancement of allochthonous water and nutrients may more speedily swell and maintain nutrient reserves of the ecosystem as a whole than if the epiphytes were absent or reduced in abundance.
An emerging concept from this and other reviews is that EM contributes significantly to ecosystem function. EM may even play keystone roles (sensu Terborgh Reference TERBORGH and Soule1986) providing critical plant resources during lean times of the year that maintain the carrying capacity of bird frugivores and foragers. Although EM makes up a small or unapparent portion relative to the total biomass of the forest, the resources they produce augment those of host trees, and their dependent organisms appear to depend upon these resources in other systems (Boucher & Nash Reference BOUCHER and NASH1990, Knops et al. Reference KNOPS, NASH, BOUCHER and SCHLESINGER1991). Although EM is clearly an important component of the TMCF ecosystem function, future research is needed to determine the vulnerability of the EM to changes in precipitation patterns and cloud base heights as well as the effects that any changes in EM communities will have on TMCF ecosystem function.
ACKNOWLEDGEMENTS
We acknowledge financial support from the National Science Foundation to the CloudNet Research Coordination Network (PIs Patrick Martin, Heidi Asbjornsen, Thomas Giambelluca, and Kenneth Young) who helped to guide the direction of this review. We acknowledge financial support from Franklin and Marshall College and the University of Utah. We are grateful for insightful comments from associate editor, Patrick Martin and two anonymous reviewers.








